Abstract
Objective
The CHanges to treatment and Outcomes in patients with type 2 diabetes initiating InjeCtablE therapy (CHOICE) study assessed time to, and reasons for, significant treatment change after patients with type 2 diabetes (T2DM) initiated their first injectable glucose-lowering therapy (exenatide twice daily [BID] or insulin) in routine clinical practice, and these patients’ clinical outcomes, in six European countries. This paper reports interim data from the first 12 months of the study.
Research design and methods
CHOICE (NCT00635492) is a prospective, noninterventional, observational study. Clinical data were collected at initiation of first injectable therapy and after approximately 3, 6, and 12 months.
Results
Of 2497 patients enrolled in CHOICE, 1096 in the exenatide BID and 1239 in the insulin cohorts had ≥1 post-baseline assessment and were included in this analysis. Overall, 32.2% of the exenatide BID cohort and 29.1% of the insulin cohort (Kaplan–Meier estimates) had significant treatment change during the first 12 months, most commonly discontinuing injectable therapy or adding new T2DM therapy, respectively. Glycemic control improved in both cohorts, but weight loss occurred only in the exenatide BID cohort (mean change −3.3 kg). Hypoglycemia occurred in 13.2% of the exenatide BID cohort and 28.6% of the insulin cohort (82.8% and 55.6% of these patients, respectively, received sulfonylureas). The post hoc endpoint of glycated hemoglobin < 7%, no weight gain, and no hypoglycemia was attained at 12 months by 24.3% and 10.3% of patients who had data at 12 months and who were receiving exenatide BID and insulin, respectively.
Conclusion
About 30% of patients in CHOICE changed treatment in the first 12 months after initiation of first injectable therapy (exenatide BID or insulin). Overall, both cohorts achieved improved glycemic control, which was accompanied by a mean weight loss in the exenatide BID cohort.
Introduction
Progressive beta-cell dysfunction prevents many patients with type 2 diabetes mellitus (T2DM) from maintaining adequate glycemic control with oral antidiabetic drugs (OADs).Citation1,Citation2 Patients whose glycemic control deteriorates despite OAD treatment require initiation of injectable glucose-lowering therapies: insulin or a glucagon-like peptide-1 (GLP-1) receptor agonist.
Increasingly, successful treatment of T2DM is seen as requiring individualization for each affected patient, with specific patient preferences, characteristics, susceptibilities to adverse events, potential for weight gain, and hypoglycemia playing a major role in drug selection.Citation1 Weight gain resulting from some antidiabetic medications may be associated with worsening markers of insulin resistance and cardiovascular risk.Citation1 In instances where weight loss is considered important, initial injectable treatment with a GLP-1 receptor agonist can be considered as an alternative to insulin therapy because GLP-1 receptor agonists generally have the potential advantage of weight loss.Citation1 Further, GLP-1 receptor agonists have been associated with a low risk of hypoglycemia (unless used with a sulfonylurea) and have been shown to have glucose-lowering efficacy similar to that of insulin glargine or biphasic insulin aspart.Citation1,Citation3–Citation8 As such, the National Institute for Health and Clinical Excellence (NICE) in the UK suggests that the patients with T2DM likely to benefit most from GLP-1 receptor agonists are those for whom excess weight is an issue.Citation9 Epidemiological data from the USACitation10,Citation11 and UKCitation12 suggest that the mean body mass index (BMI) of people prescribed GLP-1 receptor agonists is 38–40 kg/m2 – somewhat higher than estimates for the general T2DM populations in both these countries.Citation13,Citation14 Conversely, non-obese patients are more likely than obese patients to be prescribed insulin therapy in the UK, as are patients aged ≤ 60 years, those with more severe T2DM (diabetes complications and higher glycated hemoglobin [HbA1c]), and patients who have received OAD therapy, compared with those aged > 60 years, without severe illness, and who have previously received lifestyle interventions only (P ≤0.01 for all). Citation15
Preclinical data have suggested that GLP-1 and GLP-1 receptor agonists may protect beta cells, and improve beta-cell mass and function.Citation16–Citation20 These actions could potentially slow the progression of T2DM, although their clinical relevance has not been established. Results of a recent meta-analysis support the use of GLP-1 receptor agonists as the second-line therapy of choice after initial metformin therapy.Citation21
Exenatide twice daily (BID) was the first GLP-1 receptor agonist to be approved in Europe (in 2006).Citation22,Citation23 Despite accumulated clinical experience, it has not been clear how, and in whom, exenatide BID is initiated in routine clinical practice across Europe, and limited information is available regarding its effectiveness in real-life settings across Europe. Moreover, it is unclear why, whether, and how exenatide BID therapy is later modified. Well-designed, scientifically rigorous prospective observational studies of clinical practice are necessary to fill these information gaps.Citation24,Citation25 The data gathered by such studies could then be used to enhance the evidence on which the management of T2DM is based.
The American Diabetes Association and European Association for the Study of Diabetes have called for clinical practice data for newer therapies to establish safety and effectiveness alongside best current treatment, and to provide “meaningful data on meaningful outcomes.”Citation1 CHOICE (CHanges to treatment and Outcomes in patients with type 2 diabetes initiating InjeCtablE therapy) is the first prospective observational study to evaluate patterns of exenatide BID usage and the accompanying outcomes in clinical practice in multiple European countries.Citation26 CHOICE was designed to assess the time to a significant treatment change after patients initiated their first injectable, glucose-lowering therapy in clinical practice. Although additional GLP-1 receptor agonists (liraglutide and exenatide once weekly) have since been approved by the European Commission, exenatide BID and insulins were the only injectable treatments available when this study commenced, hence these agents were selected for evaluation. Exenatide BID and insulin have shown similar efficacy in clinical trials,Citation3–Citation7 but the choice of agent is likely to be based on patient characteristics in clinical practice, and it is currently unclear which patients are prescribed exenatide BID or insulin or how long exenatide BID is prescribed before insulin is initiated. Data are also limited concerning real-world effectiveness, safety, and resource use for both GLP-1 receptor agonists and insulin. Therefore, the study also aimed to describe the characteristics of patients with T2DM initiated on injectable therapy,Citation27 the factors associated with treatment changes, clinical and patient-reported outcomes, and the health care resource use observed over 24 months for patients who initiated exenatide BID or insulin.
This paper reports interim treatment change data and clinical outcomes during the first 12 months after the initiation of injectable therapy with exenatide BID or insulin, providing a report of the use of exenatide BID for a period beyond that investigated in most clinical trials (up to 6 months), and allowing comparison with a 12-month study of exenatide BID use in clinical practice in the USA.Citation10,Citation28
Patients and methods
Study design and patients
CHOICE is a prospective, multinational, noninterventional observational study that recruited patients from six European countries (Denmark, Belgium, France, Germany, Greece, and Sweden) between January 2008 and October 2009 (prior to the expanded label for exenatide BID to include adjunctive use with a thiazolidinedione or basal insulin).Citation26 The primary endpoint of the study is the time spent on the initial injectable regimen (exenatide BID or insulin) before significant treatment change, defined as at least one of the following: addition of a new medication (any route of administration) for the treatment of T2DM, a change in the number of times insulin is administered per day, discontinuation of any exenatide BID/insulin initiated at baseline, or substitution of a human insulin for an analog insulin or vice versa (not including switching between brands of the same class/type of insulin). Secondary objectives of the study include identification of factors associated with the first significant treatment change, clinical outcomes (glycemic control, weight change, and changes in other cardiovascular risk factors), and reasons for discontinuation of exenatide BID or insulin. Resource use and patient-reported outcomes (including health status, health-related quality of life, locus of health control, anxiety, depression, and weight-related quality of life) were additional secondary objectives, although these are not a focus of this paper. This interim analysis focuses on the following objectives: the number of patients who reported a significant treatment change (as defined for the primary endpoint) within the first 12 months of follow-up, the reasons for this change, and clinical outcomes at 12 months. The time to significant treatment change and the factors associated with the first significant treatment change will be reported in the final analysis.
Eligible patients were aged ≥ 18 years and initiating their first injectable glucose-lowering therapy (with exenatide BID or any type of insulin) for the treatment of T2DM in routine clinical practice. Treatment was not randomized since patients were invited to participate in CHOICE only after the clinical decision had been made to initiate exenatide BID or insulin. Physicians chose the injectable treatment to be initiated (ie, exenatide BID or insulin) following their normal treatment practice and prescribing habits. At study entry, patients could be taking any OAD. Patients gave written informed consent for the use of their data, and appropriate ethical review board approval was obtained (further details of the CHOICE study design have been published previously).Citation27
Patients were assessed at routine study visits at the time of initiation of injectable therapy (baseline) and only when they occurred as part of clinical practice at approximately 3, 6, and 12 months thereafter. Patients referred from the study site to another health care provider during the study were followed-up by contacting the new provider and by postal patient questionnaires.
Data collection
At baseline (initiation of injectable therapy), standard demographic and clinical data were collected from each patient as part of their routine clinical care.Citation27 At subsequent visits, changes to injectable therapy and the time of, and reason for, the change, were recorded. Follow-up clinical data were also collected as part of routine clinical care, including gastrointestinal (GI) adverse events, retrospectively recalled incidence of self-reported and, in most instances, self-defined hypoglycemic episodes, diabetes therapy and care, and concomitant medications.
Data analysis
Sample size justification
Based on Monte Carlo simulation and clinical data (Lilly, data on file),Citation29 the study aimed to recruit a maximum of 800 patients per country/country group, with the expectation of approximately 60% initiating insulin and 40% initiating exenatide BID. The insulin cohort was to be larger than the exenatide BID cohort because a greater variability was anticipated in the former for the time to treatment change (linked to the use of different insulin regimens). These sample sizes were chosen to achieve a 95% confidence interval (CI) width of about 3 months (±6 weeks) around the median time to significant treatment change, a level of precision considered sufficient for descriptive purposes.Citation27
Statistical analysis
All patients eligible at baseline (patients who provided consent to release information and who fulfilled study entry criteria), and with at least one post-baseline assessment, were included in the analyses of 12-month outcome data. Analyses were performed using all data up to the last data collection point for patients who were lost to follow-up, or who withdrew from the study. Missing data were not imputed, and analyses of outcomes at 12 months include only data available at 12 months.
Within each cohort, the number of patients who reported a significant treatment change within the 12 months of follow-up was estimated using Kaplan–Meier analysis. The reason for change in therapy was reported using descriptive statistics.
Analyses of the clinical endpoints were conducted using data from all eligible patients with at least one post-baseline assessment and with patients remaining in the cohort in which they were placed at baseline (initiators). Additional, post hoc, secondary analyses of key clinical data were conducted that considered only patients who initiated and continued on their baseline regimen without significant treatment change during the follow-up period (persisters) in case changing to a new treatment affected results.
Clinical outcomes data were reported using descriptive statistics and 95% CI, where appropriate, for each visit, as well as for the individual change from baseline.
For continuous variables, mean, standard deviation (SD), and 95% CI were calculated. Absolute numbers and percentages (including missing values) were given for categorical variables. In addition, the incidences of various composite endpoints were analyzed. Descriptive analyses were used to describe changes in exenatide or insulin dose, OADs, or concomitant medications. The incidence of GI events was also analyzed descriptively.
As previously reported,Citation27 analysis of the baseline data (using univariate analyses and logistic regression to compare all baseline patient characteristics between the two cohorts) indicated that the two treatment cohorts comprised substantially different patient populations (). Therefore, statistical comparison of endpoints between the two cohorts was not plausible. Logistic regression was used to derive propensity scoresCitation30 using all eligible patients from the initiators’ cohorts to create a matched subgroup (exenatide BID vs insulin). Patients from each cohort were matched 1:1 by country, based on the propensity score and optimal matching. Paired t-tests were used to compare changes in continuous variables, and McNemar’s tests were used to compare categorical variables, using clinical data (glycemic control, body weight, BMI, waist circumference, lipids, vital signs, and hypoglycemia) from these matched patients.
Table 1 Baseline clinical and demographic characteristics of patients initiated on exenatide twice daily (BID) or insulin therapy for the total CHOICE cohortCitation27 and propensity-matched subgroup
Results
A total of 2497 patients initiating injectable glucose-lowering therapy were recruited from a total of 325 sites across the six participating countries. Patient numbers varied by country: Belgium, 299 (43.1% exenatide BID); Denmark, 60 (73.3% exenatide BID); France, 295 (67.1% exenatide BID); Germany, 848 (46.5% exenatide BID); Greece, 807 (39.4% exenatide BID) and Sweden, 188 (51.1% exenatide BID); numbers reported here differ slightly from those reported previouslyCitation27 because data have been updated since baseline analysis.
As comparison of baseline patient characteristics of the exenatide BID and insulin cohorts indicated that the two treatment cohorts comprised substantially different patient populations,Citation27 statistical comparison of endpoints between the exenatide BID and insulin cohorts was not feasible. The propensity score-matched subgroup, in which exploratory between-treatment comparisons were made, included about half of the original study population and was based on baseline demographic and clinical variables (key characteristics of the total study population are summarized in ).
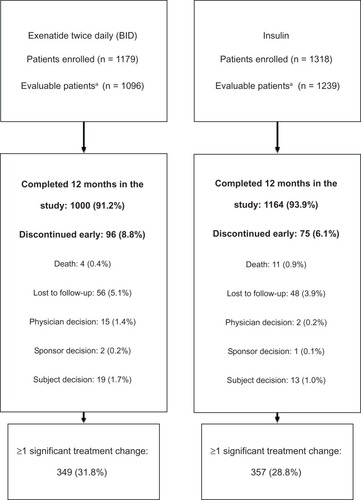
Overall, 2335 patients had sufficient data for the 12-month analysis (ie, they had data from baseline and at least one post-baseline visit): 1096 patients in the exenatide BID cohort (93.0% of the baseline population) and 1239 patients in the insulin cohort (94.0% of the baseline population). In all countries apart from France (77.3%) and Denmark (81.7%), > 90.0% of the overall baseline population was eligible for the 12-month analyses. In the exenatide BID cohort, 96 patients (8.8%) were observed to have discontinued the study before or at the 12-month visit; this was the case for 75 patients (6.1%) in the insulin cohort. Reasons for patients discontinuing the study are shown in . In addition to patients observed to have discontinued, some patients included in the 12-month analyses did not have available data at the 12-month visit (but did have data at earlier times); 968 patients (88.3%) in the exenatide BID cohort and 1128 patients (91.0%) in the insulin cohort had data available at the 12-month visit.
Figure 1 Study disposition.
Treatment change
A total of 349 patients from the exenatide BID cohort (31.8%; Kaplan–Meier estimate at 12 months: 32.2%) and 357 patients from the insulin cohort (28.8%; Kaplan–Meier estimate at 12 months: 29.1%) were observed to have a significant treatment change during the first 12 months after initiation of injectable therapy (). Therefore, in the exenatide BID cohort, there were 1096 patients in the initiators analyses (all patients who initiated exenatide BID at baseline) and 747 patients in the persisters analyses (ie, those with no significant treatment change). Corresponding numbers in the insulin cohort were 1239 (initiators) and 882 (persisters).
Figure 2 Kaplan–Meier estimates for time until significant treatment change after the initiation of first injectable therapy for the exenatide twice-daily (BID) cohort and total insulin cohort. The estimated number of patients remaining in the study with no significant treatment change is provided above each period.
Abbreviation: CI, confidence interval.
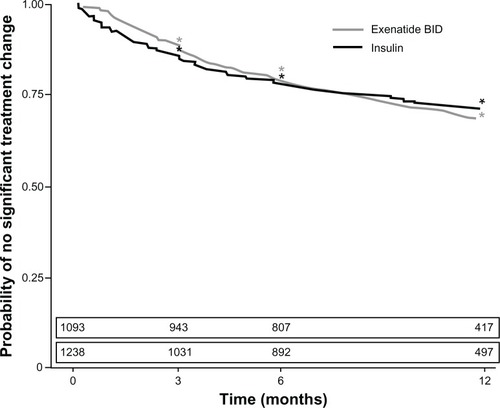
At 3, 6, and 12 months, Kaplan–Meier estimates for no significant treatment change were 88.7% (95% CI: 86.8%, 90.6%), 79.2% (76.8%, 81.6%), and 67.8% (64.9%, 70.7%) of patients, respectively, who were initiated on exenatide BID (). Corresponding figures for the insulin cohort were 85.5% (95% CI: 83.5%, 87.5%) of patients at 3 months, 78.0% (75.6%, 80.3%) at 6 months, and 70.9% (68.3%, 73.5%) at 12 months ().
The types of treatment change and reasons for discontinuation of injectable therapy are summarized in . In the exenatide BID cohort, 265 patients (24.2% of sample) added a new medication for the treatment of T2DM to their ongoing exenatide BID (for 175 patients [16.0%], this was their first significant treatment change); overall, the most commonly added injectable therapies were long-acting insulins (insulin glargine [by 5.7% of the cohort] or insulin detemir [3.6%]), and the most commonly added OADs were sulfonylureas (4.0%; most frequently glimepiride [2.8%]) and metformin (2.2%). Of the 182 patients (16.6%) who started insulin therapy during the first 12 months of the study, 168 (92.3%) discontinued exenatide BID and 14 (7.7%) added insulin to their ongoing exenatide BID regimen. Sulfonylureas (most commonly glimepiride) and metformin were also the most frequently discontinued OADs (by 7.1% [glimepiride: 4.1%] and 2.6% of the cohort, respectively).
Table 2 Treatment change occurring during the 12 months following initiation of exenatide twice daily (BID) and insulin in patients with type 2 diabetes mellitusTable Footnote a
Of the 1239 patients in the insulin cohort, 49.9% initiated insulin with long-acting insulin only, 24.5% with mixtures, 13.4% with a basal-bolus regimen, and 10.9% with short-acting insulin only (1.4% other or missing), although there was significant between-country variability (data not shown). During the study, 289 patients in the insulin cohort (23.3% of the sample) added a new therapy (this was the first significant treatment change for 251 patients [20.3%]) and 101 patients (8.2%) discontinued their initial injectable therapy (for 80 patients [6.5%], this was their first significant treatment change). The most commonly added injectable therapy was fast-acting insulin (by 7.6% of the insulin cohort: insulin aspart [by 3.1% of the cohort], insulin lispro [2.4%], or insulin glulisine [2.1%]). Overall, 5.0% of the insulin cohort added a mixture, 4.0% added long-acting insulin (insulin glargine [2.5%] or insulin detemir [1.4%]), and 3.9% added an intermediate-acting insulin (isophane). Exenatide BID and liraglutide therapy were each initiated by two patients (0.2%) in the insulin cohort; both patients who initiated exenatide BID continued their insulin therapy. The most commonly added and discontinued OADs were sulfonylureas (1.1% and 6.2%; most commonly glimepiride [0.9% and 3.6%, respectively]) and metformin (1.3% and 2.7%, respectively).
In the exenatide BID cohort, during the 12 months post-initiation of treatment, 287 patients (26.2% of 1096 patients) discontinued use of the therapy – discontinuation of exenatide BID was the first significant treatment change for 269 patients (24.5%). Adverse events were the primary stated reason for discontinuation in the first 3 months but became a less frequent reason as the study progressed (46.0% of discontinuations in the period 0 to 3 months; 33.8% and 7.3% of discontinuations during the periods > 3 to 6 months and >6 to 12 months, respectively). By contrast, inadequate response became a more common reason for discontinuing therapy as the study progressed (21.0%, 33.8%, and 54.5% of discontinuations during each time period, respectively). No other trends in reasons for stopping therapy were noted.
In the insulin cohort, discontinuation of initial insulin therapy was the first significant treatment change for 80 patients (6.5% of sample); in total, 101 patients (8.2%) discontinued initial insulin during the 12-month study period. Inadequate response became a more frequent reason for discontinuing therapy as the study progressed (52.8% and 50.0% of discontinuations in the periods 0 to 3 months and >3 to 6 months, respectively; 71.4% of discontinuations during the period > 6 to 12 months). Adverse events in the insulin cohort were an infrequent reason for discontinuing therapy (2.8%, 6.7%, and 2.9% of discontinuations during each period, respectively). Long-acting insulin was the most commonly discontinued injectable by this cohort (3.3%: insulin glargine [2.3%] or insulin detemir [0.9%]). Insulin mixtures were discontinued by 2.3%, intermediate-acting insulin (isophane) was discontinued by 2.7%, and fast-acting insulin was discontinued by 0.9% of the insulin cohort.
Clinical outcomes and adverse events: exenatide BID cohort
Glycemic control improved in the exenatide BID initiators population who had data at the 12-month visit, as shown by a mean (SD; 95% CI) absolute reduction in HbA1c of 1.0 (1.4; −1.1, −0.9)% units at 12 months, and an increase in the percentage of patients with HbA1c < 6.5% and < 7% from 5.5% and 9.8%, respectively, at baseline to 17.1% and 33.4%, respectively, at 12 months. For patients in the persisters population who had data at the 12-month visit, the mean (SD; 95% CI) reduction in HbA1c was 1.2 (1.4; −1.3, −1.0)% units at 12 months. At this time, the percentage of patients with HbA1c < 6.5% and < 7% was 19.9% and 37.9%, respectively. Of the 947 patients in the exenatide BID cohort with HbA1c ≥ 7% at baseline, 18.8% had HbA1c < 7% at both the 6- and 12-month visits, 9.4% had HbA1c ≥ 7% at the 6-month visit but had achieved HbA1c < 7% at the 12-month visit, and 9.1% had HbA1c < 7% at the 6-month but not the 12-month visit; 40.3% of patients did not achieve HbA1c < 7% at either the 6- or 12-month visit.
Patients in the exenatide BID initiators population who provided data at the 12-month visit had improvements in a number of cardiovascular risk factors, including mean body weight, BMI, waist circumference, blood pressure (BP), and lipid parameters at this time ().
Table 3 Mean changes in clinical variables from baseline to 12 months (for patients with data at 12 months) after initiation of exenatide twice daily (BID) or insulin in patients with type 2 diabetes mellitus – initiators population
Weight loss (>1.0 kg) was achieved by 64.7% of exenatide BID initiators who had data at 12 months; 2.9% had minimal change in body weight (≤1.0 kg weight gain or loss) and 15.9% had weight gain (>1.0 kg) by month 12. In the persisters population with data at 12 months, 70.7% of patients achieved a weight loss of >1.0 kg, with 3.5% having minimal change in body weight and 11.0% having weight gain by month 12. Mean (SD; 95% CI) weight change from baseline to 12 months was −3.3 (5.9; −3.7, −2.9) kg and −4.2 (5.6; −4.6, −3.8) kg for initiators and persisters, respectively.
Overall, hypoglycemia was experienced by 13.2% of patients who initiated exenatide BID (12.4% of the persisters population), with 2.2% of patients experiencing nocturnal hypoglycemia (). Of patients who experienced hypoglycemia, most (82.8%) were receiving concomitant sulfonylureas (see ).
Table 4 Hypoglycemia occurring between baseline and 12 months (or time of study discontinuation, if earlier than 12 months) after initiation of exenatide twice daily (BID) or insulin in patients with type 2 diabetes mellitus – initiators population
In a post hoc analysis, at 12 months after initiation of exenatide BID, 24.3% of the initiators population and 28.9% of the persisters population were observed to have met the composite endpoint of HbA1c < 7%, no weight gain (≤ 1 kg change), and no hypoglycemia. In the initiators population, 159 patients (14.5%) could not be assessed for this endpoint because data were missing for some parameters (available parameters may have fulfilled the criteria), and 35 patients (3.2%) had missing data for all parameters.
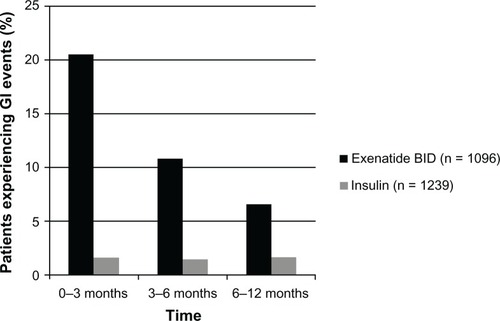
The incidences of total and individual GI events are presented in . Overall, 27.8% of the exenatide BID cohort experienced GI adverse events at some time during the study, as recorded for the 4-week period before each visit. Notably, the incidence of GI events decreased over time (). Of the 275 GI symptoms experienced in the period 0 to 3 months, 59.3% were experienced daily or on most days, whereas 41.6% of the 149 symptoms occurring in the period 3 to 6 months and 32.6% of the 86 symptoms occurring in the period 6 to 12 months occurred daily or on most days. However, GI symptoms were often not associated with meals (44.1% of all events), causing only 17.6% of affected patients to miss ≥1 meal during the study.
Table 5 Gastrointestinal (GI) events occurring during the 12 months (or time of study discontinuation, if earlier than 12 months) following initiation of exenatide twice daily (BID) and insulin in patients with type 2 diabetes mellitus – initiators population
Clinical outcomes and adverse events: insulin cohort
Glycemic control improved in the insulin initiators population with data available at the 12-month visit, as shown by a mean (SD; 95% CI) absolute reduction in HbA1c of 1.8 (1.8; −1.9, −1.7)% units at 12 months () and an increase in the percentage of patients with HbA1c < 6.5% and < 7% from 3.3% and 5.1%, respectively, at baseline to 14.5% and 32.2%, respectively, at 12 months. Similar improvements in glycemic control were seen in the persisters population with data at the 12-month visit: the mean (SD; 95% CI) reduction in HbA1c was 1.8 (1.8; −1.9, −1.6)% units at 12 months, and 14.5% and 31.4% of patients had HbA1c < 6.5% and <7%, respectively, at this time. Improvements in glycemic control did not appear to differ substantially according to the insulin regimen initiated at baseline (). Of the 1142 patients in the insulin cohort with HbA1c ≥ 7% at baseline, 20.2% had HbA1c < 7% at both the 6- and 12-month visits, 8.8% had HbA1c ≥ 7% at the 6-month visit but had achieved HbA1c <7% at the 12-month visit, and 8.7% had HbA1c < 7% at the 6-month but not the 12-month visit; 42.3% of patients did not achieve HbA1c < 7% at either the 6- or 12-month visit.
Figure 4 Change in glycated hemoglobin (HbA1c) according to the type of first insulin therapy initiated during the 12 months following initiation of that therapy.
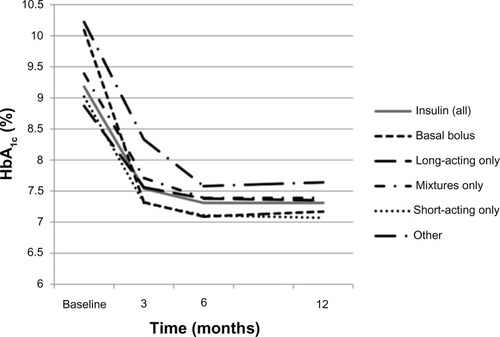
The mean (SD; 95% CI) body weight of patients in the insulin initiators and persisters populations with data available at 12 months increased between baseline and 12 months (by 1.9 [4.9; 1.6, 2.1] kg and 1.8 [4.7; 1.4, 2.1] kg, respectively). A greater mean weight gain was seen in patients receiving short-acting only (2.8 kg) or basal-bolus (2.4 kg) regimens and a lower mean weight gain was seen in those receiving mixtures (1.8 kg) or long-acting agents only (1.6 kg; see ). A total of 33.3% of patients initiated on insulin therapy achieved weight loss (> 1.0 kg), whereas 5.2% had minimal change in body weight and 45.9% had weight gain (>1.0 kg) by month 12. Similarly, 32.9% of patients remaining in the insulin cohort without significant treatment change and with data available at 12 months achieved weight loss, with 5.4% having minimal change in body weight and 44.2% having weight gain by month 12. At 12 months, patients in the insulin cohort also had improvements in mean BP and lipid parameters (see ).
Overall, 28.6% of patients who initiated insulin therapy were observed to have experienced hypoglycemia in the first 12 months after initiation (see ), with the incidence being highest in patients initiated on mixtures only (40.3%) or a basal-bolus regimen (33.1%; the 14 patients receiving “other” regimens had an incidence of 50.0%). Lower rates of hypoglycemia were reported in patients initiated on long-acting only (23.0%) or short-acting only (20.7%) regimens. Overall, 55.6% of patients who reported hypoglycemia were receiving sulfonylureas. Nocturnal hypoglycemia was reported in 11.3% of patients initiated on insulin (see ).
At 12 months after initiation of first insulin therapy, 10.3% of the initiators population and 10.9% of the persisters population were observed to have met the composite end-point of HbA1c < 7%, no weight gain (≤ 1 kg change), and no hypoglycemia. In the initiators population, 147 patients (11.9%) could not be assessed for this endpoint because data were missing for some parameters (available parameters may have fulfilled the criteria), and 22 patients (1.8%) had missing data for all parameters.
The incidences of total and individual GI events are presented in . Overall, 3.5% of the insulin cohort reported GI adverse events.
Clinical outcomes and adverse events: matched subgroup analysis
Propensity matching on baseline clinical and demographic variables, and country of participation, identified 1140 patients (570 from each cohort) who could be matched and compared at 12 months ( presents data for the matched groups). In this population, for patients with data available at 12 months, there was no significant difference between the treatment-matched groups (exenatide BID vs insulin) in mean (SD) change in HbA1c (−1.25 [1.48] % units vs −1.31 [1.52] % units; P = 0.738; n = 454 vs 477) or in the percentage of patients at 12 months with HbA1c < 7% (31.1% vs 36.5%; P = 0.265, McNemar’s test; n = 461 vs 489) or < 6.5% (15.3% vs 15.4%; P = 0.863, McNemar’s test; n = 461 vs 489). However, patients in the exenatide BID matched group had significantly greater mean (SD) weight loss (change: −2.7 [5.2] kg vs +1.6 [4.8] kg; P < 0.0001; n = 478 vs 494), BMI reduction (change: −0.9 [1.9] kg/m2 vs +0.6 [1.7] kg/m2; P < 0.0001; n = 471 vs 494) and waist circumference reduction (change: −2.0 [8.9] cm vs +0.8 [6.3] cm; P < 0.0001; n = 340 vs 358) compared with the insulin matched group. The incidence of hypoglycemia during the study was lower in the exenatide BID matched group than the insulin matched group (15.1% vs 24.6%; P < 0.0001, McNemar’s test; n = 551 vs 566). Mean changes (exenatide BID vs insulin) in total cholesterol (−0.3 [1.0] mmol/L vs −0.2 [1.2] mmol/L), high-density lipoprotein cholesterol (HDL; +0.0 [0.3] mmol/L vs +0.1 [0.4] mmol/L), low-density lipoprotein cholesterol (LDL; −0.1 [0.9] mmol/L vs −0.2 [0.9] mmol/L), triglyceride (−0.4 [1.3] mmol/L vs −0.3 [1.3] mmol/L), and BP (systolic: −2.7 [17.2] mmHg vs −2.7 [18.3] mmHg; diastolic: −1.1 [10.6] mmHg vs −1.6 [11.3] mmHg) levels did not differ significantly between the two matched groups (n = 332 to 445 patients per exenatide BID group and n = 342 to 460 per insulin group depending on endpoint of interest).
Discussion
This prospective observational study was designed to evaluate patterns of exenatide BID and insulin usage (in particular, treatment changes post-initiation) and outcomes in clinical practice in multiple European countries. We selected time to significant treatment change as the primary endpoint of the study to improve understanding of when and how treatment with exenatide BID and insulin is amended post-baseline, since changes in therapy are often associated with increased use of resources (particularly clinician’s time), device changes, and uncertainty for patients. Overall, 31.8% (Kaplan–Meier estimate: 32.2%) of patients who initiated exenatide BID as their first injectable glucose-lowering therapy had a significant treatment change (24.2% of the total exenatide BID cohort added to exenatide BID and 26.2% discontinued exenatide BID) during the first 12 months of therapy. The corresponding change value for patients whose first initiated injectable glucose-lowering therapy was insulin was 28.8% (Kaplan–Meier estimate: 29.1%), with most patients adding a new therapy for T2DM, most commonly a short-acting insulin. Discontinuations accounted for only a small proportion of the treatment changes in the insulin cohort (8.2% overall); inadequate response became a more frequent reason for discontinuing therapy as the study progressed.
We considered the rate of change in therapy for patients initiated on insulin in the CHOICE study to be relatively high, particularly when compared with similar prospective European observational studies considering treatment change post-insulin initiation,Citation31,Citation32 and to be driven primarily by the addition of a new agent rather than discontinuing the initial therapy. In the other studies, 12% of patients changed their insulin regimen at 12 months,Citation31 and 2.9% to 19.4% of patients changed, depending on insulin regimen, at 24 months.Citation32 As similar proportions of patients were initiated on long-acting insulin (50% vs 45% and 50%) and the mean time since diabetes diagnosis was similar (10 years for each study) in the CHOICE insulin cohort and these other European studies,Citation31,Citation32 we are unable to explain this finding. However, retrospective database analyses have reported wide ranges of persistence rates with insulin therapy, some of which were similar to or lower than those we report here at 6 (75% overall)Citation33 and 12 months (66%–92%, depending on insulin type; no results for the total insulin group were reported).Citation34,Citation35
The rate of treatment change appeared to be relatively stable throughout the 12-month study period in the exenatide BID cohort. It should be noted that the primary reason for discontinuation of exenatide BID in the first 3 months of the study was adverse events (assumed to be GI-related), whereas the primary reason towards the end of the 12-month follow-up was lack of efficacy. By contrast, significant treatment change occurred at almost twice the later rate during the first 3 months of the study in the insulin cohort (14.5%, compared with 7.5% and 7.1% during the periods > 3 to 6 months and > 6 to 12 months, respectively).
CHOICE considered patients initiating exenatide BID or insulin in routine clinical practice. Unlike the situation in randomized trials, compared with patients initiated on insulin, patients initiated on exenatide BID in CHOICE tended to have a younger age; higher body weight, BMI, waist circumference, and diastolic BP; lower total and LDL-cholesterol levels; a shorter time since diabetes diagnosis; and better glycemic control at baseline.Citation27 These differences are consistent with observational data from the Exenatide BID Observational Study (ExOS) in the USA.Citation10,Citation11 However, other studies have supported the use of exenatide BID at various ranges of HbA1c, including high values (> 9%).Citation36–Citation38 It is also possible that exenatide BID is used earlier in T2DM to intensify therapy, thereby delaying the need for insulin initiation.
Randomized clinical trials have shown that GLP-1 receptor agonists are at least as effective as insulin therapy, and are usually associated with weight loss.Citation3–Citation5,Citation7,Citation8 However, the observed differences in the patient populations in CHOICE make it difficult to compare the exenatide BID and insulin cohorts in a statistically meaningful manner. It should be noted that clinical findings pertaining to HbA1c and weight for the initiators and persisters populations in both the exenatide BID and insulin cohorts of CHOICE were similar to those obtained in clinical trials evaluating exenatide BIDCitation3,Citation4,Citation6,Citation36,Citation37,Citation39,Citation40 and a range of insulin regimens.Citation41–Citation43 At 12 months after initiation of injectable therapy, 24.3% and 28.9% of the initiators and persisters population, respectively, from the exenatide BID cohort, and about 10% of both populations from the insulin cohort, met the clinically relevant composite endpoint suggested by Zinman and colleaguesCitation44 of HbA1c < 7%, no weight gain (≤1 kg change), and no hypoglycemia. Analysis using initiators compared with persisters evaluable populations had little or no effect on outcomes in the insulin cohort and little effect in the exenatide BID cohort; if anything, there was a slight tendency for outcomes to be improved in the persisters compared with the initiators exenatide BID population. This observation is reassuring, as outcomes in the persisters population represent likely findings in patients actually receiving the initial injectable treatment, whereas the initiators populations better represent an intention-to-treat population that included patients who were receiving an alternative or additional treatment by 12 months.
To allow direct comparison of outcomes between the two treatment cohorts, we performed a matched subgroup analysis, which focused on the cardio-metabolic parameters that are currently a target of treatment: glycemic control while avoiding hypoglycemia, BP control, LDL-cholesterol control, and weight.Citation45 This analysis showed that, in common with findings of clinical trials,Citation43 patients in the exenatide BID matched group had greater weight loss and a lower risk of hypoglycemia than patients in the insulin matched group, although glycemic control (HbA1c change and the proportion of patients with HbA1c< 7% or < 6.5%) and changes in LDL cholesterol did not differ. However, these findings should be interpreted with caution because less than half of the patients with 12-month data (48.8%) were included in the propensity-matched analyses. CHOICE was not designed to compare the treatment groups since different patient characteristics, as anticipated, appear to have resulted in different treatment allocations. In particular, patients in the exenatide BID matched group tended to be older, have poorer HbA1c control, and lower body weight than the full exenatide BID cohort, and patients from the insulin matched group tended to be younger and have better control of HbA1c, and higher body weight than the full insulin cohort.
The CHOICE study has provided the first available data on the way exenatide BID is used in routine clinical practice across Europe. However, in common with all prospective observational studies, this study has limitations. These include the potential for unobserved factors that could have affected treatment selection or outcomes, the potential for investigators to be influenced by the scrutiny that occurs during a prospective study and the potential for bias – although the inclusion of two treatment arms should have helped to reduce prescribing bias. In addition, although the sample was designed to be representative, sample sizes were small in some countries, the ratio of exenatide BID to insulin patients varied between countries, and patients were mostly recruited in secondary care centers. Also, there may be significant unmeasured confounding factors we were unable to control for in the subgroup comparison of cohorts, and we used non-standardized measurements to assess clinical outcomes, including hypoglycemia, which relied on patient recall and self-reporting of episodes that did not have standardized diagnoses. The inclusion of several European countries may also have resulted in variations in diabetes care, including access to self-monitoring of blood glucose and the types of insulin initiated at different sites. Thus, the extent to which data can be generalized is not clear. It is also likely that treatment patterns will change to some extent following the March 2012 European Union approval of exenatide BID as adjunctive therapy in adult patients with T2DM who have not achieved adequate glycemic control with basal insulin, with or without metformin and/or pioglitazone.Citation23 Although not an approved indication for exenatide BID during the study period described here, and only two patients from the insulin cohort were initiated on this agent during the study, we expect that this new indication for exenatide BID may have a substantial impact on prescribing trends for patients with T2DM, as UK-based audits have shown that many of the patients initiated on GLP-1 receptor agonists before this indication was approved (between 30% and 40%) were also receiving basal insulin concomitantly.Citation12,Citation46 Similarly, the availability of liraglutide and exenatide once weekly has likely affected treatment patterns subsequent to these data being collected.
Conclusion
In addition to estimating the number of patients with significant treatment change and evaluating reasons for the treatment change following initiation of injectable therapy, CHOICE provided data on exenatide BID and insulin usage patterns and 12-month outcomes in clinical practice. Results show that about 30% of patients initiated on exenatide BID or insulin as their first injectable glucose-lowering therapy had a significant treatment change during the first 12 months of therapy.
Overall, both cohorts achieved improved glycemic control (in terms of mean HbA1c change and the proportion of patients achieving HbA1c < 7% or < 6.5%) and a reduced severity of cardiovascular risk factors, which included a mean weight loss in the exenatide BID cohort. There was a mean weight gain in the insulin cohort. The matched subgroup analysis found that, although patients in the two matched groups achieved similar glycemic control, patients in the exenatide BID matched group had a significantly lower incidence of hypoglycemia and greater weight loss than patients in the insulin matched group. Changes in total, HDL and LDL-cholesterol, triglyceride, and BP levels did not differ significantly between the matched groups.
Acknowledgments
The authors would like to acknowledge Caroline Spencer, Lee Baker, and Claire Lavin (Rx Communications, Mold, UK) for medical writing assistance with the preparation of this article, funded by Eli Lilly.
Disclosure
The study and the development of this manuscript were sponsored by Eli Lilly and Amylin Pharmaceuticals. The sponsors and authors were involved in the decision to submit the manuscript for publication in Diabetes, Metabolic Syndrome and Obesity: Targets and Therapy. The authors maintained complete control over the direction and content of the manuscript and did not receive financial compensation for its development.
Stephan Matthaei and Bruno Guerci have received honoraria from Eli Lilly for lectures and consultancy. Claes-Göran Östenson has received honoraria from Eli Lilly for consultancy.
Chantal Mathieu is an advisory board member for Lilly Belgium. Thure Krarup is an advisory board member for Lilly Denmark. Matthew Reaney, Jacek Kiljanski, Carole Salaun-Martin, and Hélène Sapin are employees of Eli Lilly; David Bruhn was an employee of Eli Lilly at the time of the study; and Jacek Kiljanski, Carole Salaun-Martin, and David Bruhn are holders of Eli Lilly shares and share options. Matthew Reaney and Hélène Sapin are not shareholders. Michael Theodorakis has declared that he has no conflicts of interest in this work.
References
- Inzucchi SE Bergenstal RM Buse JB American Diabetes Association (ADA) European Association for the Study of Diabetes (EASD) Management of hyperglycemia in type 2 diabetes: a patient-centered approach: position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) Diabetes Care 2012 35 6 1364 1379 22517736
- Rodbard HW Jellinger PS Davidson JA Statement by an American Association of Clinical Endocrinologists/American College of Endocrinology consensus panel on type 2 diabetes mellitus: an algorithm for glycemic control Endocr Pract 2009 15 6 540 559 19858063
- Barnett AH Burger J Johns D Tolerability and efficacy of exenatide and titrated insulin glargine in adult patients with type 2 diabetes previously uncontrolled with metformin or a sulfonylurea: a multinational, randomized, open-label, two-period, crossover noninferiority trial Clin Ther 2007 29 11 2333 2348 18158075
- Heine RJ Van Gaal LF Johns D Exenatide versus insulin glargine in patients with suboptimally controlled type 2 diabetes: a randomized trial Ann Intern Med 2005 143 8 559 569 16230722
- Nauck MA Duran S Kim D A comparison of twice-daily exenatide and biphasic insulin aspart in patients with type 2 diabetes who were suboptimally controlled with sulfonylurea and metformin: a non-inferiority study Diabetologia 2007 50 2 259 267 17160407
- Davies MJ Donnelly R Barnett AH Jones S Nicolay C Kilcoyne A Exenatide compared with long-acting insulin to achieve glycaemic control with minimal weight gain in patients with type 2 diabetes: results of the Helping Evaluate Exenatide in patients with diabetes compared with Long-Acting insulin (HEELA) study Diabetes Obes Metab 2009 11 1153 1162 19930005
- Diamant M Van Gaal L Stranks S Once weekly exenatide compared with insulin glargine titrated to target in patients with type 2 diabetes (DURATION-3): an open-label randomised trial Lancet 2010 375 9733 2234 2243 20609969
- Russell-Jones D Vaag A Schmitz O Liraglutide Effect and Action in Diabetes 5 (LEAD-5) met+SU Study Group Liraglutide vs insulin glargine and placebo in combination with metformin and sulfonylurea therapy in type 2 diabetes mellitus (LEAD-5 met+SU): a randomised controlled trial Diabetologia 2009 52 10 2046 2055 19688338
- National Institute for Health and Clinical Excellence (NICE) Type 2 diabetes: newer agents for blood glucose control in type 2 diabetes. NICE short clinical guideline 87 London NICE 2009 Available from: http://www.nice.org.uk/nicemedia/live/12165/44318/44318.pdf. Accessed January 23, 2013.
- Bergenstal RM Garrison LPJr Wintle M Exenatide bid observational study (ExOS): baseline population characteristics of a prospective research study to evaluate the clinical effectiveness of exenatide bid use in patients with type 2 diabetes in a real-world setting Curr Med Res Opin 2011 27 3 531 540 21219119
- Fabunmi R Nielsen LL Quimbo R Patient characteristics, drug adherence patterns, and hypoglycemia costs for patients with type 2 diabetes mellitus newly initiated on exenatide or insulin glargine Curr Med Res Opin 2009 25 3 777 786 19203299
- Ryder RE Thong KY Cull ML Mills AP Walton C Winocour PH The Association of British Clinical Diabetologists (ABCD) nationwide exenatide audit Pract Diab Int 2010 27 8 352 357b
- Koro CE Bowlin SJ Bourgeois N Fedder DO Glycemic control from 1988 to 2000 among US adults diagnosed with type 2 diabetes: a preliminary report Diabetes Care 2004 27 1 17 20 14693960
- Tzoulaki I Molokhia M Curcin V Risk of cardiovascular disease and all cause mortality among patients with type 2 diabetes prescribed oral antidiabetes drugs: retrospective cohort study using UK general practice research database BMJ 2009 339 b4731 19959591
- Yurgin N Secnik K Lage MJ Obesity and the use of insulin: a study of patients with type 2 diabetes in the UK J Diabetes Complications 2008 22 4 235 240 18413211
- Gedulin BR Nikoulina SE Smith PA Exenatide (exendin-4) improves insulin sensitivity and {beta}-cell mass in insulin-resistant obese fa/fa Zucker rats independent of glycemia and body weight Endocrinology 2005 146 4 2069 2076 15618356
- Nielsen LL Young AA Parkes DG Pharmacology of exenatide (synthetic exendin-4): a potential therapeutic for improved glycemic control of type 2 diabetes Regul Pept 2004 117 2 77 88 14700743
- Garber AJ Incretin effects on β-cell function, replication, and mass: the human perspective Diabetes Care 2011 34 Suppl 2 S258 S263 21525465
- Li Y Hansotia T Yusta B Ris F Halban PA Drucker DJ Glucagon-like peptide-1 receptor signaling modulates beta cell apoptosis J Biol Chem 2003 278 1 471 478 12409292
- Xu G Stoffers DA Habener JF Bonner-Weir S Exendin-4 stimulates both beta-cell replication and neogenesis, resulting in increased beta-cell mass and improved glucose tolerance in diabetic rats Diabetes 1999 48 12 2270 2276 10580413
- Liu SC Tu YK Chien MN Chien KL Effect of antidiabetic agents added to metformin on glycaemic control, hypoglycaemia and weight change in patients with type 2 diabetes: a network meta-analysis Diabetes Obes Metab 2012 14 9 810 820 22486990
- European Medicines Agency Byetta: exenatide; assessment history [web page on the Internet] London European Medicines Agency nd. Available from: http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/000698/human_med_000682.jsp&jsenabled=true. Accessed December 10, 2012.
- Lilly Byetta®approved for use with basal insulin in Europe [press release] San Diego, CA Amylin Pharmaceuticals 2012 [March 23]. Available from: https://investor.lilly.com/releasedetail2.cfm?ReleaseID=659089. Accessed December 10, 2012.
- Ligthelm RJ Borzì V Gumprecht J Kawamori R Wenying Y Valensi P Importance of observational studies in clinical practice Clin Ther 2007 29 Spec No: 1284 1292
- Mann CJ Observational research methods. Research design II: cohort, cross sectional, and case-control studies Emerg Med J 2003 20 1 54 60 12533370
- Amylin Pharmaceuticals CHOICE: CHanges to Treatment and Outcomes in Patients With Type 2 Diabetes Initiating InjeCtablE Therapy ClinicalTrialsgov [website on the Internet] Bethseda, MD US National Library of Medicine 2008 [updated April 12, 2010]. Available from: http://www.clinicaltrials.gov/ct2/show/NCT00635492. NLM identifier: NCT00635492. Accessed February 23, 2013.
- Matthaei S Reaney M Mathieu C Patients with Type 2 Diabetes Initiating Exenatide Twice Daily or Insulin in Clinical Practice: CHOICE Study Diabetes Ther 2012 3 1 6 22714818
- Bergenstal RM Garrison LPJr Miller LA Exenatide BID Observational Study (ExOS): results for primary and secondary endpoints of a prospective research study to evaluate the clinical effectiveness of exenatide BID use in patients with type 2 diabetes in a real-world setting Curr Med Res Opin 2011 27 12 2335 2342 22085180
- Jones S Benroubi M Castell C Characteristics of patients with type 2 diabetes mellitus initiating insulin therapy: baseline data from the INSTIGATE study Curr Med Res Opin 2009 25 3 691 700 19196223
- Yue LQ Statistical and regulatory issues with the application of propensity score analysis to nonrandomized medical device clinical studies J Biopharm Stat 2007 17 1 1 13 17219753
- Simpson A Smith H Nicolay C Approaches to initiation of insulin therapy in patients with type 2 diabetes and resulting outcomes at 12 months in four European countries: data from the INSTIGATE study Poster presented at the World Congress on Controversies to Consensus in Diabetes, Obesity and Hypertension (CODHy) October 30–November 2, 2008 Barcelona, Spain
- Benroubi M Schmitt H Cleall SP the TREAT Study Investigators Costs and clinical outcomes after 24 months of insulin therapy in patients with type 2 diabetes: results from the TREAT study Poster presented at the 47th European Association for the Study of Diabetes (EASD) Annual Meeting September 12–16, 2011 Lisbon, Portugal
- Blak BT Smith HT Hards M Maguire A Gimeno V A retrospective database study of insulin initiation in patients with Type 2 diabetes in UK primary care Diabet Med 2012 29 8 e191 e198 22507537
- Gordon J Pockett RD Tetlow AP McEwan P Home PD A comparison of intermediate and long-acting insulins in people with type 2 diabetes starting insulin: an observational database study Int J Clin Pract 2010 64 12 1609 1618 20946269
- Hall GC McMahon AD Dain MP Home PD A comparison of duration of first prescribed insulin therapy in uncontrolled type 2 diabetes Diabetes Res Clin Pract 2011 94 3 442 448 21963105
- Buse JB Henry RR Han J Kim DD Fineman MS Baron AD Exenatide-113 Clinical Study Group Effects of exenatide (exendin-4) on glycemic control over 30 weeks in sulfonylurea-treated patients with type 2 diabetes Diabetes Care 2004 27 11 2628 2635 15504997
- Kendall DM Riddle MC Rosenstock J Effects of exenatide (exendin-4) on glycemic control over 30 weeks in patients with type 2 diabetes treated with metformin and a sulfonylurea Diabetes Care 2005 28 5 1083 1091 15855571
- Buysschaert M Preumont V Oriot PR UCL Study Group for Exenatide One-year metabolic outcomes in patients with type 2 diabetes treated with exenatide in routine practice Diabetes Metab 2010 36 5 381 388 20598606
- Bunck MC Diamant M Cornér A One-year treatment with exenatide improves beta-cell function, compared with insulin glargine, in metformin-treated type 2 diabetic patients: a randomized, controlled trial Diabetes Care 2009 32 5 762 768 19196887
- DeFronzo RA Ratner RE Han J Kim DD Fineman MS Baron AD Effects of exenatide (exendin-4) on glycemic control and weight over 30 weeks in metformin-treated patients with type 2 diabetes Diabetes Care 2005 28 5 1092 1100 15855572
- Crasto W Jarvis J Khunti K Davies MJ New insulins and new insulin regimens: a review of their role in improving glycaemic control in patients with diabetes Postgrad Med J 2009 85 1003 257 267 19520878
- Vaag A Lund SS Insulin initiation in patients with type 2 diabetes mellitus: treatment guidelines, clinical evidence and patterns of use of basal vs premixed insulin analogues Eur J Endocrinol 2012 166 2 159 170 21930715
- Waugh N Cummins E Royle P Newer agents for blood glucose control in type 2 diabetes: systematic review and economic evaluation Health Technol Assess 2010 14 36 1 248
- Zinman B Schmidt WE Moses A Lund N Gough S Achieving a clinically relevant composite outcome of an HbA1c of <7% without weight gain or hypoglycaemia in type 2 diabetes: a meta-analysis of the liraglutide clinical trial programme Diabetes Obes Metab 2012 14 1 77 82 21883806
- American Diabetes Association Standards of medical care in diabetes-2012 Diabetes Care 2012 35 Suppl 1 S11 S63 22187469
- Ryder RE Thong K ABCD nationwide exenatide and liraglutide audit contributors ABCD nationwide exenatide and liraglutide audits Presented at Diabetes UK Annual Professional Conference March 30–April 1, 2011 London, UK