Abstract
Purpose
This study aimed to investigate the relationship between serum isthmin-1 (ISM1) and type 2 diabetes mellitus (T2DM), and the alteration of serum ISM1 level in both diabetic sensorimotor peripheral neuropathy (DSPN) and diabetic adults with obesity.
Patients and Methods
We recruited 180 participants (120 T2DM and 60 controls) in the cross-sectional study. First, we compared the serum ISM1 concentration in diabetic patients and non-diabetic controls. Secondly, according to DSPN, patients were divided into DSPN and non-DSPN groups. Last, patients were categorized as lean T2DM (15 males, 15 females), overweight T2DM (35 males, 19 females), and obese T2DM groups (23 males, 13 females) according to gender and body mass index (BMI). All participants were collected with clinical characteristics and biochemical profiles. Serum ISM1 was detected in all subjects by ELISA.
Results
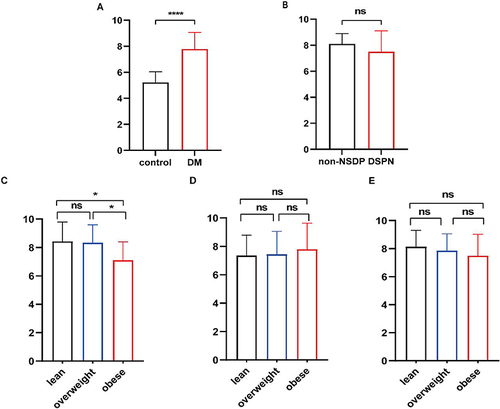
Higher serum ISM1 [7.78 ng/mL (IQR: 6.33–9.06) vs 5.22 (3.86–6.04), P <0.001] was observed in diabetic patients compared to non-diabetic controls. Binary logistic regression analysis showed that serum ISM1 was a risk factor for type 2 diabetes after adjustment (OR=4.218, 95% CI: 1.843–9.653, P=0.001). Compared to the non-DSPN group, serum ISM1 level was not changed significantly in patients who suffered from DSPN. Diabetic females with obesity had lower level of serum ISM1 (7.10±1.29 ng/mL) when compared to the lean T2DM (8.42±1.36 ng/mL, P <0.05) and the overweight T2DM (8.33±1.27 ng/mL, P <0.05). However, serum ISM1 was not changed significantly in male groups or all patients together.
Conclusion
Serum ISM1 was a risk factor for type 2 diabetes, and it was associated with diabetic adults with obesity while there was sexual dimorphism. However, serum ISM1 levels were not correlated with DSPN.
Introduction
Due to aging, diabetes affects over 10.5% of people aged 20–79 worldwide, which is a major challenge for healthcare systems worldwide. Currently, the largest number of cases is in China.Citation1 Type 2 diabetes mellitus (T2DM) is a complex disease described by insulin resistance (IR) and pancreatic β cell failure, accounting for over 90% of patients with diabetes globally.Citation2
Diabetic patients suffered from traditional and emerging complications, including cardiovascular, peripheral neuropathy diseases, and cancers.Citation3 Microvascular dysfunction and neuronal cell death induced by IR, hyperglycemia, inflammation and impaired signaling pathways of glucose and lipid metabolism led to diabetic sensorimotor peripheral neuropathy (DSPN). As the most prevalent type among diabetic neuropathies, DSPN affects about 30% of people with diabetes.Citation4 Up to 50% of DSPN is asymptomatic because it develops gradually or insidiously.Citation5 Even worse, DSPN is strongly related to an increased risk of death.Citation4
Being overweight or obese was positively associated with type 2 diabetes.Citation6 The national survey found that more than 50% of adults are overweight or obese in China.Citation7 Accumulative adiposity, assessed by high body mass index (BMI), was the most critical risk factor for type 2 diabetes.Citation2 Chinese people had a higher level of body fat when compared to Caucasians at an equal BMI.Citation7 BMI and total fat mass were strongly related to IR and inflammation.Citation8
Adipokines, mainly secreted from adipose tissue, contribute to glucose and lipid metabolism, IR, and inflammation.Citation9 Researchers found that adipokines were associated with T2DM,Citation10 obesity,Citation11 and DSPN.Citation12 Isthmin-1 (ISM1), a novel protein excreted from the nervous system, was found to regulate early embryonic development.Citation13 It was also expressed in the kidney, heart, and immune system.Citation14,Citation15 Recent evidence suggested that ISM1 acted as an adipokine to improve glucose tolerance and insulin sensitivity in mice and healthy humans.Citation16 Recent study of ISM1 revealed that it was associated with inflammatory reactions.Citation17 On the other hand, T2DM is also associated with various markers of inflammation, including neutrophil/lymphocyte count ratio,Citation18 omentin,Citation19 neuregulin-4,Citation20 frailty indexes,Citation21 serum vitamin D,Citation22 and uric acid-to-HDL ratio.Citation23 In addition, diabetic microvascular complications are associated with increased burden of inflammation too. The markers that associated with diabetic complications include C-reactive protein (CRP)-to-albumin ratio,Citation24 neuregulin-4,Citation25 monocyte-to-lymphocyte ratio,Citation26 omentin,Citation27 mean platelet volume-to-lymphocyte ratio,Citation28 and kidney injury molecule-1.Citation29 Hence, studying ISM1 in diabetes mellitus and in its complications make sense.
Recent studies reported that serum ISM1 was reduced in T2DM but elevated in diabetic kidney disease.Citation30,Citation31 A study stated that higher serum ISM1 concentration was detected in obese pubertal boys and related to BMI S-score.Citation32 So far, the role of serum ISM1 in diabetes is not fully understood, and no study has been focused on the association of serum ISM1 and DSPN. Therefore, the main objective of this study was to investigate the relationship between ISM1 and type 2 diabetes. Furthermore, this study was to explore the alteration of ISM1 in DSPN and diabetic patients with obesity.
Research Design and Population
Study Design and Population
Our analysis was an observational, prospective study. All participants were recruited from 1 October 2021 to 31 January 2023 at The Huai’an Hospital Affiliated to Xuzhou Medical University and The Second People’s Hospital of Huai’an. The criteria for selecting the subjects were as follows: 1) patients with type 2 diabetes based on the diagnostic standard of WHO 1999.Citation33 2) aged between 18 and 79 years. Exclusion criteria: 1) Estimated glomerular filtration rate (eGFR) <30 [mL•min−1• (1.73 m2)−1]. 2) A history of blood transfusion in 6 months, tumor, or other endocrine diseases. 3) An acute infectious disease or acute diabetic complications. 4) Missing critical laboratory data. The definitions of nondiabetic controls were as follows: 1) Normal blood glucose tolerance, 2) Fasting serum glucose <6.10 mmol/L, 3) HbA1c <6.1%, and 4) With no family history of diabetes. Exclusion criteria for both groups were people who suffered from chronic kidney disease and autoimmune disease.
Of the 213 participants who were screened, 180 participants were eligible for this research, which included 120 diabetic patients and 60 nondiabetic controls. Thirty-three participants were excluded based on the exclusion criteria. To determine whether serum ISM1 expression differed in DSPN, the T2DM patients were divided into two groups: the DSPN and the non-DSPN T2DM groups. DSPN was diagnosed when the following criteria were met: 1) The presence of symptoms (burning or stabbing pain, numbness, weakness, hyperalgesia, sensation loss) and signs (sensation of temperature and pain, vibration perception with 128-Hz tuning fork and 10-g monofilament test). 2) Abnormal nerve conduction studies (a reduction of limb nerve conduction and/or amplitude on electromyography).Citation34 Because all participants are Han Chinese, the BMI was categorized based on the definition of The Working Group on Obesity in China: 1) BMI ≥ 24 kg/m2 was as overweight, and 2) BMI ≥ 28 kg/m2 was as obese.Citation7
Baseline Data Collection
We gathered the baseline demographic and clinical characteristics for all people. Baseline data included age, sex, duration, drug use, and blood pressure. Measurements of height and weight were used to calculate the body mass index.
Biochemical Profiles
After a minimum of 8 hours fasting, venous samples were obtained from all participants. Blood was centrifuged at 3000 r/min for 20 minutes (ZONKIA, China), and the serum was stored at −80 degrees centigrade until further analysis. The serum was used to measure fasting plasma glucose (FPG), fasting C-peptide, urea nitrogen (BUN), uric acid (UA), serum creatinine (Cr), triglyceride (TG), total cholesterol (TC), low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C) and HbA1c by using an integrated automatic analyzer (Roche, China). The level of serum ISM1 was measured by a human ISM1-specific ELISA kit (Jiangsu Meimian Industrial Co., Ltd, China). The Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation was used to calculate the eGFR.Citation35 Fasting blood glucose and fasting C-peptide were input into the software (https://www.ox.ac.uk/), and then homeostatic model assessment (HOMA) was calculated.Citation36
Nerve Conduction Study (NCS)
Using standard electrophysiological and electromyographic equipment (Philip, China), nerve conduction tests were performed by professional electrophysiologists. Sensory nerve conduction velocity, response amplitude, and latency were measured for median nerves, ulnar nerves, sural nerves, and superficial fibular nerves. Motor nerve conduction velocity, the amplitude of action potentials and latency, and F-wave latency were detected at the median nerves and common fibular nerves. All data were gathered using the standard surface detection method and they were analyzed and standardized by healthy human age matching from our laboratory. If the muscle atrophy of the patient was severe, then needle electromyography was applied.
Statistical Analysis
SPSS 25.0 and GraphPad Prism 9 were used for statistical management and analysis. Measurement data are reported as the mean ± standard deviation (SD) if they were normally distributed. Otherwise, data are shown as the median (interquartile range). Categorical variables were analyzed by the chi-square test or Fisher’s exact test. We used the t-test or Mann‒Whitney U-test to compare continuous variables between two groups. ANOVA and the Kruskal‒Wallis H-test were performed to compare more than two groups, and the Bonferroni method was applied to correct for multiple comparisons. The Spearman correlation coefficient was calculated to analyze the correlation between serum ISM1 and other clinical variables. The relationship between ISM1 and diabetes was analyzed by logistic regression analysis. All P values were two-tailed and P <0.05 was considered statistically significant.
Results
Baseline Clinical Characteristics of the Participants
The clinic characteristics of the participants are presented in . As shown in , the mean age of the diabetic patients was 53.58 years, and that of the control group was 51.12 years. No significant difference was found in age, gender, height, SBP, DBP, Cr, uric acid, eGFR, HDL-C, LDL-C, and TC between the two groups (P>0.05). Compared to the control group, patients with diabetes were likely to have a higher body weight, BMI, FPG, C peptide, HbA1c, BUN, and TG (P<0.05). Consistent with various prior studies, patients with diabetes had lower HOMA2-β but higher HOMA2-IR (P<0.05), indicating that they suffered from worsen β-cell function and IR.
Table 1 Characteristics of All Participants
ISM1 Serum Level
The first set of analyses examined the correlation of T2DM with serum ISM1. In our study, the serum ISM1 concentration was increased significantly in the diabetic group compared to the non-diabetic control group (P<0.001, , ). Then, we explored the relationship between serum ISM1 and baseline variables in the diabetic group. Detailed information on the Spearman correlation analysis is shown in . In both mouse models and healthy controls, ISM1 transcript levels in adipose tissue were not significantly correlated with HOMA-IR.Citation16 Our experiments yield the same results. shows that BMI, FPG, C-peptide, HbA1c, and HOMA2-β did not show any correlation with ISM1. Age (r=0.197, P<0.05), gender (r=0.297, P<0.001), HDL-C (r=0.194, P<0.05), and LDL-C (r=0.205, P<0.05) showed a weak positive correlation with ISM1 levels.
Table 2 Correlation Analysis Between Serum ISM1 and Baseline Characteristics in T2DM
Figure 1 The concentration of serum ISM1 in different groups.
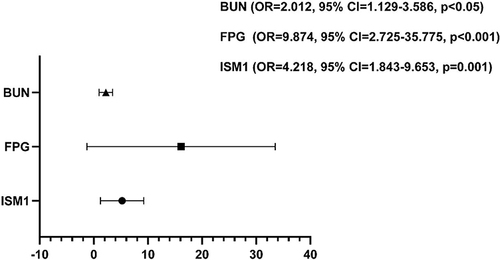
To further clarify the relationship between serum ISM1 and T2DM, a logistic regression analysis was performed. BMI, FPG, C-peptide, BUN, HDL-C, TG, and ISM1 were significantly related to T2DM in univariate analysis (P<0.05, data not shown). Binary logistic regression analysis showed that serum ISM1 (OR=4.218, 95% CI:1.843–9.653, P=0.001), FPG (OR=9.874, 95% CI:2.725–35.775, P<0.001), and BUN (OR=2.012, 95% CI:1.129–3.586, P<0.05) were positively correlated with T2DM (). The results suggested that serum ISM1 was a risk factor for type 2 diabetes.
Subgroup Analysis
A New Point of View on Type 2 Diabetic Adults with Obesity
Our study indicated that serum ISM1 was a risk factor for T2DM, but a prior study showed that it was a protective factor.Citation30 One difference between the two pieces of research was that the prior study matched their groups by age and BMI.
Previous research has established that the gene expression of ISM1 in adipose tissue is markedly elevated not only in mice fed a high-fat diet (HFD) but also in non-diabetic people with obesity. Moreover, it was significantly positively correlated with BMI significantly.Citation16 The latest study suggested that serum ISM1 level of the obese population was gender differences.Citation32 Our study showed that ISM1 had a positive correlation with gender (P=0.001, ). Therefore, we regrouped them according to gender in diabetic patients. There are two subgroups: 1) the male groups: lean T2DM (N=15), overweight T2DM (N=35), and obese T2DM (N=23), and 2) the female groups: lean T2DM (N=15), overweight T2DM (N=19), and obese T2DM (N=13).
Lower serum ISM1 concentration (7.10±1.29 ng/mL) was detected in obese females with T2DM when compared to lean T2DM (8.42±1.36 ng/mL, P<0.05, ) and overweight T2DM (8.33±1.27 ng/mL, P<0.05, ). No significant alteration of serum ISM1 was observed between lean T2DM and overweight T2DM groups in females. However, there was no significant alteration in male groups, but it was a tendency to increase (P>0.05, ). Comparing all patients together, we unexpectedly found that it was no significant alteration between three groups (P>0.05, ). The results suggest that there was a gender difference in the expression of serum ISM1 in diabetic patients with obesity.
Focus on Patients Who Suffered from DSPN
Extensive research has shown that DSPN is a complex complication of diabetes. Old age, long diabetes duration, poor glycemic control, and lower HDL-C are risk factors for T2DM.Citation4,Citation37 Data from several studies suggest that the gold standard test for the diagnosis of DSPN is nerve conduction studies.Citation34 To date, the problem of diagnosing and treating DSPN has received some attention in the research literature, but there are still no effective solutions. Therefore, we need to find novel biomarkers.
Of 120 diabetic patients, 95 patients were diagnosed with DSPN. Compared to the non-DSPN T2DM group, higher SBP and lower eGFR were observed in the DSPN group. Consistent with previous research, elderly patients were more likely to suffer from DSPN (P<0.001, ). When fasting plasma glucose is increased 1 mmol/L or HbA1c is increased 1%, the risk of developing DSPN increases by 10% to 15%.Citation38 But there was no significant alteration in FPG, HbA1c, or serum lipids (P>0.05, ). Although the concentration of ISM1 decreased in the DSPN group compared to the non-DSPN group, it was not significant (P>0.05, ). The results of binary logistic regression analysis presented that serum ISM1 was not associated with DSPN after adjusting for sex, age, BMI, blood pressure and biochemical profiles (OR=0.926, 95% CI: 0.604–1.420, P=0.725). Taken together, these results revealed that there was no association between serum ISM1 and DSPN.
Table 3 Baseline Characteristic of Patients with DSPN or Non-DSPN
Discussion
In this study, we found that serum ISM1 was significantly increased in patients with T2DM, which suggests that serum ISM1 is positively related to type 2 diabetes. Wang et al also found that elevated serum ISM1 was a risk factor in diabetic kidney disease and correlated with the severity of albuminuria.Citation31 However, J. Wang et al found that serum ISM1 acted as a protective factor in new-onset diabetic patients.Citation30 Possible reason for the inconsistent result was different duration of patients. In the researches of present and C. Wang, there was no restriction on the duration of the patients. In our research, many patients had a long duration except 23 patients whom duration was in 6 months. These observations may support a hypothesis that serum ISM1 plays different roles in the onset and progression of type 2 diabetes. Therefore, our study further supported that ISM1 was associated with T2DM no matter how it changed.
Initially, ISM1 was found to be excreted from the central nervous system to regulate embryonic and postnatal development.Citation13 Later, researchers found that ISM1 was highly expressed in brain.Citation15 Recent evidence revealed that the expression of ISM1 was elevated in adipose tissue of HFD mice, which is in parallel with leptin.Citation16 Leptin is a classical adipokine mainly excreted by adipose tissue to regulate food intake and glucose metabolism.Citation39 Prior studies have noted that leptin had a complex effect on pancreatic β cells: lower concentrations inhibited insulin release, while higher levels stimulated insulin secretion.Citation40 However, serum ISM1 was not related to serum leptin in obese children.Citation32 As mentioned in the literature reviews, leptin is transported across the blood–brain barrier (BBB) by a saturable system to partly regulate glucose metabolism. Peripheral and central leptin resistance caused by BBB disorders induced diabetes mellitus and obesity.Citation40,Citation41 However, increasing plasma ISM1 levels in HFD mice did not elevate the expression of ISM1 in brain.Citation16
ISM1 inhibits nodal signaling by downregulating the phosphorylation of mothers against decapentaplegic (SMAD) 2 during embryo development.Citation42 Nodal, a member of the transforming growth factor-β (TGF-β) superfamily, is present in human pancreatic β cells and α cells.Citation43 The activity of TGF-β signaling pathway was enhanced by ISM1.Citation44 The study reported that ISM1 activated the phosphoinositide 3-kinase (PI3K)/Akt signaling pathway.Citation16 Nodal regulates pancreatic β cell proliferation and apoptosis in different pathways. Nodal modest triggers pancreatic β cell proliferation by SMAD signaling (probably SMAD2), but it does not influence AKT signaling.Citation43 However, nodal induced β cell apoptosis by activating activin receptor-like kinase-7 (ALK7) to stimulate SMAD2/3-caspase-3 signaling and inhibiting Akt signaling.Citation45 We expect that further research could pay attention to whether and how serum ISM1 affects blood glucose through nervous system and the role of ISM1 in regulating pancreatic cell growth.
An examination of adult mice reported that ISM1 transcripts were expressed in various systems but were not detected in blood.Citation15 Jiang et al found that plasma ISM1 was 50 pg/mL in obese humans.Citation16 Recent research showed that serum ISM1 was less than 4 ng/mL in diabetic patients,Citation31 and increased higher in our study. This result suggests that the expression of serum ISM1 is different among species and nutritional states.
Obesity is one of the major risk factors for diabetes. All subjects in the study of J. Wang were matched of BMI.Citation30 However, participants in our study were selected randomly, and patients with T2DM had higher BMI than controls. There are gender differences in the incidence of obesity in China.Citation7 Moreover, serum ISM1 was associated with gender in this study. Prior study showed that serum ISM1 was elevated in obese boys but not in girls.Citation32 In this research, we showed that serum ISM1 statistically decreased in obese females with T2DM. However, it was no statistical alteration in diabetic males with obesity. The result supported that serum ISM1 was associated with patients with type 2 diabetes who suffered obesity, and the alteration of serum ISM1 was sexual dimorphism in adult. The activity of adipokines was regulated by sex hormones.Citation46 Higher serum leptin concentrations were found in females than in males at an equal weight in patients with T2DM. Serum adiponectin was decreased in patients with T2DM and lower in males.Citation47 Therefore, we could pay attention to the relationship between serum ISM1 and sex hormones in subsequent study.
The prior study reported that ISM1 was highly expressed in vitro mature adipose tissue, and HFD mice had the same expression pattern. In addition, the RNA sequence of ISM1 from adipose tissue of obese people was increased and positively correlated with BMI. However, ISM1 was no associated with FPG and HOMA-IR in obese.Citation16 Wang et al revealed that serum ISM1 was not related to C-peptide or HOMA2-IR in patients with T2DM. Consistent with prior results, serum ISM1 was also not association with C-peptide or HOMA2-IR in our study. Exogenous recombinant ISM1 therapy improved blood glucose in HFD mice, but it did not affect body weight. Therefore, the effect of ISM1 in diabetes and obesity is complex.
DSPN is one of the microvascular complications of diabetes mellitus. Cellular injury mediated by persistent hyperglycemia possibly plays the most important role in the development of DSPN. Inflammation and oxidative stress have also been implicated in diabetic polyneuropathy.Citation4 It was reported that serum ISM1 was associated with inflammation.Citation48 In addition, serum ISM1 was related to diabetic microvascular complication.Citation49 Serum ISM1 was negative correlated with myeloperoxidase (MPO) in obese children.Citation32 MPO is a peroxidase mainly secreted by neutrophils that promotes inflammation and oxidative stress, which is positively associated with atherosclerosis and IR.Citation50 However, serum ISM1 was not associated with IR or inflammation factors, such as tumor necrosis factor α (TNFα), high-sensitivity CRP, interleukin (IL) 6 or monocyte chemoattractant protein-1 (MCP-1) in obese children.Citation32
ISM1 was recognized as an important regulator of endothelial cells (EC) and angiogenesis in vitro and in vivo under both physiological and pathological conditions.Citation51 Previous research found that ISM1 has a dual role in regulating cell proliferation and apoptosis.Citation51,Citation52 ISM1 consists of a signal peptide, a conserved adhesion-associated domain in MUC4 and another proteins (AMOP) and thrombospondin type 1 repeat (TSR).Citation51 Soluble ISM1 combines with αvβ5 integrin on the EC surface to partly inhibit capillary network formation and EC proliferation and induced EC apoptosis. There is a novel “RKD” motif in the AMOP domain that interacts with αvβ5 integrin. Therefore, the AMOP domain can inhibit angiogenesis in vitro alone.Citation52 Soluble ISM1 binding to the glucose-regulated protein of 78 kDa (GRP78) targets the mitochondria to disturb ADP/ATP alternation between the cytosol and mitochondria, which significantly triggers cellular apoptosis. GRP78 and αvβ5 integrin are independent receptors, while GRP78 is the predominant factor.Citation53 However, if ISM1 is “solid”, ie, immobilized in the extracellular matrix, it contributes to EC adhesion, survival, and migration.Citation51,Citation52 Therefore, the effect of ISM on endothelial cells completely depends on its physical state.
Prior study reported that ISM1 was a downstream gene of Hypoxia-inducible factor-1α (HIF-1α) involved in the regulation of endothelial cell function.Citation54 HIF-1α is a key regulator of hypoxia.Citation55 Hypoxia induced by microvascular damage, mitochondrial dysfunction, and PI3K/Akt pathway disorder cause death of vascular endothelial cell and neuron, which lead to DSPN. Ageing is a risk factor for DSPN.Citation4 Serum ISM1 was decreased in elderly people.Citation56 Our study showed that serum ISM1 associated with age and patients in the DSPN group was older than non-DSPN group. In this study, the concentration of serum ISM1 was slightly decreased in patient with DSPN. Unexpectedly, our study revealed that serum ISM1 was not associated with DSPN after adjustment.
Isthmin-1 also associates with gastrointestinal diseases.Citation57 Accordingly, gastrointestinal diseases are reported to be related with increased inflammatory burden.Citation49 ISM1 is also secreted by NK, NKT and Th17 cells, suggesting that it is involved in the immune response.Citation14 Highly expression of ISM1 promoted cell cycle progression and inhibited cell apoptosis.Citation57 The expression of ISM1 was increased in colorectal cancer (CRC). ISM1 was positively correlated with inflammatory pathways and promoted epithelial–mesenchymal transition (EMT) in CRC, which led to worse survival and resistance to immunotherapy.Citation44 Wnt/β-catenin signaling also associates with inflammation.Citation58 Wnt/β-catenin signaling participates in cell proliferation and cellular differentiation, which is the crucial part of gastrointestinal cancer.Citation59 The overexpression of ISM1 activates Wnt/β-catenin signaling pathway to promote carcinogenesis, which is negatively regulated by miR-1307-3p.Citation57 ISM1 also promotes cell proliferation and invasion in hepatocellular cancer regulated by miR-1307.Citation60 Long noncoding RNA (lncRNA) H19 activates inflammation in gastrointestinal diseases.Citation61 The lncRNA H19 was elevated in gastric cancer and ISM1 is its binding protein. ISM1 expression positively correlated with lncRNA H19 to promote neoplasia and tumor migration.Citation62
When people develop diabetes, their risk of suffering from cardiovascular disease (CVD) is increased up to tenfold.Citation63 Endothelial injury,Citation64 mitochondrial dysfunction,Citation65 and albuminuriaCitation66 are important risks for CVD. A recent study identified that serum ISM1 was positively related to patients who suffered from albuminuria.Citation31 Research demonstrated decreasing albuminuria ameliorated cardiovascular outcomes in diabetic patients.Citation66 Serum ISM1 was associated with endothelial injury and mitochondrial dysfunction. Our study also showed that serum ISM1 had a positive correlation with T2DM. We suspect that ISM1 could be a novel target for the diagnosis and treatment of diabetic patients with CVD.
Conclusions
In summary, this research revealed that serum ISM1 was significantly elevated in T2DM patients compared to the control group and statistically decreased in obese female patients. The result suggests that the effect of ISM1 in T2DM and obesity is complex. However, serum ISM1 was not related to DSPN in this study.
Limitations of Study
There are some shortcomings of this research. First, this is a cross-sectional study that only enrolled 180 Han Chinese individuals. Additionally, we used the Chinese guideline to define overweight and obesity, and the number in each subgroup was not a perfect match. Therefore, larger studies are needed to claim whether serum ISM1 could be a diagnostic marker in diabetic patients.
Abbreviations
T2DM, type 2 diabetes; DSPN, diabetic sensorimotor peripheral neuropathy; ISM1, Isthmin-1; BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; FPG, fasting plasma glucose; HOMA2-β, homeostasis model 2 assessment-β; HOMA2-IR, homeostasis model 2 assessment of insulin resistance. HbA1c, glycated hemoglobin A1c; BUN, urea nitrogen; Cr, creatinine; eGFR, estimated glomerular filtration rate; HDL-C, high-density lipoprotein; LDL-C, low-density lipoprotein; TG, triglycerides; TC, total cholesterol.
Data Sharing Statement
We do not yet support public data, because we are also planning further extension research.
Ethics Approval and Informed Consent
The study protocol was complied with the Declaration of Helsinki and approved by the Affiliated Huai’an Hospital of Xuzhou Medical University Committee (No. HEYLL202115). The study was registered at Clinical trials (NCT: ChiCTR2200057966). All subjects obtained an informed consent form.
Disclosure
The authors declare that they have no competing interests in this work.
Acknowledgments
We are very grateful to the individuals involved in this project.
Additional information
Funding
References
- Sun H, Saeedi P, Karuranga S, et al. IDF Diabetes Atlas: global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res Clin Pract. 2022;183:109119. doi:10.1016/j.diabres.2021.109119
- Zheng Y, Ley SH, Hu FB. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat Rev Endocrinol. 2018;14(2):88–98. doi:10.1038/nrendo.2017.151
- Tomic D, Shaw JE, Magliano DJ. The burden and risks of emerging complications of diabetes mellitus. Nat Rev Endocrinol. 2022;18(9):525–539. doi:10.1038/s41574-022-00690-7
- Sloan G, Selvarajah D, Tesfaye S. Pathogenesis, diagnosis and clinical management of diabetic sensorimotor peripheral neuropathy. Nat Rev Endocrinol. 2021;17(7):400–420. doi:10.1038/s41574-021-00496-z
- Ziegler D, Tesfaye S, Spallone V, et al. Screening, diagnosis and management of diabetic sensorimotor polyneuropathy in clinical practice: international expert consensus recommendations. Diabetes Res Clin Pract. 2022;186:109063. doi:10.1016/j.diabres.2021.109063
- Longo M, Zatterale F, Naderi J, et al. Adipose tissue dysfunction as determinant of obesity-associated metabolic complications. Int J Mol Sci. 2019;20(9):2358. doi:10.3390/ijms20092358
- Pan XF, Wang L, Pan A. Epidemiology and determinants of obesity in China. Lancet Diabetes Endocrinol. 2021;9(6):373–392. doi:10.1016/S2213-8587(21)00045-0
- Zhang M, Hu T, Zhang S, et al. Associations of different adipose tissue depots with insulin resistance: a systematic review and meta-analysis of observational studies. Sci Rep. 2015;5(1):18495. doi:10.1038/srep18495
- Fasshauer M, Bluher M. Adipokines in health and disease. Trends Pharmacol Sci. 2015;36(7):461–470. doi:10.1016/j.tips.2015.04.014
- Wang K, Li F, Wang C, et al. Serum Levels of Meteorin-Like (Metrnl) are increased in patients with newly diagnosed type 2 diabetes mellitus and are associated with insulin resistance. Med Sci Monit. 2019;25:2337–2343. doi:10.12659/MSM.915331
- Du Y, Ye X, Lu A, et al. Inverse relationship between serum Metrnl levels and visceral fat obesity (VFO) in patients with type 2 diabetes. Diabetes Res Clin Pract. 2020;161:108068. doi:10.1016/j.diabres.2020.108068
- Sun Q, Yan B, Yang D, et al. Serum adiponectin levels are positively associated with diabetic peripheral neuropathy in Chinese Patients with type 2 diabetes. Front Endocrinol. 2020;11:567959. doi:10.3389/fendo.2020.567959
- Pera EM, Kim JI, Martinez SL, et al. Isthmin is a novel secreted protein expressed as part of the Fgf-8 synexpression group in the Xenopus midbrain-hindbrain organizer. Mech Dev. 2002;116(1–2):169–172. doi:10.1016/s0925-4773(02)00123-5
- Valle-Rios R, Maravillas-Montero JL, Burkhardt AM, et al. Isthmin 1 is a secreted protein expressed in skin, mucosal tissues, and NK, NKT, and Th17 cells. J Interf Cytokine Res. 2014;34(10):795–801. doi:10.1089/jir.2013.0137
- Osorio L, Wu X, Zhou Z. Distinct spatiotemporal expression of ISM1 during mouse and chick development. Cell Cycle. 2014;13(10):1571–1582. doi:10.4161/cc.28494
- Jiang Z, Zhao M, Voilquin L, et al. Isthmin-1 is an adipokine that promotes glucose uptake and improves glucose tolerance and steatosis. Cell Metab. 2021;33(9):1836–1852. doi:10.1016/j.cmet.2021.07.010
- Nguyen N, Xu S, Lam TYW, et al. ISM1 suppresses LPS-induced acute lung injury and post-injury lung fibrosis in mice. Mol Med. 2022;28(1):72–89. doi:10.1186/s10020-022-00500-w
- Duman TT, Aktas G, Atak BM, et al. Neutrophil to lymphocyte ratio as an indicative of diabetic control level in type 2 diabetes mellitus. Afr Health Sci. 2019;19(1):1602–1606. doi:10.4314/ahs.v19i1.35
- Aktas G, Alcelik A, Ozlu T, et al. Association between omentin levels and insulin resistance in pregnancy. Exp Clin Endocrinol Diabetes. 2014;122(3):163–166. doi:10.1055/s-0034-1370917
- Kocak MZ, Aktas G, Erkus E, et al. Neuregulin-4 is associated with plasma glucose and increased risk of type 2 diabetes mellitus. Swiss Med Wkly. 2019;149:w20139. doi:10.4414/smw.2019.20139
- Akan S, Aktas G. Relationship between frailty, according to three frail scores, and clinical and laboratory parameters of the geriatric patients with type 2 Diabetes Mellitus. Rev Assoc Med Bras. 2022;68(8):1073–1077. doi:10.1590/1806-9282.20220271
- Erkus E, Aktas G, Kocak MZ, et al. Diabetic regulation of subjects with type 2 diabetes mellitus is associated with serum vitamin D levels. Rev Assoc Med Bras. 2019;65(1):51–55. doi:10.1590/1806-9282.65.1.51
- Aktas G, Kocak MZ, Bilgin S, et al. Uric acid to HDL cholesterol ratio is a strong predictor of diabetic control in men with type 2 diabetes mellitus. Aging Male. 2020;23(5):1098–1102. doi:10.1080/13685538.2019.1678126
- Bilgin S, Kurtkulagi O, Atak Tel BM, et al. Does C-reactive protein to serum Albumin Ratio correlate with diabEtic nephropathy in patients with type 2 dIabetes MEllitus? The CARE TIME study. Prim Care Diabetes. 2021;15(6):1071–1074. doi:10.1016/j.pcd.2021.08.015
- Kocak MZ, Aktas G, Atak BM, et al. Is Neuregulin-4 a predictive marker of microvascular complications in type 2 diabetes mellitus? Eur J Clin Invest. 2020;50(3):e13206. doi:10.1111/eci.13206
- Kocak MZ, Aktas G, Duman TT, et al. Monocyte lymphocyte ratio as a predictor of diabetic kidney injury in type 2 diabetes mellitus; the MADKID study. J Diabetes Metab Disord. 2020;19(2):997–1002. doi:10.1007/s40200-020-00595-0
- Tekce H, Tekce BK, Aktas G, et al. Serum omentin-1 levels in diabetic and nondiabetic patients with chronic kidney disease. Exp Clin Endocrinol Diabetes. 2014;122(8):451–456. doi:10.1055/s-0034-1375674
- Kocak MZ, Aktas G, Erkus E, et al. Mean platelet volume to lymphocyte ratio as a novel marker for diabetic nephropathy. J Coll Physicians Surg Pak. 2018;28(11):844–847. doi:10.29271/jcpsp.2018.11.844
- Kin Tekce B, Tekce H, Aktas G, et al. Evaluation of the urinary kidney injury molecule-1 levels in patients with diabetic nephropathy. Clin Invest Med. 2014;37(6):E377–E383. doi:10.25011/cim.v37i6.22242
- Wang J, Du J, Ge X, et al. Circulating Ism1 reduces the risk of type 2 diabetes but not diabetes-associated NAFLD. Front Endocrinol. 2022;13:890332–890339. doi:10.3389/fendo.2022.890332
- Wang C, Xu M, Feng R, et al. Serum isthmin-1 levels are positively and independently correlated with albuminuria in patients with type 2 diabetes mellitus. BMJ Open Diabetes Res Care. 2022;10(5):e002972–e002979. doi:10.1136/bmjdrc-2022-002972
- Ruiz-Ojeda FJ, Anguita-Ruiz A, Rico MC, et al. Serum levels of the novel adipokine isthmin-1 are associated with obesity in pubertal boys. World J Pediatr. 2023. doi:10.1007/s12519-022-00665-8
- Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med. 1998;15(7):539–553. doi:10.1002/(SICI)1096-9136(199807)15:7
- Tesfaye S, Boulton AJ, Dyck PJ, et al. Diabetic neuropathies: update on definitions, diagnostic criteria, estimation of severity, and treatments. Diabetes Care. 2010;33(10):2285–2293. doi:10.2337/dc10-1303
- Matsushita K, Mahmoodi BK, Woodward M, et al. Comparison of risk prediction using the CKD-EPI equation and the MDRD study equation for estimated glomerular filtration rate. JAMA. 2012;307(18):1941–1951. doi:10.1001/jama.2012.3954
- Wallace TM, Levy JC, Matthews DR. Use and abuse of HOMA modeling. Diabetes Care. 2004;27(6):1487–1495. doi:10.2337/diacare.27.6.1487
- Jaiswal M, Divers J, Dabelea D, et al. Prevalence of and risk factors for diabetic peripheral neuropathy in youth with type 1 and type 2 diabetes: SEARCH for diabetes in youth study. Diabetes Care. 2017;40(9):1226–1232. doi:10.2337/dc17-0179
- Ziegler D, Papanas N, Vinik AI, et al. Epidemiology of polyneuropathy in diabetes and prediabetes. Handb Clin Neurol. 2014;126:3–22. doi:10.1016/B978-0-444-53480-4.00001-1
- Pereira S, Cline DL, Glavas MM, et al. Tissue-specific effects of leptin on glucose and lipid metabolism. Endocr Rev. 2021;42(1):1–28. doi:10.1210/endrev/bnaa027
- Dunmore SJ, Brown JE. The role of adipokines in β-cell failure of type 2 diabetes. J Endocrinol. 2013;216(1):T37–T45. doi:10.1530/JOE-12-0278
- Banks WA. The blood-brain barrier as an endocrine tissue. Nat Rev Endocrinol. 2019;15(8):444–455. doi:10.1038/s41574-019-0213-7
- Osorio L, Wu X, Wang L, et al. ISM1 regulates NODAL signaling and asymmetric organ morphogenesis during development. J Cell Biol. 2019;218(7):2388–2402. doi:10.1083/jcb.201801081
- Boerner BP, George NM, Targy NM, et al. TGF-β superfamily member nodal stimulates human β-cell proliferation while maintaining cellular viability. Endocrinology. 2013;154(11):4099–4112. doi:10.1210/en.2013-1197
- Wu Y, Liang X, Ni J, et al. Effect of ISM1 on the immune microenvironment and epithelial-mesenchymal transition in colorectal cancer. Front Cell Dev Biol. 2021;9:681240. doi:10.3389/fcell.2021.681240
- Zhao F, Huang F, Tang M, et al. Nodal induces apoptosis through activation of the ALK7 signaling pathway in pancreatic INS-1 beta-cells. Am J Physiol Endocrinol Metab. 2012;303(1):E132–E143. doi:10.1152/ajpendo.00074.2012
- Steiner BM, Berry DC. The regulation of adipose tissue health by estrogens. Front Endocrinol. 2022;13:889923. doi:10.3389/fendo.2022.889923
- Delaney KZ, Santosa S. Sex differences in regional adipose tissue depots pose different threats for the development of Type 2 diabetes in males and females. Obes Rev. 2022;23(3):e13393. doi:10.1111/obr.13393
- Lam TYW, Nguyen N, Peh HY, et al. ISM1 protects lung homeostasis via cell-surface GRP78-mediated alveolar macrophage apoptosis. Proc Natl Acad Sci U S A. 2022;119(4):e2019161119.
- Schmitt M, Greten FR. The inflammatory pathogenesis of colorectal cancer. Nat Rev Immunol. 2021;21(10):653–667. doi:10.1038/s41577-021-00534-x
- Lockhart JS, Sumagin R. Non-canonical functions of myeloperoxidase in immune regulation, tissue inflammation and cancer. Int J Mol Sci. 2022;23(20):12250–12267. doi:10.3390/ijms232012250
- Xiang W, Ke Z, Zhang Y, et al. Isthmin is a novel secreted angiogenesis inhibitor that inhibits tumour growth in mice. J Cell Mol Med. 2011;15(2):359–374. doi:10.1111/j.1582-4934.2009.00961.x
- Zhang Y, Chen M, Venugopal S, et al. Isthmin exerts pro-survival and death-promoting effect on endothelial cells through alphavbeta5 integrin depending on its physical state. Cell Death Dis. 2011:2e153. doi:10.1038/cddis.2011.37
- Chen M, Zhang Y, Yu VC, et al. Isthmin targets cell-surface GRP78 and triggers apoptosis via induction of mitochondrial dysfunction. Cell Death Differ. 2014;21(5):797–810. doi:10.1038/cdd.2014.3
- Li J, Xia Y, Huang Z, et al. Novel HIF-1-target gene isthmin1 contributes to hypoxia-induced hyperpermeability of pulmonary microvascular endothelial cells monolayers. Am J Physiol Cell Physiol. 2021;321(4):C671–C680. doi:10.1152/ajpcell.00124.2021
- Iacobini C, Vitale M, Haxhi J, et al. Mutual regulation between redox and hypoxia-inducible factors in cardiovascular and renal complications of diabetes. Antioxidants. 2022;11(11). doi:10.3390/antiox11112183
- Li C, Song L, Zhou Y, et al. Identification of Isthmin1 in the small annual fish, Nothobranchius guentheri, as a novel biomarker of aging and its potential rejuvenation activity. Biogerontology. 2022;23(1):99–114. doi:10.1007/s10522-021-09948-5
- Zheng Y, Zheng Y, Lei W, et al. miR-1307-3p overexpression inhibits cell proliferation and promotes cell apoptosis by targeting ISM1 in colon cancer. Mol Cell Probes. 2019:48101445. doi:10.1016/j.mcp.2019.101445
- Jang J, Jung Y, Chae S, et al. WNT/beta-catenin pathway modulates the TNF-alpha-induced inflammatory response in bronchial epithelial cells. Biochem Biophys Res Commun. 2017;484(2):442–449. doi:10.1016/j.bbrc.2017.01.156
- Cheng X, Xu X, Chen D, et al. Therapeutic potential of targeting the Wnt/beta-catenin signaling pathway in colorectal cancer. Biomed Pharmacother. 2019;110:473–481. doi:10.1016/j.biopha.2018.11.082
- Wang YG, Wang T, Ding M, et al. hsa_circ_0091570 acts as a ceRNA to suppress hepatocellular cancer progression by sponging hsa-miR-1307. Cancer Lett. 2019;460:128–138. doi:10.1016/j.canlet.2019.06.007
- Li X, Liu R, Wang Y, et al. Cholangiocyte-derived exosomal lncRNA H19 promotes macrophage activation and hepatic inflammation under cholestatic conditions. Cells. 2020;9(1):190. doi:10.3390/cells9010190
- Li H, Yu B, Li J, et al. Overexpression of lncRNA H19 enhances carcinogenesis and metastasis of gastric cancer. Oncotarget. 2014;5(8):2318–2329. doi:10.18632/oncotarget.1913
- Cole JB, Florez JC. Genetics of diabetes mellitus and diabetes complications. Nat Rev Nephrol. 2020;16(7):377–390. doi:10.1038/s41581-020-0278-5
- Yao Y, Song Q, Hu C, et al. Endothelial cell metabolic memory causes cardiovascular dysfunction in diabetes. Cardiovasc Res. 2022;118(1):196–211. doi:10.1093/cvr/cvab013
- Dillmann WH. Diabetic cardiomyopathy. Circ Res. 2019;124(8):1160–1162. doi:10.1161/CIRCRESAHA.118.314665
- Pitt B, Filippatos G, Agarwal R, et al. Cardiovascular events with finerenone in kidney disease and type 2 diabetes. N Engl J Med. 2021;385(24):2252–2263. doi:10.1056/NEJMoa2110956