Abstract
Background
One of the major issues affecting global health is Diabetes mellitus (DM), not only in terms of the disease itself but also its complications. Macrovascular complications are both common and serious, affecting many patients. This study aimed to assess fasting C-peptide levels and correlate them with the severity of the peripheral arterial disease complicating type 2 DM (T2DM).
Patients and Methods
This study included 200 participants who were categorized into two groups: Group I (n=100, patients with T2DM complicated by femoropopliteal arterial atherosclerosis) and Group II (n=100, healthy age- and sex-matched individuals serving as controls). Fasting C-peptide levels were estimated using an immunochemiluminometric assay.
Results
Fasting C-peptide levels were significantly higher in Group I than in the control group. Fasting C-peptide levels were positively correlated with the severity of atherosclerosis. In addition, the receiver operating characteristic (ROC) curve analysis revealed that fasting C-peptide levels served as a specific and sensitive marker for detecting the severity of this disease.
Conclusion
Fasting C-peptide levels can be used as a sensitive and specific indicator of the severity of femoropopliteal arteriosclerosis that complicates T2DM.
Introduction
Diabetes mellitus (DM) is a metabolic disorder characterized by hyperglycaemia resulting from defects in insulin secretion, insulin action, or both. The majority of diabetic cases (90–95%) fall under the category of type 2 DM (T2DM), which includes patients with insulin resistance (IR) and typically a deficiency of relative insulin.Citation1
In Egypt, the prevalence of T2DM is approximately 15.6% among individuals aged 20 to 79, which has a profound negative effect on ability to work, functioning, and productivity as well as morbidity and mortality rates.Citation2,Citation3
Vascular involvement is a key pathological feature of diabetes, which leads to both microvascular and macrovascular consequences, including peripheral arterial diseases (PAD), and affecting almost 15% of the ischemic Egyptian patients.Citation4,Citation5 The pathophysiological processes of both microvascular and macrovascular complications include the overproduction of endothelial growth factors and the accumulation of angiotensin-converting enzyme. In addition, dysfunction of smooth muscle cells, mediators of chronic inflammation, impaired fibrinolysis, enhanced platelet aggregation and impaired vasodilator response play as important factors in the pathogenesis of macrovascular complications of T2DM.Citation6
Prospective human studies have shown a strong association between glycaemic control levels and diabetes-related complications.Citation7,Citation8 Given the inefficiency of standard therapeutic methods for hyperglycaemic control in preventing diabetic vasculopathy, alternative treatment techniques are needed for efficient control of both hyperglycaemia and diabetes-related complications.Citation9,Citation10
Insulin secretion is accompanied by the release of a small peptide known as C-peptide, which helps ensure the proper folding of proinsulin. In clinical practice, C-peptide is frequently employed as a biomarker for the diagnosis of diabetes.Citation11,Citation12 Plasma C-peptide levels are an indirect measure of the reserve of insulin secretion.Citation13 Hyperinsulinemia in T2DM patients can result in the development of microangiopathies associated with atherosclerosis.Citation14,Citation15
Previous studies have found an association between C-peptide and cardiovascular diseases.Citation16,Citation17 Elevated levels of C-peptide may increase the risk of atherosclerosis and cardiac complications.Citation18,Citation19
However, the findings of previous studies on the association between type 2 diabetic vascular problems and serum C-peptide levels are contradictory.Citation20–22
Therefore, here we aimed to evaluate the level of C-peptide in femoropopliteal atherosclerosis among patients with T2DM and investigate whether C-peptide levels could serve as a predictive factor for the severity of arteriosclerosis in T2DM.
Patients and Methods
Selection of Participants
A total of 200 participants were selected for this case–control study from Al-Zahraa University Hospital and divided into two groups: Group I (n=100) had T2DM complicated with femoropopliteal arterial atherosclerosis, and Group II (n=100) included sex- and age-matched apparently healthy controls.
Calculation of Sample Size
The G* Power program was used to calculate the sample size. Considering an equal number of cases and controls, 80% power, and 0.05 errors, the required sample size was 87 for each group. For a better analysis, we added 20 participants to each group.
Inclusion Criteria
This study included adult patients older than 18 years of age who were diagnosed with DM according to the American Diabetes Association’s (ADA) diagnostic criteria. All selected patients were on oral hypoglycaemic therapy. The duration of diabetes was >5 and <10 years. Patients with femoropopliteal lesions, with or without infra-popliteal lesions, were selected. The lesion was diagnosed using duplex ultrasonography and computed tomography (CT) angiography. Disease severity was estimated using the Fontaine score (I, IIA, and IIB), Rutherford classification (1, 2 and 3), and Trans-Atlantic Inter-Society Consensus classification (TASC II) of femoropopliteal lesions (A, B, C, and D) associated with or without (TASC IIA) infra-popliteal lesion classification.
Exclusion Criteria
Patients with type 1 DM, T2DM with any other complications or comorbidities, steroid or insulin therapy, aortic lesions, infra-popliteal lesions TASC II (B, C, D), Rutherford (4, 5.6) and Fontaine score (III and IV) were excluded from the study.
Ethical Considerations
This study was conducted in accordance with the World Medical Association Helsinki Declaration guidelines for studies involving human subjects. The study was approved by Al-Azhar University Review Board, Cairo, Egypt (study code 1537, September 28, 2022), and the patients signed an informed consent form.
Assessment and Procedures
All participants underwent thorough clinical and radiological examinations, detailed history-taking, and the following tests:
Routine laboratory tests such as biochemical analyses of fasting blood glucose (FBG), postprandial blood glucose (PPBG), glycated haemoglobin (HbA1c), and lipid profiles were performed using the Cobas c311 system (Germany).
Fasting and 2 hours postprandial C-peptide levels were estimated using an automated immunoassay analyser (ADVIA Centaur XP, Siemens, Germany) and immunochemiluminometric assays.
Statistical Analysis
The 20th version of SPSS (IBM Corp., version 20.0. Armonk, NY, USA) was used for statistical analysis. Categorical variables are summarized as frequencies and percentages, and quantitative variables are expressed as means, standard deviations, and ranges. For comparison between groups, chi-square test for categorical variables, and Student’s t-test or ANOVA test for quantitative variables were used. Pearson’s and Spearman’s correlation tests were used to investigate the strength of the association between the quantitative and qualitative variables. In addition, we used receiver operating characteristic (ROC) curves to assess the diagnostic and predictive values of serum fasting C-peptide levels for diabetic-related complications. Youden’s index J (J= sensitivity + specificity-1) was used to choose the ideal and the most appropriate cut-off value. Statistical significance was set at p<0.05.
Results
This study included 200 participants: Group I (n=100, patients with T2DM complicated by femoropopliteal arterial atherosclerosis) and Group II (n=100, age- and sex-matched healthy controls). The age and sex of the involved participants are presented in .
Table 1 Participant’s Age and Sex
Comparison of Group I and Group II Laboratory Parameters ()
When comparing Group I to the control group, there was a significant increase in FBG, PPBG, fasting C-peptide, glycated haemoglobin (HbA1c), cholesterol, triglycerides (TG), low-density lipoprotein (LDL), and very low-density lipoprotein (VLDL) levels (p=0.000). High-density lipoprotein (HDL) and postprandial C-peptide, on the other hand, did not significantly differ between Group I and Group II.
Table 2 Comparison of Laboratory Parameters Between Group I and Group II
Association Between Fasting C-Peptide Levels and Severity Scores of Femoropopliteal Arteriosclerosis ()
According to the Fontaine score, there was a highly significant increase in fasting C-peptide levels in patients with a IIB score compared to those with an IIA score (p=0.000).
Table 3 Association Between Severity Scores of Femoropopliteal Arteriosclerosis Complications in T2DM and Fasting C-Peptide Levels
Furthermore, there was a highly significant increase in fasting C-peptide levels with increasing stage. The lowest levels were found in patients classified as score 1 according to the Rutherford classification and those in group A in the TASC II femoropopliteal classification. Conversely, the highest levels were observed in patients as score 3 according to the Rutherford classification and those in group D in the TASC II femoropopliteal classification, particularly when associated with the group A in the TASC II infra-popliteal lesion classification.
Association Between Fasting C-Peptide Levels and the Femoropopliteal Arteriosclerosis Severity Scores
There was a highly significant positive correlation between fasting C-peptide levels and all analysed severity scores: the Fontaine score, Rutherford classification, and TASC classification (r=0.738, p=0.000; r=0.758, p=0.000; and r=0.842, p=0.000, respectively).
Association Between Fasting C-Peptide Levels and Other Laboratory Parameters
There was a significant positive correlation between fasting C-peptide levels and the following parameters: postprandial C-peptide, cholesterol, TG, LDL, and VLDL levels (r=0.246, p=0.014; r=0.59, p=0.000; r=0.301, p=0.002; r=0.545, p=0.000; and r=0.298, p=0.003, respectively). In contrast, there was a significant negative correlation between fasting C-peptide levels and glycated haemoglobin levels (r=−0.434, p=0.000).
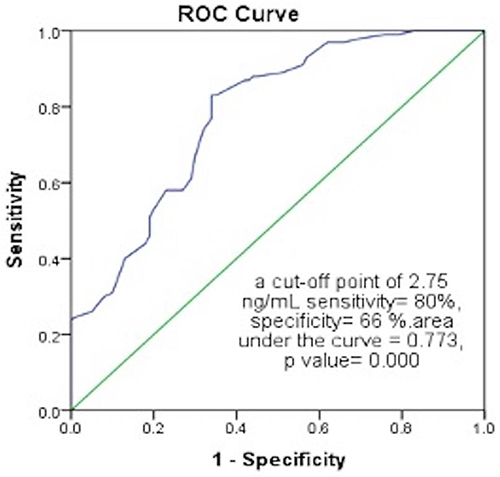
ROC Curve for the Discriminative Power of Fasting C-Peptide Levels Between T2DM Cases and the Control Group ( and )
ROC curve revealed that fasting C-peptide had a fair diagnostic value (area under the curve = 0.773) to discriminate cases from the control group at a cut-off point of 2.75 ng/mL with 80% sensitivity and 66% specificity.
Table 4 Receiver Operating Characteristic (ROC) Curve for the Discriminative Power of Fasting C-Peptide Levels Between T2DM Cases and the Control Group
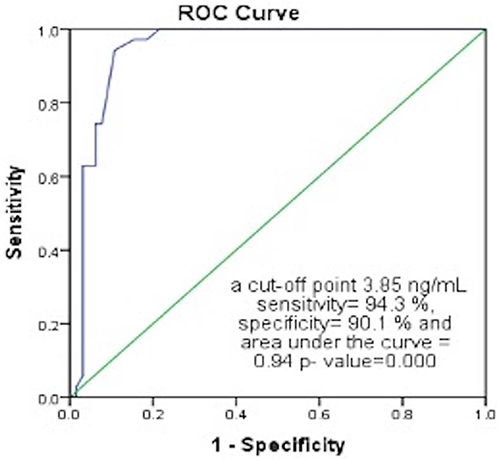
ROC Curve for the Discriminative Power of Fasting C-Peptide Levels Between Early and Late Stages of Atherosclerosis ( and )
ROC curve showed that fasting C-peptide levels can discriminate stage 1 Rutherford classification or stage IIA Fontaine score from other stages with a cut-off of 3.85 ng/mL with 94.3% sensitivity and 90.1% specificity (excellent value as area under the curve = 0.94).
Table 5 Receiver Operating Characteristic (ROC) Curve for the Discriminative Power of Fasting C-Peptide Levels Between Early- and Late-Stage Atherosclerosis
Discussion
C-peptide was initially regarded as an inactive by-product of proinsulin; however, it was later discovered to be an active product with many physiological functions.Citation23 Indeed, C-peptide significantly contributes to the development of atherosclerosis in T2DM. Following its deposition in the blood vessel wall, it exerts a chemotactic effect on inflammatory cells such as macrophages and some types of T-lymphocytes, thereby initiating atherosclerosis.Citation24
In this study, we aimed to assess the fasting C-peptide levels in individuals with T2DM complicated by PAD and determine whether it holds predictive value for the severity of this complication.
Our study found a significant increase in fasting C-peptide levels in patients with T2DM complicated by femoropopliteal arterial atherosclerosis. In addition, fasting C-peptide levels were positively correlated with the severity scores of this complication. Furthermore, analysis of the ROC curve revealed a fair diagnostic value of fasting C-peptide in T2DM complicated by PAD, with 80% sensitivity and 66% specificity, as well as a notable value in predicting the severity of the disease, with 97.5% sensitivity and 81.5% specificity.
Some studies have examined the association between fasting C-peptide levels and cardio-vascular complications and shown that increased C-peptide levels are related to cardiovascular disorders complicating T2DM.Citation25–27
Klim et al showed a correlation between C-peptide levels and the intima-media thickness of the carotid artery and cardio-vascular markers and concluded that it served as a surrogate marker for subclinical atherosclerosis in cardio-vascular diseases among patients with T2DM.Citation25 They attributed that to the important role of C-peptide in the regulation of endothelial function, smooth muscle proliferation, cell growth and immune response.
Wang et al suggested that increased C-peptide levels were correlated to a higher risk of coronary artery disease in individuals with T2DM independent of other risk factors such as smoking, hypertension and dyslipidemia.Citation26
Yan et al proposed that, in the context of macrovascular complications in T2DM, C-peptide levels play a bidirectional role, being beneficial at low levels and harmful at high levels. They also stated that the C-peptide levels and its association with cardiovascular risk may be influenced by the disease duration, the stage and the treatment of the disease. So, they suggested further studies to overcome these limitations.Citation27
To the best of our knowledge, few studies have discussed the correlation between C-peptide levels and peripheral vascular atherosclerosis complicating type 2 DM, and they have not discussed the correlation with the severity of this complication.
Sari and Balci found that the level of C-peptide increases with macrovascular but not microvascular complications of T2DM (coronary artery disease, autonomic neuropathy, and PAD). These results may be related to different responses to insulin of the macrovascular and microvascular tissues.Citation21
Further, Thippeswamy et al found that fasting C-peptide levels were higher in macrovascular disorders (including PAD) complicating T2DM than those with microvascular complications.Citation28
Previous studies discussed the theoretical relevant roles of C-peptide in microvascular and macrovascular complications and the exact mechanism and response of C-peptide in both vascular complications are still unclear and controversial.Citation12,Citation27 Future studies are recommended to detect the pathophysiological differences in microvascular and macrovascular complications that are affected by the level of C-peptide.
One of the limitations of this study is that we only included patients with diabetes complicated by femoropopliteal arteriosclerosis, but we did not study the relationship between the level of fasting C-peptide and other arterial complications, such as the aorto-iliac, carotid artery, and infra-popliteal arterial diseases. Future studies are required to assess the relationship between fasting C-peptide levels and T2DM complicated by other arteriosclerosis diseases. Another limitation of the study is the limited sample size, so other studies are recommended to confirm our findings.
Conclusion
In conclusion, our findings indicate that fasting C-peptide levels are increased in T2DM complicated by femoropopliteal arterial atherosclerosis, making it a specific and sensitive marker for assessing the severity of femoropopliteal PAD in this population.
We recommend incorporating the measurement of fasting C-peptide levels as a routine investigation and follow-up for patients with type 2 DM complicated by femoropopliteal PAD to help assess the severity of arteriosclerosis. In addition, controlling fasting C-peptide levels may contribute to the management of femoropopliteal arterial diseases that complicate type 2 DM.
Abbreviations
DM, diabetes mellitus; T2DM, type 2 diabetes mellitus; ROC curve, receiver operating characteristic curve; IR, insulin resistance; PAD, peripheral arterial disease; ADA, American Diabetes Association; TASC classification, Trans-Atlantic Inter-Society Consensus classification; FBG, fasting blood glucose; PPBG, post-prandial blood glucose; HbA1c, glycated haemoglobin; TG, triglycerides; LDL, low-density lipoprotein; VLDL, very low-density lipoprotein; HDL, high-density lipoprotein.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
All authors declare no conflicts of interest.
Acknowledgments
We would like to thank all participants in this study.
References
- Guideline of American Diabetes Association. Standards of medical care in diabetes-2016: summary of revisions. Diabetes Care. 2016;39(1):S4–5. PMID: 26696680. doi:10.2337/dc16-S003
- Hegazi R, El-Gamal M, Abdel-Hady N, Hamdy O. Epidemiology of and risk factors for type 2 diabetes in Egypt. Ann Global Health. 2015;81(6):814–820. PMID: 27108148. doi:10.1016/j.aogh.2015.12.011
- Gerbo RM, Jin CF, Clark K. Diabetes in the workplace: the hazards of hypoglycemia. Curr Diab Rep. 2019;19(11):119. PMID: 31686223. doi:10.1007/s11892-019-1234-2
- Orasanu G, Plutzky J. The pathologic continuum of diabetic vascular disease. J Am Coll Cardiol. 2009;53(5 Suppl):S35–42. PMID: 19179216; PMCID: PMC2663393. doi:10.1016/j.jacc.2008.09.055
- Basyouni MW, Shabana AM, El Kilani WM. Prevalence of lower extremities peripheral arterial disease among Egyptian ischemic patients attending cardiac rehabilitation unit. Egypt Heart J. 2018;70(4):295–299. PMID: 30591746; PMCID: PMC6303360. doi:10.1016/j.ehj.2018.06.005
- Cade WT. Diabetes-related microvascular and macrovascular diseases in the physical therapy setting. Phys Ther. 2008;88(11):1322–1335. PMID: 18801863; PMCID: PMC2579903. doi:10.2522/ptj.20080008
- Nathan DM, Genuth S, Lachin J, et al.; Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329(14):977–986. PMID: 8366922. doi:10.1056/NEJM199309303291401
- Turner Robert C, Fox C. “Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group”. Lancet. 1998;352:837–853.
- Bahtt MP, Lim Y, Ha K. C-peptide replacement therapy as an emerging strategy for preventing diabetic vasculopathy. Cardiovasc Res. 2014;104(2):234–244. PMID: 25239825. doi:10.1093/cvr/cvu211
- Reaven PD, Emanuele NV, Wiitala WL, et al. VADT Investigators. Intensive glucose control in patients with type 2 diabetes - 15-year follow-up. N Engl J Med. 2019;380(23):2215–2224. PMID: 31167051; PMCID: PMC6706253. doi:10.1056/NEJMoa1806802
- Shaw JA, Shetty P, Burns KD, Fergusson D, Knoll GA. C-Peptide as a therapy for kidney disease: a systematic review and meta-analysis. PLoS One. 2015;10(5):e0127439. PMID: 25993479; PMCID: PMC4439165. doi:10.1371/journal.pone.0127439
- Yosten GL, Maric-Bilkan C, Luppi P, Wahren J. Physiological effects and therapeutic potential of proinsulin C-peptide. Am J Physiol Endocrinol Metab. 2014;307(11):E955–68. PMID: 25249503; PMCID: PMC4254984. doi:10.1152/ajpendo.00130.2014
- Polonsky KS. The beta-cell in diabetes: from molecular genetics to clinical research. Diabetes. 1995;44(6):705–717. PMID: 7789637. doi:10.2337/diab.44.6.705
- Barakat HA, Carpenter JW, McLendon VD, et al. Influence of obesity, impaired glucose tolerance, and NIDDM on LDL structure and composition. Possible link between hyperinsulinemia and atherosclerosis. Diabetes. 1990;39(12):1527–1533. PMID: 2245877. doi:10.2337/diab.39.12.1527
- Standl E, Janka HU. High serum insulin concentrations in relation to other cardiovascular risk factors in macrovascular disease of type 2 diabetes. Horm Metab Res Suppl. 1985;15:46–51. PMID: 3908281.
- Alves MT, Ortiz MMO, Dos Reis GVOP, et al. The dual effect of C-peptide on cellular activation and atherosclerosis: protective or not? Diabetes Metab Res Rev. 2019;35(1):e3071. PMID: 30160822. doi:10.1002/dmrr.3071
- Patel N, Taveira TH, Choudhary G, Whitlatch H, Wu WC. Fasting serum c peptide levels predict cardiovascular and overall death in nondiabetic adults. J Am Heart Assoc. 2012;1(6):e003152. doi:10.1161/JAHA.112.003152
- Abdullah A, Hasan H, Raigangar V, Bani-Issa W. C-Peptide versus insulin: relationships with risk biomarkers of cardiovascular disease in metabolic syndrome in young Arab females. Int J Endocrinol. 2012;2012:420792. PMID: 22899917; PMCID: PMC3415197. doi:10.1155/2012/420792
- Harnishsingh B, Rama B. Is C-peptide a predictor of severity of coronary artery disease in metabolic syndrome? An observational study. Indian Heart J. 2018;70(Suppl 3):S105–S109. PMID: 30595240; PMCID: PMC6309290. doi:10.1016/j.ihj.2018.07.005
- Bo S, Cavallo-Perin P, Gentile L, Repetti E, Pagano G. Relationship of residual beta-cell function, metabolic control and chronic complications in type 2 diabetes mellitus. Acta Diabetol. 2000;37(3):125–129. PMID: 11277312. doi:10.1007/s005920070014
- Sari R, Balci MK. Relationship between C peptide and chronic complications in type-2 diabetes mellitus. J Natl Med Assoc. 2005;97(8):1113–1118. PMID: 16173326; PMCID: PMC2575980.
- Kim BY, Jung CH, Mok JO, Kang SK, Kim CH. Association between serum C-peptide levels and chronic microvascular complications in Korean type 2 diabetic patients. Acta Diabetol. 2012;49:9–15. PMID: 21212993. doi:10.1007/s00592-010-0249-6
- Wahren J, Shafqat J, Johansson J, Chibalin A, Ekberg K, Jornvall H. Molecular and cellular effects of C-peptide: new perspectives on an old peptide. Exp Diabesity Res. 2004;5:15–23. PMID: 15198368; PMCID: PMC2478619. doi:10.1080/15438600490424479
- Son SM. C-Peptide and vascular complications in type 2 diabetic subjects. Diabetes Metab J. 2012;36(5):345–349. PMID: 23130318; PMCID: PMC3486980. doi:10.4093/dmj.2012.36.5.345
- Kim ST, Kim BJ, Lim DM, et al. Basal C-peptide level as a surrogate marker of subclinical atherosclerosis in type 2 diabetic patients. Diabetes Metab J. 2011;35(1):41–49. PMID: 21537412; PMCID: PMC3080577. doi:10.4093/dmj.2011.35.1.41
- Wang L, Lin P, Ma A, et al. C-peptide is independently associated with an increased risk of coronary artery disease in T2DM subjects: a cross-sectional study. PLoS One. 2015;10(6):e0127112. PMID: 26098780; PMCID: PMC4476669. doi:10.1371/journal.pone.0127112
- Yan ST, Sun J, Gu ZY, et al. The bidirectional association of C-peptide with cardiovascular risk in nondiabetic adults and patients with newly diagnosed type 2 diabetes mellitus: a retrospective cohort study. Cardiovasc Diabetol. 2022;21(201). doi:10.1186/s12933-022-01636-z
- Thippeswamy T, Nithin N, Chikkegowda P. An association of fasting C-peptide levels and vascular complications in chronic type 2 diabetes mellitus patients. J Clin Diagn Res. 2021;15(2):OC10–OC13. doi:10.7860/JCDR/2021/47265.14506