Abstract
Objective
To investigate the association between plasma aldosterone concentration (PAC) and non-alcoholic fatty liver disease (NAFLD) diagnosis in Chinese hypertensive patients.
Methods
We conducted a retrospective study of all patients diagnosed with hypertension between January 1, 2010, and December 31, 2021. We included 3713 hypertensive patients based on the criteria for inclusion and exclusion. PAC measurement was performed using a radioimmunoassay. NAFLD was diagnosed using abdominal ultrasonography. Cox regression analysis was used to estimate the hazard ratios (HRs) and 95% confidence intervals (CIs) for univariable and multivariable models. A generalized additive model was used to identify nonlinear relationships between PAC and NAFLD diagnosis.
Results
A total of 3713 participants were included in the analysis. Over a median follow-up of 30 months, 1572 hypertensive individuals developed new-onset NAFLD. When PAC was used as a continuous variable, the risk of NAFLD increased by 1.04 and 1.24-fold for each 1 ng/dL and 5 ng/dL increase in PAC, respectively. When PAC was considered a categorical variable, the HR for tertile 3 was 1.71 (95% CI, 1.47–1.98, P < 0.001) compared to tertile 1. Overall, there was a J-shaped relationship between PAC and new-onset NAFLD. By fitting a two-piecewise linear regression model and using a recursive algorithm, we identified a PAC inflection point at 13 ng/dL (log-likelihood ratio test, P = 0.005). In adjusted model 3, for PAC ≥ 13 ng/dL, a 5 ng/dL increase in PAC was associated with a 30% increase in the risk of new-onset NAFLD (95% CI, 1.25–1.35, P < 0.001).
Conclusion
The study revealed a non-linear relationship between elevated PAC levels and the incidence of NAFLD in hypertensive patients. Notably, the risk of new-onset NAFLD was significantly increased when PAC levels were ≥13 ng/dL. Larger, prospective studies are necessary to confirm these findings.
Introduction
Non-alcoholic fatty liver disease (NAFLD) is a prevalent liver disease that affects over 25% of adults globally and is a leading cause of liver diseases.Citation1–3 Featuring a range of disorders from simple steatosis to the more severe non-alcoholic steatohepatitis (NASH) and fibrosis, NAFLD is a progressive disease that can result in cirrhosis and, eventually, hepatocellular carcinoma, which currently accounts for most liver transplantations.Citation2,Citation4–8 Hypertension is a highly significant risk factor for cardiovascular disease (CVD).Citation9 Additionally, NAFLD can worsen the progression of atherosclerosis, boosting the development of CVD events.Citation10,Citation11 Bi-directional relationships between NAFLD and hypertension have been observed, with evidence suggesting that NAFLD can both be a consequence and a cause of hypertension.Citation12 Moreover, the coexistence of hypertension and NAFLD is associated with considerably worse cardiovascular outcomes compared to either condition alone.Citation13 Thus, the identification and management of modifiable risk factors are of utmost importance in lowering the burden of NAFLD among hypertensive patients.Citation14
Aldosterone, a steroid hormone, is secreted by the adrenal gland to regulate volume and electrolyte balance.Citation15 Elevated levels of aldosterone have been associated with moderate to severe blood pressure elevation and severe target organ damage in numerous clinical studies.Citation16–19 In addition, aldosterone has been linked to inflammation and organ fibrosis in both epithelial and non-epithelial tissues.Citation20–22 Through the nucleotide-binding oligomerization domain-like receptor family pyrin domain-containing 3 inflammatory vesicle-associated pathways, aldosterone promotes hepatic stellate cell activation and liver fibrosis.Citation23 Recent observational studies have found an increased prevalence of NAFLD in patients with primary aldosteronism (PA),Citation24,Citation25 and a positive association between aldosterone levels and NAFLD has been demonstrated among African American women.Citation26 A selective aldosterone blocker (SAB) has been shown to attenuate fibrogenesis and suppress activated hepatic stellate cells in other studies.Citation27 However, one study suggests that aldosterone excess may not be correlated with the development of fatty liver disease in a mouse model fed a high-fat diet.Citation28 Nevertheless, there is a need for further investigation into the association between high plasma aldosterone concentrations (PAC) and NAFLD in hypertensive patients, given the controversial findings.
In this study, we conducted a retrospective cohort study to investigate the associations between PAC and the risk of NAFLD in hypertensive adults and to explore their potential dose-response relationship.
Materials and Methods
Study Design and Population
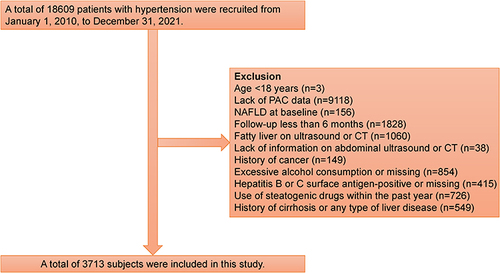
From January 1, 2010 to December 31, 2021, this study investigated hypertensive patients attending the People’s Hospital of Xinjiang Uygur Autonomous Region (n = 18,609). The study excluded subjects based on multiple criteria, including age <18 years (n = 3), lack of PAC data (n = 9118), pre-existing NAFLD (n = 1060), follow-up fewer than 6 months (n = 1828), inadequate abdominal ultrasound information (n = 38), history of cancer (n = 149), excessive alcohol consumption (>70 g/week for women or 140 g/week for men) or missing alcohol consumption (n = 854),Citation29 positive for hepatitis B or C surface antigen or missing (n = 415), use of steatogenic drugs within the past year (n = 726), and history of cirrhosis or any type of liver disease (n = 549). Ultimately, only 3713 participants were included in the analysis. shows the patient selection flowchart for this study. The Ethics Committee approved the ethics application (reference: KY2022080905). Also, all patients provided informed consent following the Helsinki Declaration.
Baseline Examination
Demographic characteristics, lifestyle factors, personal medical history, and medication use were extracted from medical records for all subjects. Details of specific measures, such as height, weight, smoking status, and baseline blood pressure, have been provided in the Supplementary Materials and referred to in prior publications.Citation30,Citation31 Fasting plasma glucose (FPG), total cholesterol (TC), high-density lipoprotein cholesterol (HDL-c), low-density lipoprotein cholesterol (LDL-c), triglycerides (TG), creatinine (Cr), blood urea nitrogen (BUN), uric acid (UA), alanine aminotransferase (ALT), aspartate aminotransferase (AST), and gamma-glutamyl transferase (GGT) levels were assessed through peripheral venous blood collection. Hormonal tests were conducted according to current guidelines and prior studies conducted at our center.Citation32,Citation33 PAC was measured by radioimmunoassay (DSL-8600 ACTIVE Aldosterone Coated Tube Radioimmunoassay Kit; Diagnostic Systems Laboratories, Webster, TX), with participants instructed to refrain from caffeine, alcohol, smoking, and strenuous physical activity for 12 hours before blood collection. Participants sat for 30 minutes after being active for at least 2 hours before blood collection, which occurred between 8:00 and 11:00 AM.
Definitions
Criteria for hypertension included self-reported hypertension, current use of anti-hypertensive medication, or systolic blood pressure (SBP) ≥ 140 mmHg and/or diastolic blood pressure (DBP) ≥ 90 mmHg recorded for at least three consecutive readings. T2DM was defined as fasting serum glucose ≥7.0 mmol/L, the 2-h serum glucose of the oral glucose tolerance test ≥11.1 mmol/L, or the current use of hypoglycaemic medication or insulin. A patient was diagnosed with PA as per the Endocrine Society’s clinical practice guidelines, based on PAC levels ≥ 12 ng/dL, aldosterone-to-renin ratio ≥ 20, and a PAC value ≥ 10 ng/dL confirmed by saline infusion test.Citation32 The BMI was calculated as weight divided by height squared.
Study Outcome
The follow-up began with an initial assessment for NAFLD conducted by a clinician, followed by annual abdominal ultrasounds to assess NAFLD.Citation34–36 Additional information on the NAFLD assessment is available in the Supplementary Materials section.
Statistical Analysis
PAC in baseline characteristics was separated into three groups: ≤ 11.55, 11.56–16.39, and ≥ 16.40 ng/dL. Kaplan-Meier survival analysis was carried out using Log rank tests. Tests for multicollinearity were performed using the variance inflation factor test, and there was no strong correlation among any of the variables in the model (variance inflation factor < 10 for all comparisons) (Table S1). Cox regression analysis was used to estimate the hazard ratios (HRs) and 95% confidence intervals (CIs) for univariable and multivariable models. PAC was described as a continuous variable (per 1 and 5 ng/dL increase) and a categorical variable (tertiles) and was placed into different models. A linear trend test was conducted by assigning medians to each tertile as a continuous variable in the models. We used a generalized additive model to identify the non-linear relationship. Heterogeneity across subgroups was assessed by Cox regression analysis and is presented in forest plots, and interactions between subgroups and PAC were examined by likelihood ratio testing. Lastly, to address potential unmeasured confounding, we calculated E-values.Citation37 Details of the statistical analysis are shown in the Supplementary Materials. All analyses were computed with R software, version 4.1.1, at a 2-sided, 5% α level of significance.
Results
Characteristics of Participants
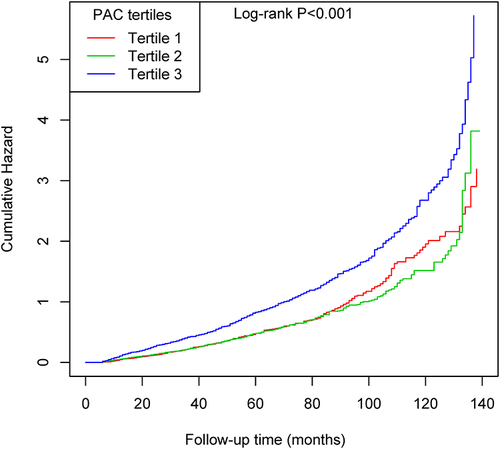
This cohort study included 3713 participants. The average age of the participants was 52.00 ± 11.80 years, with males accounting for 61.76%. Baseline characteristics of the participants grouped by tertiles of PAC can be found in . Participants in higher tertiles of PAC had higher tendencies for current smoking, a higher BMI, elevated levels of ALT, Cr, and UA, and were more likely to use insulin and spironolactone when compared with those in the tertile 1 group. 1572 (42.3%) participants were diagnosed with NAFLD during the median follow-up of 30 months. The numbers of patients with incident first NAFLD and the corresponding cumulative incidence in tertiles 1 to 3 were 411 (33.28%), 393 (31.82%), and 768 (61.79%), respectively. The Kaplan-Meier curve demonstrated that participants in tertile 3 of PAC had a higher risk of developing new-onset NAFLD events than those in other groups (Log rank test, P < 0.001) ().
Table 1 Baseline Characteristics of the Study Participants According to Plasma Aldosterone Concentrations Categories
Relationship Between PAC and NAFLD Diagnosis
Furthermore, we assessed the risk of NAFLD according to the presence of PAC (). When PAC was used as a continuous variable, it appeared that the risk of NAFLD was higher for each 1 ng/dL and 5 ng/dL increase in PAC; the HR were 1.04 (95% CI, 1.04–1.05, P < 0.001) and 1.24 (95% CI, 1.19–1.29, P < 0.001) respectively. And as a categorical variable, compared with tertile 1, the HR was 1.71 (95% CI, 1.47–1.98, P < 0.001) for tertile 3. Since the association between PAC and new-onset NAFLD had an E-value of 1.59, it seemed unlikely that unmeasured confounding could have caused the results (Figure S1).
Table 2 Regression Models of Effects of PAC on New-Onset NAFLD
Nonlinearity and Threshold Effect Analysis
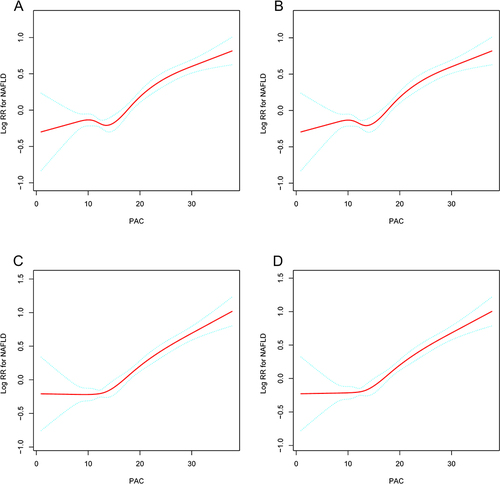
Both unadjusted and adjusted smoothing curves demonstrated that the relationship between PAC and new-onset NAFLD was nonlinear (). Overall, there was a J-shaped relationship between PAC and new-onset NAFLD. We used a two-piecewise linear regression model and recursive algorithm to gain the inflection point of PAC at 13 ng/dL (log-likelihood ratio test, P = 0.005) (). In adjusted model 3, for PAC ≥ 13 ng/dL, a 5 ng/dL increase in PAC was associated with a 30% increase in the risk of NAFLD (95% CI, 1.25–1.35, P < 0.001).
Table 3 Threshold Effect Analyses of PAC (per 5 Ng/dL Increase) on the Risk of New-Onset NAFLD Using Two-Piecewise Regression Models
Stratification Analysis and Test for Interaction
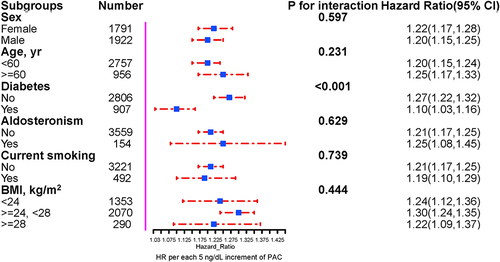
Stratified analyses were performed to assess the association of PAC (per 5 ng/dL increment) with new-onset NAFLD in various subgroups (). We observed a significant interaction between diabetes and non-diabetes (P for interaction < 0.001), with the non-diabetes group associated with a greater increment in NAFLD (HR 1.27, 95% CI 1.22–1.32) than those with diabetes (HR 1.10, 95% CI 1.03–1.16). None of the other variables, including sex, age, duration of hypertension, PA, diabetes, current smoking, and BMI, significantly modified the association of PAC with new-onset NAFLD (all P-interactions > 0.05).
Discussion
NAFLD has emerged as the most prevalent chronic liver disease worldwide. Its incidence and prevalence are incessantly increasing, resulting in enormous clinical and economic burdens.Citation1–4,Citation6 Growing evidence indicates that patients with NAFLD are at increased risk for the development of hypertension, coronary heart disease, cardiomyopathy, and cardiac arrhythmias, which clinically result in increased cardiovascular morbidity and mortality.Citation38–41 While lifestyle modifications remain the cornerstone of the management of NAFLD,Citation42 there has been a marked absence of effective pharmacological therapies approved for this condition.Citation43 Therefore, identifying emerging serum markers for NAFLD might help find high-risk individuals and develop auxiliary prevention or treatment strategies.
Aldosterone excess enhances oxidative stress and inflammation, induces reduction of both circulating adiponectin and adiponectin expression in visceral adipose tissue,Citation44,Citation45 and directly promotes HSC activation and liver fibrosis by inducing the activation of the NLRP3 inflammasome.Citation23 Accumulating evidence has suggested that decreased adiponectin expression could enhance hepatic oxidative stress and play a role in the development and progression of NAFLD, from simple steatosis to NASH.Citation46,Citation47 The in vivo findings revealed that adiponectin deficiency not only aggravated liver injury and steatosis but also stimulated palmitate-mediated NLRP3 inflammasome activation in hepatocytes and induced severe liver fibrosis.Citation48 Moreover, elevated aldosterone levels were independently associated with insulin resistance in a 10-year prospective study of non-diabetic subjects from the general population.Citation49 However, there has been little research into the relationship between aldosterone and NAFLD in population studies.
In the present large cohort study, we found a meaningful relationship between high levels of PAC and NAFLD risk in hypertensive patients. And either as a continuous or categorical variable, elevated PAC was independently associated with new-onset NAFLD; this association was independent of other risk factors. With the PAC increasing, those in the highest tertile of the PAC had a 1.71-fold greater risk of NAFLD (T3 vs T1). This study is the first to examine the nonlinear association between PAC and the onset of NAFLD. Besides assessing the independent effects of PAC and new-onset NAFLD in hypertensive patients, we also explored the dose-response association between them. We calculated the inflection point of PAC at 13 ng/dL When PAC ≥ 13 ng/dL, PAC was significantly positively associated with the risk of NAFLD. However, when PAC < 13 ng/dL, there was no significant association between PAC and the risk of NAFLD.
Growing research has directly or indirectly found a relationship between aldosterone and NAFLD. A large retrospective cohort study demonstrated that angiotensin-converting enzyme inhibitor (ACEI) therapy was associated with a lower risk of NAFLD-related events.Citation50 Spironolactone is a potent mineralocorticoid receptor antagonist and may also improve glucose and lipid metabolism.Citation51 A small randomized controlled trial showed that the combined low-dose spironolactone and vitamin E therapeutic regimen had a favorable effect on serum insulin and HOMA-IR in NAFLD, possibly attributable to spironolactone action.Citation52 An in vitro study showed that SAB inhibited aldosterone-induced HSC proliferation and in vitro angiogenesis in a dose-dependent manner. Together, these treatment results indicated that aldosterone plays a pivotal role in the progression of NAFLD.Citation27 In subgroup analysis, our study found that the risk of NAFLD was associated with a higher PAC in hypertensive patients without diabetes than in those with diabetes. The reason underlying the association was the number of diabetes patients who accepted antidiabetic agents, including metformin, insulin, pioglitazone, glucagon-like peptide-1 agonists, sodium-glucose co-transporter-2, and dipeptidyl peptidase-4 inhibitors, which had a beneficial effect on the prevention of NAFLD.Citation53–56
Our study has the strengths of a large sample size, a population-based cohort, and strict inclusion criteria. Furthermore, this research identifies the J-shaped relationship between PAC and NAFLD diagnosis in hypertensive patients for the first time. And it is important to identify and intervene with the high-risk groups for NAFLD in hypertensive patients. Despite this, study limitations require consideration. First, our study is limited by its retrospective, observational nature. Second, although unobserved confounding is inevitable, the estimated E-values in our study indicate that confounders must have a relatively strong association with both PAC and NAFLD risk to completely clarify the observed associations. Third, abdominal ultrasonography is used to diagnose fatty liver rather than a liver biopsy. However, ultrasonography is widely used in clinical practice and epidemiological studies because of its acceptable accuracy in non-invasive fatty liver detection. Fourth, our research is restricted to Chinese hypertensive patients, and additional research is needed to validate our findings in a more extensive and diverse patient population. Fifth, this study only explored the relationship between PAC and new-onset NAFLD, without mentioning the relationship between PAC and other comorbidities associated with NAFLD. Finally, PAC obtained with a single measurement may not reflect changes over time or cumulative exposure. Future, well-designed prospective cohort studies are needed to explore whether cumulative exposure to PAC is associated with new-onset NAFLD.
Conclusion
In conclusion, this study revealed a nonlinear relationship between elevated PAC and new-onset NAFLD in patients with hypertension. Higher PAC was significantly associated with new-onset NAFLD when PAC was ≥ 13 ng/dL. Further prospective studies are needed to confirm the current findings.
Disclosure
The authors report no conflicts of interest in this work.
Acknowledgment
Xintian Cai and Junli Hu are regarded as co-first authors.
Additional information
Funding
References
- Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease—Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64(1):73–84. doi:10.1002/hep.28431
- Li J, Zou B, Yeo YH, et al. Prevalence, incidence, and outcome of non-alcoholic fatty liver disease in Asia, 1999–2019: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2019;4(5):389–398. doi:10.1016/S2468-1253(19)30039-1
- Zhou J, Zhou F, Wang W, et al. Epidemiological Features of NAFLD From 1999 to 2018 in China. Hepatology. 2020;71(5):1851–1864. doi:10.1002/hep.31150
- Sanyal AJ, Van Natta ML, Clark J, et al. Prospective study of outcomes in adults with nonalcoholic fatty liver disease. N Engl J Med. 2021;385(17):1559–1569. doi:10.1056/NEJMoa2029349
- Huang DQ, El-Serag HB, Loomba R. Global epidemiology of NAFLD-related HCC: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol. 2021;18(4):223–238. doi:10.1038/s41575-020-00381-6
- Estes C, Anstee QM, Arias-Loste MT, et al. Modeling NAFLD disease burden in China, France, Germany, Italy, Japan, Spain, United Kingdom, and United States for the period 2016–2030. J Hepatol. 2018;69(4):896–904. doi:10.1016/j.jhep.2018.05.036
- Cai X, Wang M, Liu S, et al. Establishment and validation of a nomogram that predicts the risk of type 2 diabetes in obese patients with non-alcoholic fatty liver disease: a longitudinal observational study. Am J Transl Res. 2022;14(7):4505–4514.
- Gutiérrez-Cuevas J, Lucano-Landeros S, López-Cifuentes D, Santos A, Armendariz-Borunda J. Epidemiologic, Genetic, Pathogenic, Metabolic, Epigenetic Aspects Involved in NASH-HCC: current Therapeutic Strategies. Cancers. 2022;15(1):23. doi:10.3390/cancers15010023
- Mancia G, Fagard R, Narkiewicz K, et al. 2013 ESH/ESC Guidelines for the management of arterial hypertension: the Task Force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J Hypertens. 2013;31(7):1281–1357. doi:10.1097/01.hjh.0000431740.32696.cc
- Targher G, Byrne CD, Tilg H. NAFLD and increased risk of cardiovascular disease: clinical associations, pathophysiological mechanisms and pharmacological implications. Gut. 2020;69(9):1691–1705. doi:10.1136/gutjnl-2020-320622
- Gutiérrez-Cuevas J, Santos A, Armendariz-Borunda J. Pathophysiological molecular mechanisms of obesity: a link between MAFLD and NASH with cardiovascular diseases. Int J Mol Sci. 2021;22(21):11629. doi:10.3390/ijms222111629
- Ma J, Hwang S-J, Pedley A, et al. Bi-directional analysis between fatty liver and cardiovascular disease risk factors. J Hepatol. 2017;66(2):390–397. doi:10.1016/j.jhep.2016.09.022
- Johnston MP, Patel J, Byrne CD. Causes of mortality in Non-Alcoholic Fatty Liver Disease (NAFLD) and Alcohol Related Fatty Liver Disease (AFLD). Curr Pharm Des. 2020;26(10):1079–1092. doi:10.2174/1381612826666200128094231
- Lonardo A, Nascimbeni F, Mantovani A, Targher G. Hypertension, diabetes, atherosclerosis and NASH: cause or consequence? J Hepatol. 2018;68(2):335–352. doi:10.1016/j.jhep.2017.09.021
- Gao X, Yamazaki Y, Tezuka Y, et al. Pathology of aldosterone biosynthesis and its action. Tohoku J Exp Med. 2021;254(1):1–15. doi:10.1620/tjem.254.1
- Yuan Y, Li N, Liu Y, et al. Positive association between plasma aldosterone concentration and white matter lesions in patients with hypertension. Front Endocrinol. 2021;12:753074. doi:10.3389/fendo.2021.753074
- Zhu Q, Heizhati M, Lin M, et al. Higher plasma aldosterone concentrations are associated with elevated risk of aortic dissection and aneurysm: a case-control study. Hypertension. 2022;79(4):736–746. doi:10.1161/HYPERTENSIONAHA.121.18342
- Lin M, Heizhati M, Gan L, et al. Higher aldosterone is associated with increased renal impairment risk in patients with hypertension and abnormal glucose metabolism: a longitudinal study. J Hypertens. 2022;40(3):561–569. doi:10.1097/HJH.0000000000003049
- Kim KJ, Hong N, Yu MH, et al. Time-dependent risk of atrial fibrillation in patients with primary aldosteronism after medical or surgical treatment initiation. Hypertension. 2021;77(6):1964–1973. doi:10.1161/HYPERTENSIONAHA.120.16909
- Mathew JT, Patni H, Chaudhary AN, et al. Aldosterone induces mesangial cell apoptosis both in vivo and in vitro. Am J Physiol Renal Physiol. 2008;295(1):F73–F81. doi:10.1152/ajprenal.00435.2007
- Gilbert KC, Brown NJ. Aldosterone and inflammation. Curr Opin Endocrinol Diabetes Obes. 2010;17(3):199–204. doi:10.1097/med.0b013e3283391989
- Briet M, Schiffrin EL. Vascular actions of aldosterone. J Vasc Res. 2013;50(2):89–99. doi:10.1159/000345243
- Li Y, Zhang Y, Chen T, et al. Role of aldosterone in the activation of primary mice hepatic stellate cell and liver fibrosis via NLRP3 inflammasome. J Gastroenterol Hepatol. 2020;35(6):1069–1077. doi:10.1111/jgh.14961
- Chen Y, Chen X, Chen Q, et al. Non-alcoholic fatty liver disease and hypokalemia in primary aldosteronism among Chinese population. Front Endocrinol. 2021;12:565714. doi:10.3389/fendo.2021.565714
- Fallo F, Dalla Pozza A, Tecchio M, et al. Nonalcoholic fatty liver disease in primary aldosteronism: a pilot study. AM J HYPERTENS. 2009;23(1):2–5. doi:10.1038/ajh.2009.206
- Kumar A, Blackshear C, Subauste JS, et al. Fatty liver disease, women, and aldosterone: finding a link in the Jackson Heart Study. J Endocr Soc. 2017;1(5):460–469. doi:10.1210/js.2017-00055
- Noguchi R, Yoshiji H, Ikenaka Y, et al. Selective aldosterone blocker ameliorates the progression of non-alcoholic steatohepatitis in rats. Int J Mol Med. 2010;26(3):407–413.
- Gamliel-Lazarovich A, Raz-Pasteur A, Coleman R, et al. The effects of aldosterone on diet-induced fatty liver formation in male C57BL/6 mice: comparison of adrenalectomy and mineralocorticoid receptor blocker. Eur J Gastroenterol Hepatol. 2013;25(9):1086–1092. doi:10.1097/MEG.0b013e328360554a
- Farrell GC, Chitturi S, Lau GK, Sollano JD. Asia-Pacific Working Party on N Guidelines for the assessment and management of non-alcoholic fatty liver disease in the Asia-Pacific region: executive summary. J Gastroenterol Hepatol. 2007;22:775–777. doi:10.1111/j.1440-1746.2007.05002.x
- Hu J, Cai X, Li N, et al. Association between triglyceride glucose index-waist circumference and risk of first myocardial infarction in Chinese hypertensive patients with obstructive sleep apnoea: an observational cohort study. Nat Sci Sleep. 2022;14:969–980. doi:10.2147/NSS.S362101
- Cai X, Li N, Hu J, et al. Nonlinear relationship between Chinese visceral adiposity index and new-onset myocardial infarction in patients with hypertension and obstructive sleep apnoea: insights from a Cohort Study. J Inflamm Res. 2022;15:687–700. doi:10.2147/JIR.S351238
- Funder JW, Carey RM, Mantero F, et al. The management of primary aldosteronism: case detection, diagnosis, and treatment: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2016;101(5):1889–1916. doi:10.1210/jc.2015-4061
- Wang X, Luo Q, Wang M, et al. Long-term impact of spironolactone compliance on microalbuminuria in patients with primary aldosteronism. Hypertens Res. 2021;44(4):426–434. doi:10.1038/s41440-020-00589-8
- Gao X, Fan JG; Study Group of Liver and Metabolism, Chinese Society of Endocrinology. Diagnosis and management of non-alcoholic fatty liver disease and related metabolic disorders: consensus statement from the Study Group of Liver and Metabolism, Chinese Society of Endocrinology. J Diabetes. 2013;5(4):406–415. doi:10.1111/1753-0407.12056
- Dasarathy S, Dasarathy J, Khiyami A, et al. Validity of real time ultrasound in the diagnosis of hepatic steatosis: a prospective study. J Hepatol. 2009;51(6):1061–1067. doi:10.1016/j.jhep.2009.09.001
- Fan JG, Jia JD, Li YM, et al. Guidelines for the diagnosis and management of nonalcoholic fatty liver disease: update 2010: published in Chinese on Chinese Journal of Hepatology 2010; 18:163–166. J Dig Dis. 2011;12(1):38–44. doi:10.1111/j.1751-2980.2010.00476.x
- VanderWeele TJ, Ding P. Sensitivity analysis in observational research: introducing the E-Value. Ann Intern Med. 2017;167(4):268–274. doi:10.7326/M16-2607
- Wu S, Wu F, Ding Y, et al. Association of non-alcoholic fatty liver disease with major adverse cardiovascular events: a systematic review and meta-analysis. Sci Rep. 2016;6:33386. doi:10.1038/srep33386
- Niederseer D, Wernly S, Bachmayer S, et al. Diagnosis of Non-Alcoholic Fatty Liver Disease (NAFLD) is independently associated with cardiovascular risk in a large Austrian screening cohort. J Clin Med. 2020;9(4):1065. doi:10.3390/jcm9041065
- Møller S, Kimer N, Kronborg T, et al. Nonalcoholic fatty liver disease and cardiovascular disease: overlapping mechanisms. Semin Liver Dis. 2021;41(3):235–247. doi:10.1055/s-0041-1725022
- Song QR, Liu SL, Bi YG, et al. Non-alcoholic fatty liver disease is associated with cardiovascular outcomes in subjects with prediabetes and diabetes: a prospective community-based cohort study. Front Cardiovasc Med. 2022;9:889597. doi:10.3389/fcvm.2022.889597
- Deng YY, Zhong QW, Zhong HL, Xiong F, Ke YB, Chen YM. Higher Healthy Lifestyle Score is associated with lower presence of non-alcoholic fatty liver disease in middle-aged and older Chinese adults: a community-based cross-sectional study. Public Health Nutr. 2021;24(15):5081–5089. doi:10.1017/S1368980021000902
- Younossi Z, Anstee QM, Marietti M, et al. Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol. 2018;15(1):11–20. doi:10.1038/nrgastro.2017.109
- Moustaki M, Paschou SA, Vakali EC, et al. Secondary diabetes mellitus due to primary aldosteronism [published online ahead of print, 2022 Aug 24]. Endocrine. 2022. doi:10.1007/s12020-022-03168-8
- Li P, Zhang XN, Pan CM, et al. Aldosterone perturbs adiponectin and PAI-1 expression and secretion in 3T3-L1 adipocytes. Horm Metab Res. 2011;43(7):464–469. doi:10.1055/s-0031-1277226
- Ma H, Gomez V, Lu L, et al. Expression of adiponectin and its receptors in livers of morbidly obese patients with non-alcoholic fatty liver disease. J Gastroen Hepatol. 2008;24(2):233–237. doi:10.1111/j.1440-1746.2008.05548.x
- Fukushima J, Kamada Y, Matsumoto H, et al. Adiponectin prevents progression of steatohepatitis in mice by regulating oxidative stress and Kupffer cell phenotype polarization. Hepatol Res. 2009;39(7):724–738. doi:10.1111/j.1872-034X.2009.00509.x
- Dong Z, Zhuang Q, Ye X, et al. Adiponectin Inhibits NLRP3 inflammasome activation in nonalcoholic steatohepatitis via AMPK-JNK/ErK1/2-NFκB/ROS signaling pathways. Front Med. 2020;7:546445. doi:10.3389/fmed.2020.546445
- Kumagai E, Adachi H, Jacobs DR, et al. Plasma aldosterone levels and development of insulin resistance: prospective study in a general population. Hypertension. 2011;58(6):1043–1048. doi:10.1161/HYPERTENSIONAHA.111.180521
- Zhang X, Wong GL, Yip TC, et al. Angiotensin-converting enzyme inhibitors prevent liver-related events in nonalcoholic fatty liver disease. Hepatology. 2022;76(2):469–482. doi:10.1002/hep.32294
- Cai X, Li N. Association between use of spironolactone and risk of stroke in hypertensive patients: a cohort study. Pharmaceuticals. 2022;16(1):57. doi:10.3390/ph16010057
- Polyzos SA, Kountouras J, Zafeiriadou E, et al. Effect of spironolactone and vitamin E on serum metabolic parameters and insulin resistance in patients with nonalcoholic fatty liver disease. J Renin Angiotensin Aldosterone Syst. 2011;12(4):498–503. doi:10.1177/1470320311402110
- Wabitsch S, McCallen JD, Kamenyeva O, et al. Metformin treatment rescues CD8+ T-cell response to immune checkpoint inhibitor therapy in mice with NAFLD. J Hepatol. 2022;77(3):748–760. doi:10.1016/j.jhep.2022.03.010
- Musso G, Cassader M, Paschetta E, Gambino R. Thiazolidinediones and advanced liver fibrosis in nonalcoholic steatohepatitis: a meta-analysis [published correction appears in JAMA Intern Med. 2017 May 1;177(5):747]. JAMA Intern Med. 2017;177(5):633–640. doi:10.1001/jamainternmed.2016.9607
- Ito D, Shimizu S, Inoue K, et al. Comparison of Ipragliflozin and pioglitazone effects on nonalcoholic fatty liver disease in patients with type 2 diabetes: a randomized, 24-week, open-label, active-controlled trial. Diabetes Care. 2017;40(10):1364–1372. doi:10.2337/dc17-0518
- Kumar J, Memon RS, Shahid I, et al. Antidiabetic drugs and non-alcoholic fatty liver disease: a systematic review, meta-analysis and evidence map. Dig Liver Dis. 2021;53(1):44–51. doi:10.1016/j.dld.2020.08.021