Abstract
Purpose
We aimed to investigate the risk factors associated with revitrectomy in eyes with diabetic vitreous hemorrhage and to determine the prognosis of these patients at least one year postoperatively.
Patients and Methods
This retrospective case-control study had a minimum follow-up period of one year. Patients were divided into single vitrectomy group (control group, n=202) and revitrectomy group (case group, n=36) for analysis. The indications, number, and timing of revitrectomies were documented. And the revitrectomy group was further divided into two vitrectomies group (n=30) and three or more vitrectomies group (n=6). The best-corrected visual acuity (BCVA) at the last follow-up and the occurrence of neovascular glaucoma (NVG) were compared among the single vitrectomy, two vitrectomies and three or more vitrectomies groups. We conducted a thorough collection of patient data and used univariate and binary logistic regression analyses to identify the risk factors associated with revitrectomy.
Results
A total of 197 patients (238 eyes) were included. Thirty-six eyes (15.1%) required revitrectomy with six eyes (2.5%) undergoing three or more vitrectomies during the follow-up period. The median duration of the second vitrectomy was 3 (2–6) months. The indications for a second vitrectomy included 28 eyes (77.8%) of postoperative vitreous hemorrhage and 7 eyes (22.2%) combined with tractional retinal detachment. Patients undergoing three or more vitrectomies had significantly worse postoperative BCVA and a higher incidence of NVG (P<0.01). Fibrinogen> 4 g/L (P<0.001) and preoperative anti-vascular endothelial growth factor intravitreal injection (P=0.015) were independent risk factors for revitrectomy, and glycated hemoglobin A1c (HbA1c)>10% (P=0.049) showed significant difference only in univariate analysis.
Conclusion
Patients requiring revitrectomy tended to have higher fibrinogen levels, tightly adhered fibrovascular membranes, higher HbA1c levels, and worse prognoses.
Introduction
Diabetic retinopathy (DR), a microvascular complication of diabetes, is the leading cause of blindness among working-age adults.Citation1 Pars plana vitrectomy (PPV) is widely used to treat proliferative DR (PDR) complications, including non-clearing vitreous hemorrhage (NCVH), proliferative fibrovascular membrane, and tractional retinal detachment (TRD). Although the use of anti-vascular endothelial growth factor (VEGF) drugs and microinvasive vitrectomy systems (MIVS) has significantly improved the efficacy and safety of surgical procedures,Citation2,Citation3 a subset of patients requires revitrectomy for postoperative complications.
Postoperative vitreous hemorrhage (PVH) is one of the most common complications of diabetic vitrectomy, with an incidence ranging from 21.6% to 40% in studies using MIVS.Citation4–7 PVH that persists and negatively affects visual acuity often requires further surgical intervention, which is the most common reason for revitrectomy.Citation8 Repeated operations can lead to decreased postoperative visual function than a single vitrectomy operation,Citation6,Citation9–11 and consequently increase the psychological and economic burden on patients. Therefore, interventions can be implemented to prevent vision loss by identifying the risk factors associated with revitrectomy.
Numerous studies have investigated the risk factors associated with PVH, and approximately one-third of patients requiring reoperation.Citation12 However, limited research has been conducted on risk factors associated with revitrectomy. A multicentre study by Takayama et alCitation9 indicated that low baseline visual acuity and air tamponade were independent risk factors for revitrectomy. The follow-up period in this study was relatively short (six months), and the initial surgical indications were not analyzed independently. Schreur et alCitation11 conducted a long-term retrospective study that revealed that poor contralateral visual acuity was the only risk factor for revitrectomy, and a subgroup analysis based on initial vitrectomy indications was not performed. Our study aimed to investigate the risk factors for revitrectomy in eyes with diabetic vitreous hemorrhage with or without a fibrovascular membrane as the initial surgical indication, and to explore the prognosis.
Materials and Methods
Study Design and Participants
This was a single center, retrospective, case-control study. This study was approved by the Ethics Committee of Tianjin Medical University Eye Hospital (approval number: 2022KY-27) and conducted in accordance with the Declaration of Helsinki. Patients with PDR who underwent microinvasive PPV at Tianjin Medical University Eye Hospital between January 2017 and May 2022 were included in this study. The inclusion criteria were: (1) age ≥18 years old; (2) diagnosed with type I or II diabetes mellitus; and (3) presence of NCVH for at least one month. The key exclusion criteria were: (1) a follow-up period of <12 months since the first vitrectomy; (2) prior intraocular surgeries in the study eye (except cataract surgery); (3) existence of TRD; (4) inert gas or silicon oil as fillers for vitreous cavity; (5) missing medical records; (6) combined with other vision-threatening ocular complications (eg, pathologic myopia, retinal vein occlusion, age-related macular degeneration); and (7) surgeries in the study eye within 3 months before the last follow-up (eg, cataract surgery, intravitreal injection, revitrectomy). Patients were divided into the single vitrectomy group (control group, n=202) and revitrectomy group (case group, n=36), and the revitrectomy group was further divided into two vitrectomies (n=30) and three or more vitrectomies (n=6) groups.
Primary Surgical Procedures
Standard 23- and 25-gauge PPV were used. In eyes with fibrovascular proliferative membranes tightly adhered to the retina, an anti-VEGF drug was injected into the vitreous cavity 3–5 days before surgery. The vitreous and proliferative membranes were removed as completely as possible. Endolaser photocoagulation was performed based on the preoperative laser status, ensuring that pan-retinal photocoagulation (PRP) was performed to the fullest extent possible. The vitreous cavity was filled with balanced salt solution (BSS) or clean air at the end of the surgery. Intravitreal anti-VEGF drugs were injected as required. Phacoemulsification and intraocular lens implantation were combined to treat visually significant cataracts. All surgical procedures were performed by the same experienced surgeon.
Revitrectomy
Revitrectomy was performed for PVH that persisted for at least one month without self-absorbing tendency, postoperative progressive fibrovascular membranes and TRD. Revitrectomy mainly involved vitreous cavity lavage, fibrovascular membrane peeling, and supplementary endolaser treatment. The choice of filling material for the vitreous cavity–BSS, air, C3F8 gas, or silicone oil–depended on the status of the retina. A subsequent simple silicone oil removal without additional treatment of the retina (eg, membrane peeling, laser photocoagulation) was not taken as a revitrectomy.
Data Collection
All data were collected by reviewing electronic medical records. The baseline characteristics included sex, age, diabetes type, diabetes duration, hypertension, diabetic nephropathy, dialysis, coronary heart disease, cerebral infarction, blood parameters within three months prior to the first vitrectomy (including complete blood count, coagulation, glycated hemoglobin A1c and biochemistry), symptom duration, best-corrected visual acuity (BCVA) of the study eye, history of PRP and anti-VEGF intravitreal injections, and lens status (phakic or pseudophakic). Surgical information included preoperative and intraoperative anti-VEGF intravitreal injections, cataract surgery and vitreous cavity tamponade. The number, time, and indications for revitrectomies were recorded. Prognostic data included BCVA at the last follow-up and incidence of neovascular glaucoma (NVG) during the follow-up period.
Statistical Analysis
All statistical analyses were performed using IBM SPSS Statistics v 25.0, and R software v 4.0.1. The decimal visual acuity was transformed to the logarithmic minimum angle of resolution (logMAR) for statistical analysis, and values of 1.85, 2.3, 2.6, and 2.9 logMAR were assigned to counting fingers, hand movement, light perception, and no light perception, respectively.Citation13 The Shapiro–Wilk test was used to assess the normality of the data distribution. Categorical variables were presented as frequencies and percentages. Normal and non-normal distributed continuous variables were presented as mean ± standard deviation (SD), and median and interquartile range, respectively. The Student’s t-test and analysis of variance (ANOVA) were used to compare continuous variables with a normal distribution, and the Mann–Whitney U-test was used for non-normal data. The chi-square or Fisher’s exact test was used to compare categorical variables. Bonferroni correction and least significant difference post hoc tests were used for multiple comparisons. The cut-off value of continuous variables was determined by both clinical experience and the critical value of the receiver operating characteristic (ROC) curve. Binary logistic regression analysis was used to determine independent risk factors for revitrectomy. The Kaplan-Meier curve was used to depict the time-dependent probability of revitrectomy. The level of statistical significance was set at P<0.05.
Results
Patient Characteristics
In total, 197 patients (238 eyes) who met the eligibility criteria were included in this study. The mean follow-up period was 24.6 ± 16.9 months. For all eyes, the mean BCVA improved from 1.48 ± 0.65 logMAR preoperatively to 0.59 ± 0.68 logMAR postoperatively (P<0.01). NVG developed in 22 (9.2%) eyes during follow-up. Throughout the follow-up period, a single vitrectomy was performed in 202 eyes (84.9%), while 36 eyes (15.1%) required revitrectomy. In the revitrectomy group, 30 eyes (83.3%) underwent two vitrectomies and 6 eyes (16.7%) underwent three or more vitrectomies to restore vision. During the follow-up period, a total of 8 eyes (4 in the single vitrectomy group and 4 in the revitrectomy group) underwent phacoemulsification combined with intraocular lens implantation due to progressive cataracts. At the last follow-up, the revitrectomy group had a significantly higher proportion of eyes that were pseudophakic compared to the single vitrectomy group (69.4% versus 45.5%, P=0.008).
Occurrence Time and Indication of Revitrectomy
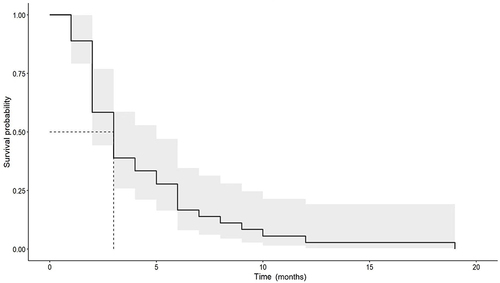
The mean time to the second vitrectomy was 4.28 ± 3.69 months, with a median time of 3 (2–6) months. Thirty eyes (83.3%) underwent a second vitrectomy within 6 months and 35 eyes (97.2%) experienced a second vitrectomy within one year. The Kaplan-Meier curve illustrates the probability of revitrectomy over time and a 95% confidence interval ().
In the revitrectomy group, the indications for a second vitrectomy included 28 eyes (77.8%) of PVH and 7 eyes (22.2%) combined with TRD. Of the 6 eyes that underwent three or more vitrectomies, 5 (83.3%) eventually developed TRD.
Visual Outcome and Occurrence of NVG
shows the prognosis of the three groups of patients who underwent different numbers of surgeries.
Table 1 Prognosis According to Number of Surgeries
There was no significant difference in the preoperative BCVA among the three groups (P=0.955); however, there were significant differences in the postoperative BCVA, mean BCVA change, and occurrence of NVG (P<0.01). Multiple comparison results showed that the prognosis of patients in the three or more vitrectomies group was significantly worse than that of patients in the other two groups (P<0.05). Although the prognosis of patients in the two revitrectomies groups was better than that of patients in the single vitrectomy group, the difference was not significant (P>0.05). The incidence of NVG in pseudophakic eyes was higher than that of phakic eyes (63.6% versus 47.7%), but the difference was not statistically significant (P=0.154).
Revitrectomy Risk Factors
The baseline characteristics and surgical information of the single vitrectomy and revitrectomy groups are shown in .
Table 2 Characteristics of the Single Vitrectomy and Revitrectomy Groups
For better clinical analysis and interpretation, we transformed the continuous variables into categorical variables and conducted a univariate analysis. The results are summarized in . In the univariate analysis, preoperative anti-VEGF, glycated hemoglobin A1c (HbA1c)>10%, and fibrinogen (FIB)> 4 g/L were identified as risk factors for revitrectomy (P<0.05).
Table 3 Univariate Analysis on Stratified Continuous Variables
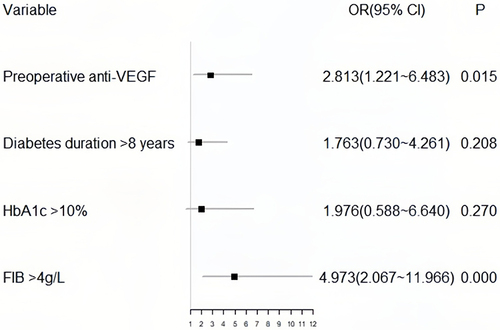
Binary multivariate logistic regression analysis included predictors with P<0.15, and a forest plot () was generated to visualize the results, which identified preoperative anti-VEGF (P=0.015) and FIB> 4 g/L (P<0.001) as independent risk factors for revitrectomy.
Discussion
In this study, the incidence of revitrectomy in patients with diabetic vitreous hemorrhage was 15.1%, similar to but slightly lower than that reported in previous studies.Citation9–11,Citation14 This is because our study only included patients with NCVH as an indication for initial vitrectomy, while TRD patients had a higher incidence of revitrectomy.Citation9,Citation10 The median time for the second vitrectomy in our study was 3 (2–6) months. Most patients (83.3%) underwent a second vitrectomy within six months, and nearly all (97.2%) underwent a second vitrectomy within one year. The most common reason for revitrectomy is the presence of PVH, which cannot self-absorb after more than one month. This can mainly be attributed to fibrovascular ingrowth at sclerotomy sites, retinal fibrovascular proliferation, and inadequate retinal photocoagulation.Citation12,Citation15 Although the vitreous was completely removed and retinal photocoagulation was extensively performed, there was still a possibility of developing postoperative vitreous hemorrhage and retinal detachment due to anterior hyaloidal fibrovascular proliferation.Citation16–18 Previous studiesCitation12,Citation19 have indicated that PVH tends to occur 6–8 weeks after surgery, which explains the peak revitrectomy time observed in our study.
Eyes undergoing two vitrectomies showed a worse visual outcome and a higher incidence of NVG, although these differences were not statistically significant. In eyes undergoing three or more vitrectomies, BCVA even decreased by 0.08±0.17 logMAR at the last follow-up, and 4 eyes (66.7%) developed NVG. In patients with PDR requiring revitrectomy, the disease course tends to be prolonged, and retinal circulation disturbances are often more severe. This promotes the synthesis of VEGF by the retinal pigment epithelial and Müller cells, thereby facilitating neovascularization.Citation20,Citation21 The surgical procedure itself induces the release of inflammatory mediators and VEGF, further accelerating this process and leading to a higher incidence of NVG.Citation22 In addition, vitrectomy can accelerate the progression of cataract.Citation23 After lens removal, the resistance to communication between the vitreous cavity and anterior chamber decreases. This allows the VEGF present in the vitreous cavity to enter the anterior chamber more easily, promoting iris neovascularization.Citation24 In our search, the incidence of NVG was higher in pseudophakic eyes, although there was no statistical difference. Functional outcomes were determined based on multiple factors. First, NVG is difficult to treat and is associated with a high rate of blindness. Further, the complications of vitrectomy itself, such as elevated intraocular pressure and retinal damage, can lead to visual decline. Among the six patients who underwent three or more vitrectomies, five developed TRD. Patients with TRD have more severe retinal lesions, and the use of long-acting vitreous fillers can affect their visual acuity.
In both univariate and multivariate analyses, FIB levels > 4 g/L were found to be closely associated with revitrectomy (P<0.01). According to the ROC curve, the optimal cutoff value for FIB to predict revitrectomy was 3.91, with a sensitivity of 0.471 and a specificity of 0.851. Since the normal range of FIB is 2–4 g/L, we used 4 g/L as the grouping criterion. Our study revealed that the incidence of revitrectomy in patients with FIB > 4 g/L was 4.973 times higher than that in other patients. FIB is a protein synthesized by the liver, indicating hypercoagulability and decreased fibrinolytic activity, which can significantly affect blood coagulation and hemorheology. It has been confirmed that FIB levels are closely associated with DR development.Citation25,Citation26 Although the specific mechanisms are not fully understood, there are hypotheses suggesting that FIB levels may contribute to the progression of DR postoperatively, leading to the need for revitrectomy. Elevated FIB levels can lead to thrombus formation and vascular occlusion, affecting retinal blood flow and oxygen supply, which can result in the disruption of blood-retinal barrier and formation of retinal neovascularization.Citation26,Citation27 Additionally, FIB is known to interact with various growth factors and cytokines involved in angiogenesis and inflammation,Citation28–30 which can also contribute to the progression of DR. Many prognostic studies have overlooked FIB. Our study is the first to propose that a FIB level > 4 g/L is a high-risk factor for revitrectomy.
Preoperative intravitreal injection of anti-VEGF drugs was another independent risk factor for revitrectomy (P=0.015). However, numerous studies have shown that preoperative combination therapy with anti-VEGF drugs can promote the regression of neovascularization, reduce intraoperative bleeding, shorten surgical time, decrease the occurrence of intraoperative and postoperative complications, and improve prognosis.Citation31,Citation32 This is because our preoperative use of anti-VEGF drugs in clinical practice is not random but only applied when there is a need to peel off tightly adhered fibrovascular membranes, leading to selection bias. Therefore, the severity of PDR, rather than preoperative anti-VEGF therapy, determines the increased risk of revitrectomy.
In terms of HbA1c, HbA1c>10% only showed a statistically significant difference in univariate analysis (P=0.049). HbA1c level is considered the gold standard for evaluating long-term blood sugar control in clinical settings. The risk of microvascular complications in patients with diabetes increases exponentially with increasing HbA1c levels.Citation33 However, changes in HbA1c levels cannot explain approximately one-third of the actual blood glucose levels and cannot reflect blood glucose fluctuations. Multiple studies have shown that patients with higher glycemic variability, despite having similar average HbA1c levels, have a higher risk of developing DR.Citation34,Citation35 In addition to the effect of blood glucose concentration, HbA1c is also related to internal environmental differences, red blood cell membrane permeability, levels and activities of enzymes involved in glucose metabolism, and individual heterogeneity.Citation36 These explanations may account for why HbA1c levels did not show a significant difference in the multivariate analysis. Although an extensively researched prognostic factor, HbA1c has not been found to be an independent risk factor for reoperation or recurrent VH in previous studies.Citation9,Citation11,Citation37 According to the ROC curve, the optimal cut-off value for HbA1c to predict revitrectomy was 10.35, with a sensitivity of 0.167 and a specificity of 0.996. For clinical application, we adjusted the grouping criteria to 10% per group. Thus, preoperative blood glucose management in patients with high HbA1c levels, especially in those with HbA1c>10%, should be improved to reduce the likelihood of revitrectomy.
Numerous studies have investigated PVH after microinvasive PPV in patients with PDR. Other potential risk factors associated with revitrectomy may overlap with those for PVH, including younger age, longer duration of diabetes, hypertension, renal disease, cardiovascular disease, use of antiplatelet or anticoagulation agents, neovascularization of the optic disc, and the absence of posterior vitreous detachment.Citation6,Citation7,Citation37–41 However, no significant differences were observed in this study. Management of PVH includes observation, oral medication, laser supplementation, intravitreal injection of anti-VEGF drugs, and revitrectomy. The diversity of treatment options may contribute to the differences in risk factors for revitrectomy and PVH.
Compared with previous studies, we conducted an analysis and comparison of coagulation and lipid indicators, which is one of the strengths of our study. Furthermore, we excluded patients with TRD because DR progression and therapeutic measures differ in the presence of TRD, which could affect the analysis of prognostic factors.
Our study has certain limitations. First, we did not consider factors that may be associated with revitrectomy, including the use of antiplatelet or anticoagulation agents, and the status of posterior vitreous detachment. However, this did not affect the reliability of the results. Second, the follow-up durations of the eyes included in our study varied significantly. However, previous studies have indicated that vision tends to stabilize at one year postoperatively,Citation11,Citation23 and revitrectomy mostly occurs within the first year after surgery. Therefore, the difference in follow-up duration may have had some impact on the research results, but it should be minimal because the minimum follow-up time of our eyes was one year. Finally, due to the retrospective nature of the study, there may have been some confounding factors and biases.
Conclusion
In patients with diabetic vitreous hemorrhage, our study identified FIB> 4 g/L and preoperative injection of anti-VEGF drugs (referring to the existence of fibrovascular membrane tightly adhered to the retina) as independent risk factors for revitrectomy, and HbA1c>10% showed statistical significance in the univariate analysis. The postoperative period of three months was a high-risk period for revitrectomy. Patients who underwent three or more vitrectomies often had significantly poorer visual acuity and a higher incidence of NVG. This study can assist clinicians in predicting patient prognosis and improving preoperative management by identifying risk factors, ultimately reducing the need for revitrectomy.
Abbreviations
ANOVA, analysis of variance; BCVA, best-corrected visual acuity; BSS, balanced salt solution; DR, diabetic retinopathy; HbA1c, hemoglobin A1c; logMAR, logarithmic minimum angle of resolution; MIVS, microinvasive vitrectomy systems; NCVH, non-clearing vitreous hemorrhage; NVG, neovascular glaucoma; PDR, proliferative DR; PPV, pars plana vitrectomy; PRP, pan-retinal photocoagulation; PVH, postoperative vitreous hemorrhage; ROC, receiver operating characteristic; TRD, tractional retinal detachment; VEGF, vascular endothelial growth factor.
Data Sharing Statement
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Ethics Approval and Informed Consent
Ethics Committee of Tianjin Medical University Eye Hospital has granted the study ethical approval (2022KY-27). Patient consent was waived due to the retrospective nature of this study.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
Additional information
Funding
References
- Tsiknakis N, Theodoropoulos D, Manikis G, et al. Deep learning for diabetic retinopathy detection and classification based on fundus images: a review. Comput Biol Med. 2021;135:104599. doi:10.1016/j.compbiomed.2021.104599
- Yokota R, Inoue M, Itoh Y, Rii T, Hirota K, Hirakata A. Comparison of microincision vitrectomy and conventional 20-gauge vitrectomy for severe proliferative diabetic retinopathy. Jpn J Ophthalmol. 2015;59(5):288–294. doi:10.1007/s10384-015-0396-y
- Smith JM, Steel DH. Anti-vascular endothelial growth factor for prevention of postoperative vitreous cavity haemorrhage after vitrectomy for proliferative diabetic retinopathy. Cochrane Database Syst Rev. 2015;2015(8):Cd008214. doi:10.1002/14651858.CD008214.pub3
- Lee BJ, Yu HG. Vitreous hemorrhage after the 25-gauge transconjunctival sutureless vitrectomy for proliferative diabetic retinopathy. Retina. 2010;30(10):1671–1677. doi:10.1097/IAE.0b013e3181dcfb79
- Fujii GY, De Juan E, Humayun MS, et al. Initial experience using the transconjunctival sutureless vitrectomy system for vitreoretinal surgery. Ophthalmology. 2002;109(10):1814–1820. doi:10.1016/S0161-6420(02)01119-3
- Khuthaila MK, Hsu J, Chiang A, et al. Postoperative vitreous hemorrhage after diabetic 23-gauge pars plana vitrectomy. Am J Ophthalmol. 2013;155(4):757–763. doi:10.1016/j.ajo.2012.11.004
- Ding Y, Yao B, Hang H, Ye H. Multiple factors in the prediction of risk of recurrent vitreous haemorrhage after sutureless vitrectomy for non-clearing vitreous haemorrhage in patients with diabetic retinopathy. BMC Ophthalmol. 2020;20(1):292. doi:10.1186/s12886-020-01532-8
- Brown GC, Tasman WS, Benson WE, McNamara JA, Eagle RC. Reoperation following diabetic vitrectomy. Arch Ophthalmol. 1992;110(4):506–510. doi:10.1001/archopht.1992.01080160084037
- Takayama K, Someya H, Yokoyama H, et al. Prognostic factors of revitrectomy for complications in eyes with proliferative diabetic retinopathy: a retrospective multicentre study. Acta Ophthalmol. 2020;98(4):e434–e439. doi:10.1111/aos.14292
- Al-Khersan H, Venincasa MJ, Kloosterboer A, et al. Pars plana vitrectomy reoperations for complications of proliferative diabetic retinopathy. Clin Ophthalmol. 2020;14:1559–1563. doi:10.2147/OPTH.S252285
- Schreur V, Brouwers J, Van Huet RAC, et al. Long-term outcomes of vitrectomy for proliferative diabetic retinopathy. Acta Ophthalmol. 2021;99(1):83–89. doi:10.1111/aos.14482
- Yan H, Cui J, Lu Y, Yu J, Chen S, Xu Y. Reasons for and management of postvitrectomy vitreous hemorrhage in proliferative diabetic retinopathy. Curr Eye Res. 2010;35(4):308–313. doi:10.3109/02713680903572491
- Gupta B, Wong R, Sivaprasad S, Williamson TH. Surgical and visual outcome following 20-gauge vitrectomy in proliferative diabetic retinopathy over a 10-year period, evidence for change in practice. Eye. 2012;26(4):576–582. doi:10.1038/eye.2011.348
- Nishi K, Nishitsuka K, Yamamoto T, Yamashita H. Factors correlated with visual outcomes at two and four years after vitreous surgery for proliferative diabetic retinopathy. PLoS One. 2021;16(1):e0244281. doi:10.1371/journal.pone.0244281
- Gündüz K, Bakri SJ. Management of proliferative diabetic retinopathy. Compr Ophthalmol Update. 2007;8(5):245–256.
- Lewis H, Abrams GW, Foos RY. Clinicopathologic findings in anterior hyaloidal fibrovascular proliferation after diabetic vitrectomy. Am J Ophthalmol. 1987;104(6):614–618. doi:10.1016/0002-9394(87)90174-7
- Kobayashi T, Machida S, Fujiwara T, Ishibe T, Kurosaka D. Vitreous levels of vascular endothelial growth factor in eyes with anterior hyaloidal fibrovascular proliferation. Clin Ophthalmol. 2010;4:1043–1046. doi:10.2147/opth.s13193
- Lewis H, Abrams GW, Williams GA. Anterior hyaloidal fibrovascular proliferation after diabetic vitrectomy. Am J Ophthalmol. 1987;104(6):607–613. doi:10.1016/0002-9394(87)90173-5
- Bhende M, Agraharam SG, Gopal L, et al. Ultrasound biomicroscopy of sclerotomy sites after pars plana vitrectomy for diabetic vitreous hemorrhage. Ophthalmology. 2000;107(9):1729–1736. doi:10.1016/S0161-6420(00)00213-X
- Funatsu H, Yamashita H, Noma H, Mimura T, Sakata K, Hori S. Risk evaluation of outcome of vitreous surgery for proliferative diabetic retinopathy based on vitreous level of vascular endothelial growth factor and angiotensin II. Br J Ophthalmol. 2004;88(8):1064–1068. doi:10.1136/bjo.2003.032656
- Wakabayashi Y, Usui Y, Okunuki Y, et al. Intraocular VEGF level as a risk factor for postoperative complications after vitrectomy for proliferative diabetic retinopathy. Invest Ophthalmol Vis Sci. 2012;53(10):6403–6410. doi:10.1167/iovs.12-10367
- Song S, Yu X, Zhang P, Dai H. Increased levels of cytokines in the aqueous humor correlate with the severity of diabetic retinopathy. J Diabetes Complications. 2020;34(9):107641. doi:10.1016/j.jdiacomp.2020.107641
- Ostri C, Lux A, Lund-Andersen H, la Cour M. Long-term results, prognostic factors and cataract surgery after diabetic vitrectomy: a 10-year follow-up study. Acta Ophthalmol. 2014;92(6):571–576. doi:10.1111/aos.12325
- Poliner LS, Christianson DJ, Escoffery RF, Kolker AE, Gordon ME. Neovascular glaucoma after intracapsular and extracapsular cataract extraction in diabetic patients. Am J Ophthalmol. 1985;100(5):637–643. doi:10.1016/0002-9394(85)90617-8
- Zhao H, Zhang LD, Liu LF, et al. Blood levels of glycated hemoglobin, D-Dimer, and fibrinogen in diabetic retinopathy. Diabetes Metab Syndr Obes. 2021;14:2483–2488. doi:10.2147/DMSO.S309068
- Chen X, Zhao J, You Y, Li Z, Chen S. The ratio of fibrinogen to albumin is related to the occurrence of retinopathy in type 2 diabetic patients. Diabetes Metab Syndr Obes. 2023;16:1859–1867. doi:10.2147/DMSO.S407391
- Huang Q, Wu H, Wo M, Ma J, Song Y, Fei X. Clinical and predictive significance of plasma fibrinogen concentrations combined monocyte-lymphocyte ratio in patients with diabetic retinopathy. Int J Med Sci. 2021;18(6):1390–1398. doi:10.7150/ijms.51533
- Luyendyk JP, Schoenecker JG, Flick MJ. The multifaceted role of fibrinogen in tissue injury and inflammation. Blood. 2019;133(6):511–520. doi:10.1182/blood-2018-07-818211
- Sui J, Noubouossie DF, Gandotra S, Cao L. Elevated plasma fibrinogen is associated with excessive inflammation and disease severity in COVID-19 patients. Front Cell Infect Microbiol. 2021;11:734005. doi:10.3389/fcimb.2021.734005
- Tomić M, Ljubić S, Kaštelan S, Gverović Antunica A, Jazbec A, Poljičanin T. Inflammation, haemostatic disturbance, and obesity: possible link to pathogenesis of diabetic retinopathy in type 2 diabetes. Mediators Inflamm. 2013;2013:818671. doi:10.1155/2013/818671
- Zhao XY, Xia S, Chen YX. Antivascular endothelial growth factor agents pretreatment before vitrectomy for complicated proliferative diabetic retinopathy: a meta-analysis of randomised controlled trials. Br J Ophthalmol. 2018;102(8):1077–1085. doi:10.1136/bjophthalmol-2017-311344
- Simunovic MP, Maberley DA. Anti-vascular endothelial growth factor therapy for proliferative diabetic retinopathy: a systematic review and meta-analysis. Retina. 2015;35(10):1931–1942. doi:10.1097/IAE.0000000000000723
- Stratton IM, Adler AI, Neil HA, et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ. 2000;321(7258):405–412. doi:10.1136/bmj.321.7258.405
- Hirsch IB. glycemic variability and diabetes complications: does It matter? Of course it does! Diabetes Care. 2015;38(8):1610–1614. doi:10.2337/dc14-2898
- Ceriello A, Kilpatrick ES. Glycemic variability: both sides of the story. Diabetes Care. 2013;36(Suppl 2):S272–275. doi:10.2337/dcS13-2030
- Hare MJ, Shaw JE, Zimmet PZ. Current controversies in the use of haemoglobin A1c. J Intern Med. 2012;271(3):227–236. doi:10.1111/j.1365-2796.2012.02513.x
- Baget-Bernaldiz M, Romero-Aroca P, Mira-Puerto A, et al. Risk factors for recurrent vitreous hemorrhage in type 2 diabetes mellitus patients after posterior vitrectomy. J Clin Med. 2023;12(8):2989. doi:10.3390/jcm12082989
- Sato T, Tsuboi K, Nakashima H, Emi K. Characteristics of cases with postoperative vitreous hemorrhage after 25-gauge vitrectomy for repair of proliferative diabetic retinopathy. Graefes Arch Clin Exp Ophthalmol. 2017;255(4):665–671. doi:10.1007/s00417-016-3522-8
- Zhao M, Chandra A, Xu J, Li J. Factors related to postoperative vitreous hemorrhage after small-gauge vitrectomy in proliferative diabetic retinopathy patients. BMC Ophthalmol. 2023;23(1):215. doi:10.1186/s12886-023-02940-2
- Motoda S, Shiraki N, Ishihara T, et al. Predictors of postoperative bleeding after vitrectomy for vitreous hemorrhage in patients with diabetic retinopathy. J Diabetes Investig. 2018;9(4):940–945. doi:10.1111/jdi.12791
- Tandias R, Lemire CA, Palvadi K, Arroyo JG. Posterior vitreous detachment status as a predictive factor for outcomes of vitrectomy for diabetic vitreous hemorrhage. Retina. 2022;42(6):1103–1110. doi:10.1097/IAE.0000000000003453