Abstract
Background
Solute carrier family 22 member 3 (SLC22A3) gene had been reported to be associated with the efficacy of metformin in type 2 diabetes mellitus (T2DM). However, few studies reported the relationship between SLC22A3 polymorphism and T2DM. The aim of this study was to investigate the association of SLC22A3 polymorphism and susceptibility to T2DM in Chinese population.
Methods
We identified SLC22A3 rs555754, rs3123636, rs3088442 genotypes of 450 T2DM patients and 220 healthy controls from the Chinese population. The association between SNPs of SLC22A3 and susceptibility of T2DM was evaluated.
Results
The clinical characteristics were significantly different between T2DM patients and healthy controls. The polymorphisms of SLC22A3 rs555754 and rs3123636 were obviously associated with the susceptibility of T2DM which was adjusted for age, sex and BMI, while rs3088442 did not. And there was haplotype association of SLC22A3 rs3088442-rs3123636 with T2DM susceptibility.
Conclusion
SLC22A3 rs555754 and rs3123636 polymorphisms were associated with the susceptibility to T2DM in Chinese Han population. Large sample size studies would be required to verify this association.
Introduction
As one of the most common chronic metabolic diseases, diabetes characterized by hyperglycemia and abnormal insulin reaction, which severely threaten human health worldwide. It is estimated that 536.6 million people among 20–79 years were diagnosed with diabetes globally in 2021, accounting for about 10.5% of the global population, and type 2 diabetes mellitus (T2DM) is the major.Citation1 As we all known, T2DM is the results caused by the interaction of environmental factors and genetics.Citation2 Organic Cation Transporters (OCT) is a factor transferring many endogenous small molecules, drugs and environmental toxins, which will influence the development of various diseases and the efficacy of multiple drugs.Citation3
The OCT family includes three organic cation transporters, and OCT3 is included. OCT3, encoded by the gene SLC22A3, which contains 12 putative transmembrane domains. SLC22A3 is highly expressed at human blood–brain barrier, which suggest that OCT3 may be involved in the entry of diversity substrates into the CNS.Citation4 And it is reported that OCT3 can regulate the uptake of dopaminergic neurotransmission and behavior.Citation5 And it is a high-capacity transporter OCT3 is a critical transporter regulating the cardiac accumulation of doxorubicin, the deficiency of OCT3 can protect heart from acute and chronic doxorubicin-related damage.Citation6 What is more, SLC22A3 is reported to be associated with esophageal cancer,Citation7,Citation8 pancreatic adenocarcinomaCitation9 and acute myeloid leukemia.Citation10 SLC22A3 is widely expressed in various tissues including liver, kidney, intestine, and other organs.Citation11 Any changes of SLC22A3, such as polymorphisms, methylation or epigenetic modification may influence the structure or function of OCT3, and then influence the transporting efficacy of many endogenous compounds.
It is reported that the polymorphisms of SLC22A3 were related to various disease including colon cancer, coronary artery diseaseCitation12 and T2DM.Citation3 As an organic cation transporter, more studies reported the influence of SLC22A3 polymorphisms on metformin efficacy in T2DM,Citation13–15 but very few studies focused on the relationship between SLC22A3 polymorphisms and T2DM,Citation11 especially in Chinese population. Thus, the aim of this study was to elucidate the association between SLC22A3 polymorphisms and the susceptibility of T2DM in Chinese population.
Materials and Methods
Study Population and Design
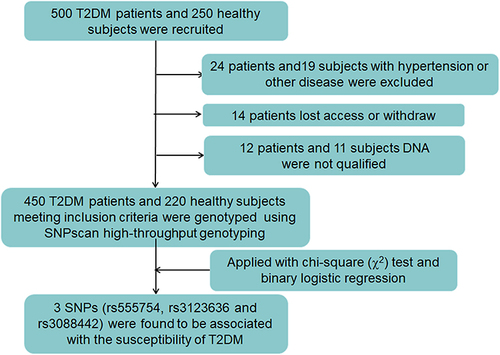
This study includes 450 T2DM patients (281 males and 169 females, mean age 56.98±13.60) and 220 healthy controls (111 males and 109 females, mean age 52.92 ± 12.83). All patients were diagnosed with T2DM according to the ADA standardCitation16 as fasting plasma glucose (FPG) ≥7.0 mmol ⁄ L or 2h plasma glucose (2h PG) during oral glucose tolerance test (OGTT) ≥11.1 mmol ⁄ L or glycosylated hemoglobin (HbA1c) ≥6.5%. And the healthy controls were recruited from health management center without history of diabetes and cardiovascular disease. The exclusion criteria of this study listed as follows, type 1 diabetes mellitus, chronic kidney disease, liver disease, myocardial infarction, stroke, and pregnancy. The demographic and clinical characteristics, including gender, age, body mass index (BMI), HbA1c, FBG, postprandial blood glucose (PBG), C-Peptide (CPO), postprandial C-Peptide (PCPO), total cholesterol (TC), triglycerides (TG), low-density lipoprotein cholesterol (LDL) and high-density lipoprotein cholesterol (HDL) of all participants are listed in . This study complies with the Declaration of Helsinki. The informed consent has obtained from all participants and this study was approved by the Ethics Committee of the Affiliated Zhuzhou Hospital of Xiangya School of Medicine CSU (Zhuzhou, Hunan, China) (2019–05031).
Table 1 The Demographic and Clinical Characteristics of T2DM Patients and Healthy Controls
DNA Isolation and Genotyping
Three-milliliter peripheral blood was donated from each participant. Genomic DNA was isolated by the SQ Blood DNA Kit II (Omega Bio-Tek, Guangzhou, China) according to the manufacturer’s protocol and stored at −20°C. Three SNPs of SLC22A3 gene (rs555754, rs3123636 and rs3088442) was selected for further investigation according to bioinformatics analysis and previous studies. High-throughput genotyping was relegated to Genesky Biotechnologies Inc. in Shanghai. The primers of these three SNPs were supplied in Table S1.
Statistics
The Hardy–Weinberg equilibrium (HWE) of SNPs was examined by a goodness-of-fit chi-square (χ2) test. The demographic and clinical characteristics (apart from gender) of T2DM patients and health controls were compared using independent sample T-test and shown as mean ± SD. And the χ2-test also conducted to compare the frequencies of genotypes and alleles between T2DM patients and healthy controls. The association between three SNPs of SLC22A3 gene and susceptibility to T2DM was performed by binary logistic regression adjusted for age, gender, and BMI. Furthermore, the homozygote comparison, heterozygote comparison, dominant model, recessive model and haplotype analysis were also analyzed by binary logistic regression. Two-tailed p values less than 0.05 were considered to significance. All statistical analysis was performed by SPSS 23.0 and PLINK v1.07.
Results
Characteristics of Subjects
To find out the difference between healthy controls and T2DM patients, the characteristic analysis was conducted. After filtered, there were 220 healthy controls and 450 T2DM patients in accordance with the inclusion criteria (). The differences of demographic and clinical characteristics between T2DM patients and healthy controls are displayed in . There were significant differences in gender, age, weight, HbA1c, FBG, PBG and TC between two groups (all p values < 0.05). In T2DM groups, there were 281 males and 169 females, aged 56.98 ± 13.60 years old, while in the healthy controls, 111 males and 109 females, aged 52.92 ± 12.83 years old were enrolled. Apart from these, the weight, HbA1c level, FBG, PBG of T2DM groups were significantly higher than the healthy controls (all p < 0.001). However, TC level of T2DM groups was lower than the healthy controls (p = 0.024).
Association Study
In order to investigate the association of SLC22A3 polymorphism and susceptibility to T2DM, χ2-test and logistic regression was chosen for us. The genotypes and allelic frequencies of SLC22A3 rs555754, rs3123636 and rs3088442 polymorphisms in the healthy controls and T2DM patients are listed in . All three SNPs were in accordance with the Hardy–Weinberg equilibrium (HWE p > 0.05, ) and had a minor allele frequency > 5%. As for rs555754, the genotype frequencies of healthy controls were significantly different with T2DM patients (p = 0.034). The allelic frequencies were also different between healthy controls and T2DM patients (p = 0.012). As for rs3123636, there was a significant difference of genotype frequencies and allelic frequencies between the healthy controls and T2DM patients (p = 0.045 and p = 0.014, separately). However, we found no difference of rs3088442 in these two groups.
Table 2 Comparisons of Allelic Frequencies of SLC22A3 rs555754, rs3123636 and rs3088442 Polymorphisms in T2DM Patients and Healthy Controls
Further, we had applied different models to verify the influence of SLC22A3 genetic polymorphisms on T2DM after adjusted for age, gender, and BMI (). In accordance with the results of χ2-test, the influence of rs555754 and rs3123636 on the risk of T2DM was also confirmed by the results of binary logistic analysis adjusted by age, gender and BMI. The rs555754 genetic polymorphism was significantly associated with the increased susceptibility of T2DM in the homozygote comparison (AA vs GG: adjusted OR = 1.409, 95% CI = 1.049–1.892, p = 0.023) and recessive model (AA vs.GA/GG: adjusted OR = 1.365, 95% CI = 1.032–1.805, p = 0.029). As for rs3123636, there was a significant association of homozygote comparison with the decreased susceptibility to T2DM (CC vs TT: adjusted OR = 0.645, 95% CI = 0.449–0.927, p = 0.018). In the dominant model, TT genotype of rs3123636 was selected as the reference, we found that the TC/CC genotype was shown to be the protective factor of T2DM (TC/CC vs TT: adjusted OR = 0.824, 95% CI = 0.694–0.978, p = 0.027). In the recessive model, when rs3123636 TC/TT genotype was used as the reference, the CC genotype was associated with a decreased risk of T2DM (CC vs TC/TT: adjusted OR = 0.705, 95% CI = 0.497–0.999, p = 0.049). However, there was no significant association between rs3088442 of SLC22A3 and T2DM risk when analyzed by logistic regression adjusted for age, gender and BMI.
Table 3 The Association Between SLC22A3 Genetic Polymorphisms and the Risk of T2DM (Adjusted for Age, Gender and BMI)
Haplotype Analysis
After correlation analysis, we also conducted the linkage disequilibrium (LD) analysis by the online software, SHEsisCitation17 (Figures S1 and S2). And we found there was LDs between SLC22A3 rs3123636 and rs3088442, with the r square value of rs3088442-rs3123636 is 0.332, and the D′ value is 0.948.
Then, haplotype analysis between T2DM and healthy controls was conducted. According to the results, there were only SLC22A3 rs3088442-rs3123636 in a block, and the frequency of haplotypes with p values <0.05 was ignored. So, there were only three haplotypes analyzed in this study. Among them, only one haplotype was significantly related to the risk of T2DM (). The haplotype of AC with rs3088442-rs3123636 was considered as the protective factor of the susceptibility to T2DM (OR = 0.713, 95% CI = 0.548–0.929, p = 0.011).
Table 4 Haplotype Analysis of SLC22A3 rs3088442 and rs3123636 with the Risk of T2DM
Discussion
SLC22A3 is a candidate gene related to various diseases, including cancer,Citation8,Citation18 cardiovascular diseaseCitation6,Citation19 and metabolic disease.Citation20,Citation21 Ren et al found that rs420038 in SLC22A3 was related to lower risk of colorectal cancer and influenced the expression of this gene.Citation18 In addition, Wang et al revealed that rs3088442 in SLC22A3 was associated with both plasma lipoprotein(a) level and the risk of coronary artery disease in Chinese population.Citation19 Paquette et al demonstrated that rs2048327 SNP of SLC22A3 gene was significantly associated with lipoprotein(a) level and cardiovascular disease events in hypercholesterolemia subjects.Citation22 Further, rs3088442 G>A and rs2292334 G>A in SLC22A3 had been reported to be associated with the susceptibility of T2DM.Citation11 At the same time, these two variants (rs2292334Citation23 and rs3088442Citation24) had been reported to be related to the efficacy of metformin in different population. As for Indian males, rs2292334 was identified as the risk factor for the development of T2DM.Citation25 In Iranians, rs3088442 G>A was identified as a protective factor with T2DM, while rs2292334 G>A as the risk.Citation11 In Pakistan population, rs3088442 was suggested to be a protective allele and was associated with metformin efficacy with T2DM patients.Citation26 In the latest follow-up study, rs543159 and rs1317652 in SLC22A3 gene was suggested to be associated with variability in response to metformin therapy in T2DM patients.Citation20 However, no studies have shown the relationship between SLC22A3 polymorphism and the risk of T2DM in Chinese.
In the present study, we firstly investigated the distribution of rs555754, rs3123636 and rs3088442 genetic polymorphism of SLC22A3 gene, and discussed the roles of these three SNPs on the susceptibility of T2DM on 450 T2DM patients and 220 healthy controls in Chinese population. And we found significant difference of rs555754 and rs3123636 distribution between T2DM patients and healthy controls (). Compared to the wild-type, our data indicated the increased risk of T2DM patients carrying mutant-type of rs555754. In other words, the allele A and genotype AA of rs555754 in SLC22A3 gene could lead to the occurrence of T2DM in Chinese population. As for rs3123636 genetic polymorphism, the mutant type was a significantly protective factor of T2DM. What’s more, the haplotype of AC with rs3088442-rs3123636 was deemed as the protective factor of T2DM in our study. By means of bioinformatics analysis, we found the rs555754 polymorphism was the 5′ UTR variant, and rs3123636 was the intron variant. Like other reported regulatory genetic variants (such as SLC22A3 −1603 G>ACitation26 and rs2229611Citation27), rs555754 and rs3123636 could also influence the expression or function of SLC22A3 gene by affecting the activity of splicing site or transcription factor. Thus, we hypothesized that rs555754 and rs3123636 genetic polymorphism of SLC22A3 play similar roles in the susceptibility of T2DM. And the function of these two variants needs further investigations in the future.
In conclusion, this is the first time to analyze the effect of rs555754, rs3123636 and rs3088442 genetic polymorphisms in SLC22A3 gene on the susceptibility of T2DM, especially in Chinese population. We found that rs555754 and rs3123636 of SLC22A3 gene were associated with the susceptibility of T2DM in Chinese population. Further studies in larger different population are needed to confirm our finding, and the research of the molecular mechanisms of these two variants will be the next steps of our team.
Disclosure
The authors declare no conflict of interests in this study.
Acknowledgments
We thank the patients and volunteer blood donors and the involved staff of Zhuzhou Central Hospital.
Data Sharing Statement
The data used to support the findings of this study are available from the corresponding author upon request.
Additional information
Funding
References
- Sun H, Saeedi P, Karuranga S, et al. IDF diabetes atlas: global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res Clin Pract. 2022;183:109119. doi:10.1016/j.diabres.2021.109119
- Chiasson JL, Rabasa-Lhoret R. Prevention of type 2 diabetes: insulin resistance and beta-cell function. Diabetes. 2004;53 Suppl 3:S34–S38.
- Li C, Yang Y, Liu X, Li Z, Liu H, Tan Q. Glucose metabolism-related gene polymorphisms as the risk predictors of type 2 diabetes. Diabetol Metab Syndr. 2020;12(1):97. doi:10.1186/s13098-020-00604-5
- Chen EC, Matsson P, Azimi M, et al. High throughput screening of a prescription drug library for inhibitors of organic cation transporter 3, OCT3. Pharm Res. 2022;39(7):1599–1613. doi:10.1007/s11095-022-03171-8
- Gasser PJ. Roles for the uptake(2) transporter OCT3 in regulation of dopaminergic neurotransmission and behavior. Neurochem Int. 2019;123:46–49.
- Huang KM, Zavorka TM, Magdy T, et al. Targeting OCT3 attenuates doxorubicin-induced cardiac injury. Proc Natl Acad Sci U S A. 2021;118(5). doi:10.1073/pnas.2020168118
- Fu L, Qin YR, Ming XY, et al. RNA editing of SLC22A3 drives early tumor invasion and metastasis in familial esophageal cancer. Proc Natl Acad Sci U S A. 2017;114(23):E4631–E4640. doi:10.1073/pnas.1703178114
- Xiong JX, Wang YS, Sheng J, et al. Epigenetic alterations of a novel antioxidant gene SLC22A3 predispose susceptible individuals to increased risk of esophageal cancer. Int J Biol Sci. 2018;14(12):1658–1668. doi:10.7150/ijbs.28482
- Cervenkova L, Vycital O, Bruha J, et al. Protein expression of ABCC2 and SLC22A3 associates with prognosis of pancreatic adenocarcinoma. Sci Rep. 2019;9(1):19782. doi:10.1038/s41598-019-56059-w
- Gu Y, Xu ZJ, Zhou JD, et al. SLC22A3 methylation-mediated gene silencing predicts adverse prognosis in acute myeloid leukemia. Clin Epigenetics. 2022;14(1):162. doi:10.1186/s13148-022-01373-w
- Mahrooz A, Alizadeh A, Hashemi-Soteh MB, Ghaffari-Cherati M, Hosseyni-Talei SR. Polymorphic variants rs3088442 and rs2292334 in the Organic Cation Transporter 3 (OCT3) gene and susceptibility against type 2 diabetes: role of their interaction. Arch Med Res. 2017;48(2):162–168. doi:10.1016/j.arcmed.2017.03.010
- Koepsell H. Organic cation transporters in health and disease. Pharmacol Rev. 2020;72(1):253–319. doi:10.1124/pr.118.015578
- Shirasaka Y, Lee N, Zha W, Wagner D, Wang J. Involvement of organic cation transporter 3 (Oct3/Slc22a3) in the bioavailability and pharmacokinetics of antidiabetic metformin in mice. Drug Metab Pharmacokinet. 2016;31(5):385–388. doi:10.1016/j.dmpk.2016.04.005
- Chen EC, Liang X, Yee SW, et al. Targeted disruption of organic cation transporter 3 attenuates the pharmacologic response to metformin. Mol Pharmacol. 2015;88(1):75–83. doi:10.1124/mol.114.096776
- Kwon EY, Chung JY, Park HJ, Kim BM, Kim M, Choi JH. OCT3 promoter haplotype is associated with metformin pharmacokinetics in Koreans. Sci Rep. 2018;8(1):16965. doi:10.1038/s41598-018-35322-6
- Care, Diabetes. Diabetes: standards of medical care in diabetes-2022. Diabetes Care. 2022;45(Suppl 1):S17–S38. doi:10.2337/dc22-S002
- Li Z, Zhang Z, He Z, et al. A partition-ligation-combination-subdivision EM algorithm for haplotype inference with multiallelic markers: update of the SHEsis. Cell Res. 2009;19(4):519–523. doi:10.1038/cr.2009.33
- Ren A, Sun S, Li S, et al. Genetic variants in SLC22A3 contribute to the susceptibility to colorectal cancer. Int J Cancer. 2019;145(1):154–163. doi:10.1002/ijc.32079
- Wang L, Chen J, Zeng Y, et al. Functional variant in the SLC22A3-LPAL2-LPA gene cluster contributes to the severity of coronary artery disease. Arterioscler Thromb Vasc Biol. 2016;36(9):1989–1996. doi:10.1161/ATVBAHA.116.307311
- Taheri R, Kazerouni F, Mirfakhraei R, Kalbasi S, Shahrokhi SZ, Rahimipour A. The influence of SLC22A3 rs543159 and rs1317652 genetic variants on metformin therapeutic efficacy in newly diagnosed patients with type 2 diabetes mellitus: 25 weeks follow-up study. Gene. 2022;823:146382. doi:10.1016/j.gene.2022.146382
- Huang LO, Rauch A, Mazzaferro E, et al. Genome-wide discovery of genetic loci that uncouple excess adiposity from its comorbidities. Nat Metab. 2021;3(2):228–243.
- Paquette M, Bernard S, Baass A. SLC22A3 is associated with lipoprotein (a) concentration and cardiovascular disease in familial hypercholesterolemia. Clin Biochem. 2019;66:44–48. doi:10.1016/j.clinbiochem.2019.02.008
- Hosseyni-Talei SR, Mahrooz A, Hashemi-Soteh MB, Ghaffari-Cherati M, Alizadeh A. Association between the synonymous variant organic cation transporter 3 (OCT3)-1233G>A and the glycemic response following metformin therapy in patients with type 2 diabetes. Iran J Basic Med Sci. 2017;20(3):250–255. doi:10.22038/IJBMS.2017.8351
- Ghaffari-Cherati M, Mahrooz A, Hashemi-Soteh MB, Hosseyni-Talei SR, Alizadeh A, Nakhaei SM. Allele frequency and genotype distribution of a common variant in the 3 -untranslated region of the SLC22A3 gene in patients with type 2 diabetes: association with response to metformin. J Res Med Sci. 2016;21:92. doi:10.4103/1735-1995.192508
- Ghasan AAS, Ramachandran V, Inche ML, Mohamad NA, Mohamed MH, Wan SW. Analysis of OCT1, OCT2 and OCT3 gene polymorphisms among type 2 diabetes mellitus subjects in Indian ethnicity, Malaysia. Saudi J Biol Sci. 2022;29(1):453–459. doi:10.1016/j.sjbs.2021.09.008
- Moeez S, Riaz S, Masood N, et al. Evaluation of the rs3088442 G>A SLC22A3 gene polymorphism and the role of microRNA 147 in groups of adult Pakistani populations with type 2 diabetes in response to metformin. Can J Diabetes. 2019;43(2):128–135.
- Karthi S, Rajeshwari M, Francis A, et al. 3’-UTR SNP rs2229611 in G6PC1 affects mRNA stability, expression and glycogen storage disease type-Ia risk. Clin Chim Acta. 2017;471:46–54. doi:10.1016/j.cca.2017.05.016