Abstract
Purpose
The pandemic of coronavirus disease 2019 (COVID-19) has highlighted the intricate relationship between underlying conditions and death. We designed this study to determine whether metformin therapy for type 2 diabetes mellitus (T2D) is associated with low in-hospital mortality in patients hospitalized for COVID-19.
Materials and Methods
This was a retrospective study including patients with COVID-19 and T2D in Wuhan, from February 4th to April 11th, 2020. Patients were divided into two groups according to metformin exposure. The hazard ratio (HR) of COVID-19-related mortality and invasive mechanical ventilation was estimated using Cox regression.
Results
There were 571 T2D patients among the 4330 confirmed COVID-19 patients. Of those patients, 241 received metformin therapy. The in-hospital mortality and invasive mechanical ventilation of metformin group was lower than non-metformin group. In the multivariate model, metformin use was linked to a decreased in-hospital mortality and invasive mechanical ventilation when compared with that of the control group (HR: 0.376 [95% CI 0.154–0.922]; P = 0.033).
Conclusion
Our study indicated that metformin therapy was associated with decreased death risk in COVID-19 patients with T2D.
Introduction
Coronavirus disease 2019 (COVID-19) was brought on by the severe acute respiratory syndrome coronavirus 2 (SARS-COV-2) infection, which has never before spread on such a massive worldwide scale. Type 2 diabetes (T2D) has been proven to be one of the most significant and frequent risk factors for COVID-19 mortality in numerous observational studies around the world,Citation1 which significantly raises the risk of death and adverse complications in people with COVID-19.Citation2,Citation3 Besides, diabetes increases the risk of acute respiratory distress syndrome and artificial ventilation in intensive care units and almost double the mortality rate.Citation4,Citation5 Pharmacological treatment of T2D now includes several new medications that compete with previous oral antidiabetic drugs. Nevertheless, metformin continues to be the first-line anti-diabetic medication for the treatment of hyperglycemia in patients with T2D.Citation6 As a first-line hypoglycemic therapy with good safety profile, a broad spectrum of clinical benefits and low cost, it is extensively used around the world, including in developing countries.Citation7 The potent immunomodulatory and anti-inflammatory properties of metformin imply that they might be useful against coronaviral infections.Citation8 In fact, observational studies have shown that metformin significantly improves immune cell activity and the generation of pro-inflammatory cytokines in individuals with viral and bacterial pneumonia.Citation8,Citation9
Studies have shown that molecular mechanism of metformin intervention in SARS-COV2 pathogenesis in humans: it increases the stability of membrane angiotensin-converting enzyme 2 (ACE2) in respiratory system epithelial cells, through 5’-AMP-activated protein kinase (AMPK) signaling and subsequently reduces the rate of SARS-COV2 infection.Citation10 In addition, metformin lessens the activity of dipeptidyl peptidase-4 (DPP4, another receptor for viruses) and decreases the adhesion of SARS-COV2 on T cells. Along the way, the modulatory effects of metformin on ACE2 and DPP4 could modulate the immune response to SARS-COV2 and control the inflammatory processes.Citation8 Thus, it is hypothesized that the immunomodulatory properties of metformin and the management of blood glucose levels may have a beneficial effect on patient prognosis.Citation8 On March 6, 2023, a joint study from institutions including the University of Minnesota and Johns Hopkins University was published on the preprints with The Lancet. This multicenter, four-blind, parallel-group randomized Phase III clinical trial (COVID-OUT) showed that metformin can reduce the risk of long COVID by 42% when given early in COVID-19 infection, and it can further reduce the incidence of long COVID by 63% if given within four days of the onset of symptoms.Citation11
The large-scale vaccine inoculation has established an effective immune barrier. But considering the symbiotic possibility of the COVID-19, and the sizeable population of people with COVID-19 and pre-existing T2D who could be treated by metformin, information on the clinical impact of its usage in the context of COVID-19 would have significant and immediate implications. Our aims of this study were to evaluate the association of metformin with clinical outcomes in COVID-19 patients with T2D.
Materials and Methods
Study Design
This was a retrospective cohort study of T2D patients confirmed COVID-19 between February 4th to April 11th, 2020, who admitted to Taikang Tongji and Huoshenshan hospital in Wuhan city, China. The two hospitals are general hospitals that focused on the treatment of COVID-19 patients. The WHO interim recommendations and the Clinical Guideline for COVID-19 Diagnosis and Treatment Protocol released by the National Health Commission of China were followed in the diagnosis of COVID-19. The management of COVID-19 patients with T2D was in accordance with the guidelines recommended by the Chinese Diabetes Society (Diabetes Branch of Chinese Medical Association, 2020).Citation12 What’s more, this study was approved by the Ethics Committee of Army Medical center of Army Medical Center of People’s Liberation Army (2021(154)). Written consent was waived because the data were anonymous and retrospective; the study involved no more than minimal risk; and the waiver would not adversely affect the rights and welfare of the participants. All clinical investigations were carried out in conformity with the principles outlined in the Helsinki Declaration.
Inclusion and Exclusion Criteria
We used the following inclusion and exclusion criteria to identify the study cohort. Inclusion criteria included 1) 18 years of age or older; 2) a diagnosis of COVID-19 and admission to both hospitals between February 4th to April 11th, 2020; and 3) a diagnosis of diabetes and/or a history of diabetes. Exclusion criteria included pregnancy, type 1 diabetes, acute heart failure, greater than stage 4 renal insufficiency, acute liver failure, missing all or almost all data on laboratory and clinical characteristics and patients who were not on antidiabetic medications during hospitalization. Due to the emergency nature of the patient’s income and lack of HbA1c, we diagnosed diabetic patients by questioning those previously diagnosed with diabetes and random blood glucose levels. A confirmed COVID-19 case was defined by RT-PCR results of SARS-CoV-2 virus nucleic acid testing in respiratory specimens. Confirmed patients were divided into four categories: mild form, moderate form, severe form, and critical form, according to the Novel Coronavirus Pneumonia Diagnosis and Treatment plan (8th).
Patient Assessment and Data Collection
The clinical characteristics (age, gender and height, weight), clinical symptoms (fever, cough, and dyspnea), comorbidities (hypertension, diabetes and coronary heart disease [CHD]), antidiabetic therapies during hospitalization, non-antidiabetic therapies (traditional Chinese medicine, antiviral drugs and glucocorticoids), and clinical outcomes were extracted from the electronic medical system. Laboratory data on the routine blood test, blood glucose, creatine kinase-myocardial band (CK-MB), alanine aminotransferase (ALT), lactic dehydrogenase (LDH), and blood urea nitrogen (BUN) were collected from the laboratory information system. Chest severity was determined by CT of the lungs. The laboratory data in this study were collected when patients with COVID-19 were admitted. In order to prevent the potential of identifying specific patients, personnel information on patients was anonymous, and each patient was given a unique ID. The above data are extracted from standardized electronic medical records, and the data are carefully reviewed and validated by a group of knowledgeable doctors meticulously analyze, confirm, and double-check the information to assure correctness.
Definition of Clinical Endpoint
The primary endpoint was a composite endpoint of in-hospital mortality and invasive mechanical ventilation. The secondary endpoints were the occurrence of acute cardiac events, severe respiratory failure, acute respiratory distress syndrome (ARDS), and secondary infection. Acute cardiac events are defined as all cardiac conditions, including major adverse cardiovascular events (MACE), major adverse cardiac and cerebrovascular events (MACCE), acute exacerbation of heart failure, cardiac arrest, malignant arrhythmia, heart attack, acute exacerbation of heart failure, and cardiac arrest. Severe respiratory failure (Brescia-COVID ≥ 3, defined by the necessity of high frequency nasal ventilation, CPAP, non-invasive ventilation, mechanical ventilation, or mortality due to respiratory failure),Citation13 ICU admission, or death. ARDS was defined according to the WHO interim guideline “Clinical management of severe acute respiratory infections in the setting of suspected novel coronavirus (2019-nCoV) infection”.Citation14
Cox Regression Analysis
The risk of primary and secondary endpoints were calculated using the Cox proportional regression model comparing the metformin group versus the non-metformin group. Metformin exposure status was defined daily during follow-up based on medication history until the emergence of outcomes (in-hospital mortality and invasive mechanical ventilation, acute cardiac events, respiratory failure, ARDS, acute kidney injury, and secondary infection). In the Cox analysis, individuals discharged from the hospital were considered “0 risk” for two main reasons but data were not reviewed. First, patients with COVID-19 were discharged only if they had significant resolution of symptoms due to two consecutive negative viral PCRs. Second, individuals discharged from the hospital are quarantined for an additional 2 weeks. Therefore, individuals who are discharged from the hospital are unlikely to die from COVID-19, and information about their survival can still be obtained after being discharged.Citation14 The association of the treatment and clinical outcomes was assessed by multivariate Cox regression analysis before and after propensity score matching between the metformin group and the non-metformin group. We performed a multivariate Cox regression model adjusted for these baseline. These potential confounding variables included in the multivariate Cox regression model included age, sex, CHD, COPD, blood glucose, insulin, Acarbose. Adjusted hazard ratios (95% confidence intervals) were calculated for mortality using Cox models.
Statistical Analysis
Data were presented as the median and interquartile range (IQR) for continuous variables and frequency rates and percentage (%) for categorical variables. Student’s t-tests (normally distributed) or Mann–Whitney U-test (nonnormally distributed) were used for comparison of continuous variables between 2 groups, and χ2-test or Fisher’s exact test were used to analyze the comparison of categorical variables. Survival curves was described by the Kaplan-Meier method and compared with the Log rank test. p < 0.05 was considered statistically significant. Statistical analyses were performed using SPSS (IBM Corp, Armonk, NY, USA) version 23.0.
Results
Demographics and Characteristics of Study Cohort
A total of 571 (13.2%) consecutive patients with T2D from a cohort of 4330 hospitalized patients with confirmed COVID-19 from Taikang Tongji and Huoshenshan hospital, Wuhan, China, were included in this study. Among of the 571 confirmed patients, 306 patients (53.6%) were male (). The median age was 65 (IQR 57–72) years. Among those patients, 241 subjects (42.2%) treated with metformin or metformin plus other anti-diabetic drugs (referred to as the metformin group) and 330 (57.8%) individuals treated with anti-diabetic drugs other than metformin (referred to as the non-metformin group). The baseline characteristics of the two groups are described in . The median age was 63 (IQR, 56–70) and 66 (IQR, 60–73) years in the metformin and the non-metformin groups, respectively. At hospital admission, the distribution of severity category was: 62.5% mild, 0.7% moderate, 30.5% severe, and 6.3% critical COVID-19 patients. No significant differences in major symptoms at baseline were found between the two groups either (). The most common symptoms were cough, fever and malaise, similar to a general fever. There were no significant differences between the two groups in other underlying diseases, including hypertension (62.7% versus 61.0%, p = 0.725), cerebrovascular disease (6.6% versus 10.6%, p = 0.105) and chronic kidney disease (4.6% versus 2.5%, p = 0.065). But the non-metformin group had a higher rate of coronary heart disease (15.8% versus 9.1%, p = 0.023).
Table 1 Baseline Demographics and Clinical Characteristics in COVID-19 Patients with T2D Stratified by Metformin
Laboratory Indices
The baseline laboratory test results of all patients and the propensity score-matched subpopulations in the two groups were shown in . Major lab examinations results and chest computed tomography (CT) scan were similar or marginally different between the two groups. After propensity score matching, comparing the metformin group to the non-metformin group, the baseline characteristics for the matched subpopulations of patients were comparable (almost all p > 0.05). The levels of lymphocyte, monocyte, basophil count in the metformin group were higher than those in the non-metformin group. There was no difference in the overall WBC count. Meanwhile, the level of RBC in the metformin group (4.12×1012/L, IQR, 3.77×1012/L–4.48×1012/L) was higher than that in the non-metformin group (3.92×1012/L, IQR, 3.53×1012/L–4.32×1012/L) (p < 0.001). What’s more, the level of hemoglobin in the metformin group (126.00 g/L, IQR, 114.00–135.00 g/L) was higher than that in the non-metformin group (119.00 g/L, IQR, 106.00–131.00 g/L) (p < 0.001). On admission, the median fasting blood glucose level in the metformin group (7.96 mmol/L, IQR, 6.05–11.76 mmol/L) was higher than that in the non-metformin group (7.14 mmol/L IQR, 5.31–9.14 mmol/L) (p < 0.001). The absolute values of the median and IQR for laboratory test were shown in .
Table 2 Laboratory Parameters of COVID-19 Patients with T2D in the Metformin and Non-Metformin Groups
Drug Therapy
According to the Clinical Guidelines for the Diagnosis and Treatment of COVID-19 published by the National Health Council of China (National Health Council of the People’s Republic of China, 2020), all patients received standard treatment for COVID-19 symptoms. Among subjects receiving glucose-lowering medications, in addition to metformin (42.2% of all glucose-lowering medication users), acarbose was prescribed most frequently (45.2% of all glucose-lowering medication users), followed by insulin (30.5% of all glucose-lowering medication users) (), and finally gliclazide (10.2% of all glucose-lowering medication users). (10.2% of all glucose-lowering drug users). In addition, as shown in , there were significant differences between the metformin group and the non-metformin group for certain treatments, including the type of antidiabetic drug (68.9% versus 27.9% for acarbose, p < 0.001; 36.9% versus 25.8% for insulin, p < 0.001; Gliclazide (17.0% vs 5.2%, p < 0.001), and Calcium antagonists treatment (48.1% vs 35.6%, p < 0.05). There were no differences between the metformin and non-metformin groups in the application of herbal treatments (p = 0.700), antiviral treatments (p = 0.384), antibiotics (p = 0.190) and glucocorticoids (p = 0.725).
Table 3 Treatment and Clinical Outcome in T2DM Patients Between the Metformin and Non-Metformin Groups
Clinical Outcomes
Among the entire cohort of 571 patients with COVID-19 and T2D, a total of 39 patients met the primary outcome, including 8 out of 241 in the metformin group (3.3%) and 31 out of 343 in the non-metformin group (9.4%). Comparing to the individuals without metformin use, the individuals with metformin therapy had a lower in-hospital mortality and invasive mechanical ventilation rate than those without metformin treatment (p = 0.004). However, for discharged patients, median hospitalization time/ hospital stay durations (in-hospital days) was significantly longer for the metformin-treated patients than the non-metformin-treated patients (16 days versus 14 days, p = 0.012). The Kaplan-Meier survival analysis showed a significantly higher survival rate in patients with T2D treated with metformin compared with patients with T2D without metformin treatment (log-rank, p = 0.006) ().
Figure 1 Kaplan-Meier Survival Curves for patients with COVID-19 and T2D with and without Metformin treatment.
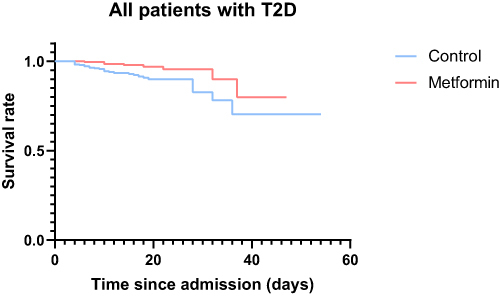
Using a Cox model accounting for metformin as a time-varying exposure and with adjustment for baseline differences (including age, sex, hypertension, COPD, blood glucose), metformin treatment was associated with lower mortality (aHR:0.376[95% CI 0.154–0.922]; p =0.033) compared to non-metformin users (). Furthermore, metformin treatment group showed a lower incidences of acute cardiac events (aHR:0.216[95% CI 0.078–0.595]; p =0.003) and severe respiratory failure (aHR:0.189[95% CI 0.063–0.561]; p =0.003) compared with non-metformin-treated patients.
Table 4 Hazard Ratios for Primary and Secondary Outcomes Between Individuals in the Metformin and the Non-Metformin Groups
Discussion
In this retrospective cohort study, we evaluated the association between metformin treatment and adverse clinical outcomes in patients with COVID-19 and T2D. Our results suggest that metformin reduces in-hospital mortality and invasive mechanical ventilation.
It is worth noting that patients with COVID-19 had underlying health conditions.Citation15 Diabetes was found to be a major risk factor for severe acute respiratory syndrome (SARS) and adverse clinical outcome in patients with COVID-19.Citation16 Because of deficiencies in innate immunity that affect phagocytosis, neutrophil chemotaxis, and cell-mediated immunity, people with all forms of diabetes are generally more susceptible to infection. However, the high prevalence of diabetes in serious cases of COVID-19 may reflect the higher prevalence of T2D in older people. Additionally, diabetes in older age is associated with cardiovascular disease, which by itself may assist to explain the association with fatal outcomes of COVID-19.Citation12,Citation17 Those COVID-19 patients with poorly controlled blood glucose had a higher risk of severity and mortality when compared with patients with well-controlled blood glucose.
It is obvious that blood glucose management is very important for the prognosis of COVID-19 patients.Citation12 Metformin therapy has been correlated to a decreased death rate among DM patients, according to some cohort studies.Citation18–20 However, there still have some studies did not confirm such an association between metformin therapy and hospital mortality among type 2 diabetes.Citation21,Citation22 For the virus to attach to this cellular receptor, the ACE2 must be glycosylated, a process that can be triggered by hyperglycemia.Citation23 According to previous studies, diabetic mice had higher levels of glycosylated ACE2 protein expression in lung tissue than non-diabetic controls.Citation24 Metformin, which is the most widely used hypoglycemic medication, stimulates AMPK, which phosphorylates the ACE2 protein on its Ser-680 residue and increases ACE2 stability by preventing its ubiquitination and proteasomal destruction.Citation25 Additionally, several prior papers advised against taking metformin plus SGLT-2 inhibitors due to the increased risk of lactic acidosis associated with metformin or moderate hyperglycemic diabetic ketoacidosis associated with SGLT-2 inhibitors.Citation7 As a result, the relationship between COVID-19 risk and metformin use in people with type 2 diabetes is still debatable and needs more research. Although metformin may be beneficial as monotherapy or in combination with other medications, randomized trials with multiple drug combinations need to be conducted before definitive conclusions can be drawn. At present, the COVID-OUT study led by Assistant Professor Carolyn Bramante from the University of Minnesota Medical School has been preprinted and is still undergoing peer review. If officially published, it would become one of the most influential results for the treatment of post-COVID-19 symptoms to date. However, further validation of the research findings and identification of the most suitable population for metformin therapy before wider clinical application are still crucial.Citation11 Our study population was an early case during the COVID-19 outbreak in China. After adjusting for some relevant clinical factors that may contribute to disease severity, mortality was significantly lower in patients with COVID-19 and T2D treated with metformin than in those not treated with metformin.
Our study has some limitations. First, due to the emergency nature of the COVID-19 pandemic, some important data, such as body mass index (BMI), smoking, and drinking history, were not included in the analysis. Among these missing data, BMI had a relatively high proportion of missing values which may lead to uncertainty association between metformin use and reduced risk of in-hospital mortality. Second, the relatively small sample size and clinical center size were the main limitation of the study, which might affect the accuracy of our findings and prevented a more detailed analysis. Third, the outcomes of this study may have been affected by combination of metformin and other antidiabetic medications, which we did not consider. Fourth, we did not assess some important information reflecting the severity of DM, such as the duration of diabetes and HbA1c levels; therefore, the results of this study may have been influenced by the proper glycemic control of patients with type 2 diabetes. Fifth, the study population included only hospitalized subjects, and hospitalization or nonhospitalization may have influenced the association between metformin use and hospital mortality. Therefore, caution is advised when extrapolating these results to the broader population with COVID-19-related problems in a non-hospital situation. The findings of this study should be regarded cautiously in light of its limitations, and additional prospective, sizable population-based cohort studies are required in order to support these conclusions.
Conclusion
In brief, our study suggests that metformin treatment reduces the risk of death and poor prognosis in COVID-19 patients with type 2 diabetes mellitus. There is no clear indication to alter the prescription of glucose-lowering medications in patients with type 2 diabetes in the context of the COVID-19 pandemic. Due to the nature of such retrospective studies, these results should be interpreted with caution. Therefore, more studies are needed.
Abbreviations
T2D, type 2 diabetes mellitus; COVID-19, coronavirus disease 2019; HR, hazard ratio; SARA-COV-2, severe acute respiratory syndrome coronavirus 2; ACE2, angiotensin-converting enzyme 2; AMPK, 5’-AMP-activated protein kinase; DPP4, dipeptidyl peptidase-4; CK-MB, creatine kinase-myocardial band; ALT, alanine aminotransferase; LDH, lactic dehydrogenase; BUN, blood urea nitrogen; ARDS, acute respiratory distress syndrome; MACE, major adverse cardiovascular events; MACCE, major adverse cardiac and cerebrovascular events; IQR, interquartile range; CT, computed tomography; SARS, severe acute respiratory syndrome; BMI, body mass index.
Disclosure
The authors declare that they have no known competing commercial or financial relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
We would like to thank the staff of the Department of Taikang Tongji and Huoshenshan Hospital in Wuhan city, Hubei Province, who contributed to this study by collecting the required data in the hospital data system.
Additional information
Funding
References
- Apicella M, Campopiano MC, Mantuano M, et al. COVID-19 in people with diabetes: understanding the reasons for worse outcomes. Lancet Diabetes Endocrinol. 2020;8(9):782–792. doi:10.1016/S2213-8587(20)30238-2
- Richardson S, Hirsch JS, Narasimhan M, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020;323(20):2052–2059. doi:10.1001/jama.2020.6775
- Zhu L, She ZG, Cheng X, et al. Association of blood glucose control and outcomes in patients with COVID-19 and pre-existing type 2 diabetes. Cell Metab. 2020;31(6):1068–77.e3. doi:10.1016/j.cmet.2020.04.021
- Targher G, Mantovani A, Wang XB, et al. Patients with diabetes are at higher risk for severe illness from COVID-19. Diabetes Metab. 2020;46(4):335–337. doi:10.1016/j.diabet.2020.05.001
- Singh AK, Khunti K. Assessment of risk, severity, mortality, glycemic control and antidiabetic agents in patients with diabetes and COVID-19: a narrative review. Diabetes Res Clin Pract. 2020;165:108266. doi:10.1016/j.diabres.2020.108266
- Buse JB, Wexler DJ, Tsapas A, et al. 2019 update to: management of hyperglycaemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia. 2020;63(2):221–228. doi:10.1007/s00125-019-05039-w
- Cheng X, Liu YM, Li H, et al. Metformin is associated with higher incidence of acidosis, but not mortality, in individuals with COVID-19 and Pre-existing type 2 diabetes. Cell Metab. 2020;32(4):537–47.e3. doi:10.1016/j.cmet.2020.08.013
- Hashemi P, Pezeshki S. Repurposing metformin for covid-19 complications in patients with type 2 diabetes and insulin resistance. Immunopharmacol Immunotoxicol. 2021;43(3):265–270. doi:10.1080/08923973.2021.1925294
- Sharma S, Ray A, Sadasivam B. Metformin in COVID-19: a possible role beyond diabetes. Diabetes Res Clin Pract. 2020;164:108183. doi:10.1016/j.diabres.2020.108183
- Zhang J, Dong J, Martin M, et al. AMP-activated protein kinase phosphorylation of angiotensin-converting enzyme 2 in endothelium mitigates pulmonary hypertension. Am J Respir Crit Care Med. 2018;198(4):509–520. doi:10.1164/rccm.201712-2570OC
- Bramante C. The COVID-OUT study: a randomized, double-blind, placebo-controlled trial of metformin for the treatment of COVID-19 sequelae. medRxiv; 2021.
- Yu B, Li C, Sun Y, et al. Insulin treatment is associated with increased mortality in patients with COVID-19 and type 2 diabetes. Cell Metab. 2021;33(1):65–77.e2. doi:10.1016/j.cmet.2020.11.014
- Sancho-López A, Caballero-Bermejo AF, Ruiz-Antorán B, et al. Efficacy and safety of sarilumab in patients with COVID19 pneumonia: a randomized, phase III clinical trial (SARTRE study). Infect Dis Ther. 2021;2021:1–14.
- Zhang XJ, Qin JJ, Cheng X, et al. In-hospital use of statins is associated with a reduced risk of mortality among individuals with COVID-19. Cell Metab. 2020;32(2):176–87.e4. doi:10.1016/j.cmet.2020.06.015
- Remuzzi A, Remuzzi G. COVID-19 and Italy: what next? Lancet. 2020;395(10231):1225–1228. doi:10.1016/S0140-6736(20)30627-9
- Iacobellis G, Penaherrera CA, Bermudez LE, et al. Admission hyperglycemia and radiological findings of SARS-CoV2 in patients with and without diabetes. Diabetes Res Clin Pract. 2020;164:108185. doi:10.1016/j.diabres.2020.108185
- Bornstein SR, Rubino F, Khunti K, et al. Practical recommendations for the management of diabetes in patients with COVID-19. Lancet Diabetes Endocrinol. 2020;8(6):546–550. doi:10.1016/S2213-8587(20)30152-2
- Cariou B, Hadjadj S, Wargny M, et al. Phenotypic characteristics and prognosis of inpatients with COVID-19 and diabetes: the CORONADO study. Diabetologia. 2020;63(8):1500–1515. doi:10.1007/s00125-020-05180-x
- Crouse AB, Grimes T, Li P, et al. Metformin use is associated with reduced mortality in a diverse population with COVID-19 and diabetes. Front Endocrinol. 2020;11:600439. doi:10.3389/fendo.2020.600439
- Nguyen NN, Ho DS, Nguyen HS, et al. Preadmission use of antidiabetic medications and mortality among patients with COVID-19 having type 2 diabetes: a meta-analysis. Metabolism. 2022;131:155196. doi:10.1016/j.metabol.2022.155196
- Wang J, Cooper JM, Gokhale K, et al. Association of metformin with susceptibility to COVID-19 in people with type 2 diabetes. J Clin Endocrinol Metab. 2021;106(5):1255–1268. doi:10.1210/clinem/dgab067
- Bramante CT, Huling JD, Tignanelli CJ, et al. Randomized trial of metformin, ivermectin, and fluvoxamine for Covid-19. N Engl J Med. 2022;387(7):599–610. doi:10.1056/NEJMoa2201662
- Ceriello A. Hyperglycemia and the worse prognosis of COVID-19. Why a fast blood glucose control should be mandatory. Diabetes Res Clin Pract. 2020;163(163):108186. doi:10.1016/j.diabres.2020.108186
- Roca-Ho H, Riera M, Palau V, et al. Characterization of ACE and ACE2 expression within different organs of the NOD mouse. Int J Mol Sci. 2017;18(3):563. doi:10.3390/ijms18030563
- Ursini F, Ciaffi J, Landini MP, et al. COVID-19 and diabetes: is metformin a friend or foe? Diabetes Res Clin Pract. 2020;164:108167. doi:10.1016/j.diabres.2020.108167
