Abstract
Obesity,and metabolic dysfunction-associated fatty liver disease (MAFLD) have reached epidemic proportions globally. Obesity and MAFLD frequently coexist and act synergistically to increase the risk of adverse clinical outcomes (both hepatic and extrahepatic). Type 2 diabetes mellitus (T2DM) is the most important risk factor for rapid progression of steatohepatitis and advanced fibrosis. Conversely, the later stages of MAFLD are associated with an increased risk of T2DM incident. According to the proposed criteria, MAFLD is diagnosed in patients with liver steatosis and in at least one in three: overweight or obese, T2DM, or signs of metabolic dysregulation if they are of normal weight. However, the clinical classification and correlation between obesity and MAFLD is more complex than expected. In addition, treatment for obesity and MAFLD are associated with a reduced risk of T2DM, suggesting that liver-based treatments could reduce the risk of developing T2DM. This review describes the clinical classification of obesity and MAFLD, discusses the clinical features of various types of obesity and MAFLD, emphasizes the role of visceral obesity and insulin resistance (IR) in the development of MAFLD,and summarizes the existing treatments for obesity and MAFLD that reduce the risk of developing T2DM.
Introduction
Obesity is defined as excessive accumulation or improper distribution of body fat (BF).Citation1 Other concomitant illnesses include type 2 diabetes mellitus (T2DM), hepatic steatosis, cardiovascular disease,Citation2,Citation3 stroke, dyslipidemia, and hypertension, making obesity treatment more essential.Citation4 Conventional classifications of overweight and obesity have been developed based on the Body Mass Index (BMI) and ethnicity-specific thresholds. Adults with a BMI of 25 to 29.9 kg/m2 were categorized as overweight, those with a BMI of 30 kg/m2 as obese, and those with a BMI of 18.5 to 24.9 kg/m2 as normal (ie, lean) weight.Citation5 For Asian populations, BMI from 23.0 to 24.9 kg/m2 is considered overweight, BMI ≥25.0 kg/m2 is considered obese, and 18. 5–22. 9 kg/m2 were regarded as normal weight.Citation6 The most reported adult subtypes of obesity and heterogeneity used for research were as follows: (i) normal-weight obesity (NWO) syndrome; (ii) metabolically obese normal weight (MONW); metabolically unhealthy normal-weight (MUHNW); (iii) metabolically healthy obesity (MHO); (iv) metabolically unhealthy obesity (MUO), metabolically obese (MO), metabolically abnormal obese (MAO), (v) sarcopenic obesity (SO).Citation7
In the past several decades, the prevalence of obesity has increased nearly three times, reaching epidemic levels. According to the World Health Organization(WHO), more than 650 million adults, or over 13% of the world’s population, had this chronic illness in 2016.Citation8 According to previous reports,Citation9 up to 463 million individuals globally and 1 in 11 adults have TD2M.Citation10 Patients with non-alcoholic fatty liver disease (NAFLD) may have a higher risk of developing diabetes because they often exhibit aberrant glucose metabolism, which is indicative of T2DM and is characterized by elevated blood glucose levels, insulin resistance(IR), and impaired islet cell function.Citation11 The prevalence rates of NAFLD and non-alcoholic steatohepatitis(NASH) in T2DM were 65.04% and 31.55%, respectively, according to a meta-analysis of 156 studiesCitation12 including 1,832,125 individuals. Clinically significant fibrosis (F2-F4) was observed in 35.54% of the patients with T2DM and NAFLD, whereas advanced fibrosis (F3-F4) was present in 14.95% of these patients.
Obese patients frequently have “fatty liver.” A global panel of experts redefined fatty liver disease in 2020 from a negative state, excluding diagnosis, to a positive state of fatty liver disease coupled with metabolic dysfunction.Citation13 Clinical Practice Guidelines for the Diagnosis and Management of Metabolically Related Fatty Liver Diseases issued by the Asian Pacific Association for the Study of the Liver (APASL) were adopted as a new consensus suggestion.Citation14 While the American Society of Liver Disease (AASLD) and the European Association for the Study of Liver Diseases (EASL) have not yet approved the nomenclature change from metabolic dysfunction-associated fatty liver disease(MAFLD) to NAFLD. The current proposal to rename NAFLD as metabolic dysfunction-associated steatosis liver disease(MASLD)is the result of the recent multinational Delphi consensus.Citation15 As previously described, NAFLD and MAFLD have a noticeable overlapCitation13,Citation16,Citation17 and based on a Cohen kappa value of up to 0.92, the two definitions have a high level of overall concordance.Citation18 Furthermore, a meta-analysis based on information fromCitation19 research covering 1,088,677 individuals globally revealed that the prevalence of MAFLD was comparable to that of NAFLD. Only 4.0% of NAFLD patients fail to fulfill the new MAFLD diagnostic standards.Citation19 MAFLD represents the vast majority of NAFLD to some extent; therefore, in our review, previous NAFLD studies were also included in the analysis of MAFLD.
A recent systematic review and meta-analysisCitation20 of MAFLD prevalence in an Asian context, comprising 13,044,518 individuals, suggested that the prevalence of MAFLD in this region was 29.62%. Another 29 studiesCitation21 comprising 6,095 individuals reported that MAFLD prevalence in overweight or obese children and adolescents from the general population was 33.78%, regardless of diagnostic techniques. One metaCitation22 identified 116 relevant studies comprising 2,667,052 participants in the general population with an estimated global MAFLD prevalence of 51.3% among overweight/obese adults using ultrasound diagnostic technique, and the generating prevalence rate of males (59.0%) had a significantly higher MAFLD prevalence than females (47.5%).
Steatosis, steatohepatitis, and accompanying fibrosis can be used as pathological lesion forms of MAFLD, such as metabolic associated steatohepatitis (MASH),Citation23,Citation24 MAFLD with significant fibrosis,Citation25 and MAFLD associated with advanced liver fibrosis.Citation26 It arises in the setting of poor metabolic status, such as being overweight or obese, and is linked to lipid manipulation and abnormalities in glucose homeostasis. MAFLD is part of a complex, multi-organic set of disorders, rather than merely a liver condition.Citation27 It is fueled by intricate gene-environment interactions, creating a dysfunctional metabolic medium with a range of outcomes.Citation28 Based on the proposed criteria, MAFLD is diagnosed when patients with hepatic steatosis meet one or more of the following three criteria: Overweight or obese, T2DM, or signs of metabolic dysregulation (Increased waist circumference, high blood pressure, low HDL cholesterol levels, hypertriglyceridemia, impaired fasting plasma glucose, IR, and chronic subclinical inflammation are at least two of the risk factors.)Citation13,Citation14 This leads us to believe that there are three different types of MAFLD: I, MALFD with obesity or overweight; II, normal weight MAFLD (Lean MAFLD, both hepatic steatosis and evidence of metabolic dysregulation must be present); and III, MAFLD with T2DM.
The categorization of MAFLD and metabolic phenotypes of obesity are closely associated [], and the proper classification of obesity facilitates the diagnosis and categorization of metabolic fatty liver disease and gives more insight into the MAFLD treatment strategy. The metabolic phenotypes of obesity [] and particular MAFLD types [] are the main focus of this review. The significance of various definitions, clinical characteristics, genes and molecules, phenotypes, prognosis, and other metabolic disorders is of particular note. Here, we highlight some pathomechanisms and assess their clinical utility.
Table 1 Definitions Used for Heterogeneity Subtypes in Obese Individuals
Table 2 The Types and Subtypes in MAFLD Individuals
Figure 1 Different forms of metabolic fatty liver disease interchanging with one another. (By Figdraw. ID:AAPSIddcb1).
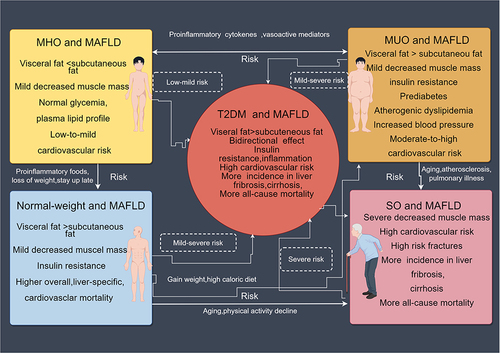
Visceral obesity is the principal contributor to insulin resistance, which is the pathophysiological underpinning of Obesity, MAFLD, and T2DMCitation2 []. As a result, treating metabolic fatty liver includes treating both IR and obesity, in addition to the liver itself, and weight loss continues to be the cornerstone of MAFLD treatment, as is prompt and adequate treatment. Not only do ideal therapies result in considerable weight loss, hepatic steatosis remission, and fibrosis regression, but they also reduce IR and prevent the onset of T2DM []. As a result, alternative treatments are advised.
Table 3 Treatments for Obesity and MAFLD, Considering Pathological Processes That are Targets for Therapy
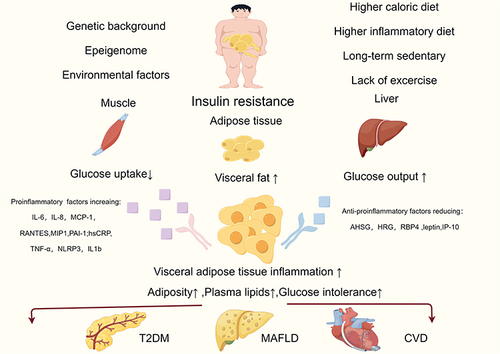
Figure 2 The underlying causes of MAFLD and T2DM include visceral obesity and insulin resistance, and inflammatory factors play a significant role in the onset and progression of the illness. (By Figdraw. ID:IIAPIadaa4).
Metabolic Phenotypic Obesity
The diversity of obesity, which includes a wide range of potential causes, is highlighted by the occurrence of several “phenotypes of obesity” with varying metabolic and cardiovascular disease (CVD)risks.Citation7,Citation31,Citation41,Citation124,Citation125 [] De Lorenzo et alCitation126 identified three distinct obesity phenotypes: MHO, NWO, and MUO. The National Cholesterol Education Program Adult Treatment Panel III (NCEP ATP III) criteria for differentiating between MHO and MUO as well as the International Diabetes Federation (IDF) criteria for the differential diagnosis of NWO syndrome and MONW were used to evaluate metabolic diseases.Citation127 Particularly, two subclasses of NWO have been identified:Citation128 normal weight healthy metabolic obesity, typical of normal weight obesity syndrome, with a high CVD risk index,Citation38 and normal weight obesity associated with metabolic syndrome (MetS) and IR,Citation129 defined as MONW.Citation37 In contrast, SO is characterized by a loss of lean mass, BF accumulation, a decline in skeletal muscle mass, and a loss of muscular strength.Citation75,Citation130
Normal Weight Obesity (NWO) Syndrome
Individuals with normal weight who have hereditary obesity and are in the early stages of low-grade inflammation are considered to have Normal Weight Obesity (NWO) syndrome.Citation31 There is a considerable loss of lean mass, equivalent to at least 1.5 kg (FFM kg), especially in the lower limb muscle mass, when the percentage of body fat (PBF) approaches 30%.Citation29 They exhibit elevated levels of TNF-α, IL-1, and IL-8 as well as oxidative stress caused by metabolic anomalies.Citation32 An alteration in a set of genes linked to aging and inflammation was revealed by NWO. Cardiovascular risk scores and fat distribution were strongly correlated in NWO patients.Citation33 NWO patients had more vascular inflammation than normal lean subjects, according to a study by Kang et al.Citation131 After accounting for the impact of abdominal obesity, the prevalence of NWO in 23,748 Chinese was 6.6% in women and 9.5% in men, and it was linked to an increase in MetS and CVD risk.Citation30
Metabolically Obese Normal Weight (MONW)/The Metabolically Unhealthy Normal-Weight Phenotype (MUHNW)
Metabolically Obese Normal Weight (MONW), metabolically abnormal with no obesity (MANO), and metabolically unhealthy normal weight phenotype (MUHNW) are other names for this condition.Citation35 The phenotype has an elevated Visceral Adipose Tissue (VAT) and abdominal Subcutaneous Adipose Tissue (SAT), a normal BMI (18.5–25 kg/m2), and decreased lean mass. Adiposity and ectopic fat distribution also increased.Citation34 Diet quality may be an independent determinant of metabolic health. A studyCitation36 showed that components of dietary intake, such as high intake of sugar and low intake of cereals, fish, and root vegetables, were associated with normal-weight obesity, high body fat percentage, and poor metabolic health.
Individuals with MetS have a higher incidence of clinical features that are frequently identified because they tend to overlook clinical therapy or prevention. They are typically young and exhibit early symptoms of IR, hyperinsulinemia, and dyslipidemia, all of which may be associated with a higher risk of T2DM and CVD.Citation37 Additionally, elderly individuals with this genotype are more likely to die from CVD-related causes.Citation40 In obese individuals, adipose tissue is a significant source of pro-inflammatory cytokines,Citation2 and high levels of hypersensitive C-reactive protein(hsCRP), tumor necrosis factor alpha (TNF-α), interleukin (IL)-1, IL-6, and IL-8 are observed in the blood.Citation38,Citation39
Metabolically Healthy Obesity (MHO)
At present, there is no international standard for the identification of MHO, and more than 30 distinct criteria have been used to operationalize symptoms.Citation132 The MHO has been proposed to have a combination of obesity and absence of components of metabolic syndrome (in some definitions with the exception of waist circumference), with the exception of normal lipid and blood pressure profiles, good insulin sensitivity, obesity with BMI over 30 kg/m2, and no metabolic anomalies.Citation41 Reduced visceral adiposity in relation to high total fat levels may contribute to increased insulin sensitivity and decreased inflammation.Citation42 MHO are often young, physically active, and have good eating habits. Their livers operated correctly, their blood pressure was normal, their serum lipid profile was stable, and their level of inflammation was low.Citation43
According to various classification criteria, the MHO group comprises 6–75% of the obese population.Citation43–45 Several underlying reasons, including reduced VAT and ectopic fat deposition (including less hepatic steatosis) compared to the more expandable subcutaneous fat depots, have been hypothesized to explain the better profile in those with MHO.Citation41 Compared to healthy individuals of normal weight, patients with MHO have a higher risk of developing metabolic syndrome.Citation133 The fatty acid composition of myristic, palmitic, stearic, oleic, and linoleic acids may help explain why MHO has a lower inflammatory state.Citation46 All MHO patients have a healthy Homeostasis Model Assessment of Insulin Resistance (HOMA-IR), Quantitative Insulin Sensitivity Check Index(QUICKI), insulin sensitivity index (ISI) (Mffm/l), hsCRP, and IL-6.Citation38 Other biomarkers, such as leptin, have increased in MHO patients.Citation47 Pro-inflammatory proteins such as histamine releasing peptide (HRP), hsCRP, Complement Component 4a (C4A), and inter-alpha-trypsin inhibitor heavy chain H4 (ITIH4) are downregulated, while anti-inflammatory molecules such as Alpha-2 Heremans Schmid Glycoprotein (AHSG), Heregulin (HRG), and retinol-binding protein -4(RBP4) are overexpressed in MHO.Citation48 MHO still has the risk of developing into an unhealthy phenotype and is linked to a number of serious chronic illnesses, such as CVD, hypertension, T2DM, chronic kidney disease, and several types of cancer. Therefore, it should not be regarded as a benign condition.Citation49
The Metabolically Unhealthy Obese Phenotype (MUO)/MAO
Metabolically unhealthy obesity (MUO), also known as metabolically abnormal obesity (MAO), is characterized by a BMI greater than 30 kg/m2 and a body fat percentage greater than 30%.Citation50 Patients with MUO typically have ectopic fat distribution and excessive VAT accumulation, and they are thought to be at a higher risk of mortality due to serious health issues such as T2DM and CVD.Citation51 This group differed considerably from the MHO subtype in terms of body fat (%), high-density lipoprotein cholesterol, systolic blood pressure, triglycerides, glucose, and insulin.Citation134
Obesity-related inflammation and metabolic problems are exacerbated by macrophage infiltration into adipose tissue, which is a significant pathogenic component.Citation135,Citation136 Alanine aminotransferase (ALT) is a metabolic syndrome-related biomarker that can be significantly increased. The pro-inflammatory cytokines IL-6, IL-8, Monocyte chemoattractant protein-1 (MCP-1), regulated upon activation normal T cell expressed and presumably secreted(RANTES), Mps1 interacting protein-1 (MIP1), and plasminogen activator inhibitor-1 (PAI-1) are more incidents of heterogeneous expression seen in VAT, whereas leptin and interferon-gamma (IFN-gamma)-induced protein 10 (IP-10) are mostly expressed in SAT.Citation7 Leucine rich repeat-containing receptor family NACHT, LRR, and PYD domain-containing protein-3 (NLRP3)gene and IL-1b are increased in VAT, which is infiltrated by pro-inflammatory macrophages in the MUO/MAO subgroup. VAT is associated with metabolic problems and its activation and expression are upregulated.Citation137 Increased levels of hsCRP and TNF-α were linked to higher waist circumference (WC) in males and BMI in women, according to Marques-Vidal et al.Citation52 The number of genomic alterations that can be connected to various MAO symptoms rises as a result of epigenetic mechanisms, WC and levels of fasting triglycerides are two characteristics of the phenotypic hypertriglycéridaemic-waist (HTGW), for instance. It is believed to be associated with the genes for ATP binding cassette subfamily G 1 (ABCG1) and carnitine palmitoyltransferase 1A (CPT1A).Citation53
Sarcopenic Obesity (SO)
Sarcopenic obesity (SO), which is characterized by a loss of lean mass and an increase in the percentage of fat mass, is associated with risk factors such as advanced age, a decline in physical activity, atherosclerosis, and pulmonary illness.Citation54 Individuals of various ages, not just older adults, can develop SO. According to Kim et al’s research,Citation57 the prevalence of sarcopenic non-obese, sarcopenic, and non-sarcopenic obesity in patients was 10%, 15%, and 20%, respectively. The subquintile of the skeletal muscle index (skeletal muscle/BMI), along with the measurement of grasping force (30 kg for men and 20 kg for women), is frequently used for diagnosis.Citation55 Perna et alCitation56 stated that according to dual-energy X-ray absorptiometry (DXA) or bioelectrical impedance analysis (BIA), SO refers to those who have extra body fat that is higher than the median or >27% for men and 38% for women, as well as loss of muscle mass and strength. Age-related reductions in muscle mass and strength may then cause a reduction in physical activity in the elderly, which in turn leads to weight gain and an increase in abdominal fat.Citation138
Perna et alCitation59 reported that sarcopenic visceral obesity is a phenotype that seems to be linked to inflammation, higher risk of fractures, and worse metabolic pattern. SO is associated with increased levels of serum hs-CRP among males,Citation58 and an increase in MCP-1 levels in the serum indicates a pro-inflammatory state. SO is linked to several loci, including those in the protein tyrosine phosphatase receptor type D(PTPRD), cyclin-dependent kinase 14 (CDK14), and inner mitochondrial membrane peptidase 2-like (IMMP2L) genes.Citation7 Pro-inflammatory cytokines, including TNF-α, IL-6, IL-1, MCP-1, and fetuin-A (FetA), are secreted more frequently when there is an increase in fat tissue or when macrophages invade adipocytes.Citation59 Additionally, adipokines that induce lipotoxicity in skeletal muscle cells are produced by adipose tissue, which contributes to the pathophysiology of sarcopenia.Citation139 An imbalance between pro-inflammatory adipokines and anti-inflammatory myokines is caused by the transition of adipose tissue from subcutaneous to visceral adipose sites, as well as skeletal muscle atrophy during the aging process.Citation140 Furthermore, as people age, their adipocyte hormone leptin production increases, which can result in resistance to leptin, impaired fatty acid oxidation in the muscles, ectopic fat deposition in these tissues, and muscular atrophy.Citation60,Citation61
Dynamic Nature of Weight-Metabolic Phenotypes
The dynamic and ever-changing characteristics of metabolic weight phenotypes make it difficult to predict outcomes. An individual’s health state can transition from metabolically healthy to metabolically unhealthy, for example, from NWO to MONW or MHO to MAO. MHO, NWO, MUO, and MONW may transform into SO as people age. A third to 50% of individuals with MHO eventually reach an unhealthy metabolic state.Citation61,Citation141–143 Additionally, these results imply that metabolic phenotypes of weight are dynamic phenomena that need to be monitored over time.
Overweight or Obese and MAFLD
Given the worldwide epidemic incidence of MAFLDCitation144 and obesity,Citation145 it is necessary to clarify the pathophysiological relationship between these two conditionsCitation146 []. The cliche “fat people have fatty livers” does not explain how anything comes about.
Association Between MHO and MAFLD
Currently, there is no understanding of how MHO affects MAFLD risk. However, according to recent epidemiological research, MHO can be significantly linked to a higher chance of developing MAFLD.Citation62,Citation63,Citation147 For instance, a study of 270 individuals with MHO who underwent bariatric surgery found that 35.5% (96/270) of obese individuals had NAFLD, 8.2% (22/270) had NASH, and 4.4% (12/270) had liver fibrosis.Citation62 Sung et al found that 45% of MHO individuals had ultrasonically defined MAFLD in a large cohort survey of South Koreans.Citation63 Chang et al discovered that an increase in BMI was independently related to an increased incidence of MAFLD at a mean follow-up of 4.5 years in a cohort of 77,425 South Koreans who were metabolically healthy and free from MAFLD at baseline.Citation147
Association Between MUO and MAFLD
Approximately 90%-95% of individuals with extreme obesity and associated MetS characteristics have imaging-defined MAFLD, and more than a third of these patients develop histological NASH.Citation64 Increased BMI and waist measurements are linked not only to MAFLD, but also to a higher risk of liver disease progression, especially in elderly individuals.Citation148 This may be due in part to the fact that visceral fat has a stronger correlation with MAFLD than subcutaneous fat does.Citation31 Visceral adipose tissue differs from subcutaneous fat in that it releases more pro-inflammatory and pro-fibrogenic mediators, has greater lipolytic rates, and is associated with increased insulin resistance. These factors may contribute to MAFLD development and progression.Citation31 All of these processes have demonstrated that one of the most significant risk factors for developing more severe types of MAFLD is the presence of expanded and inflamed (dysfunctional)visceral adipose tissue.Citation68,Citation69 Patients with “cryptogenic” cirrhosis also frequently exhibit MetS characteristics, indicating that “burned-out” NASH may account for the majority of these instances.Citation65
The Correlation Factors to MUO and MAFLD
In addition to aggravating liver/systemic insulin resistance and predisposing to dyslipidemia, MAFLD releases several pro-inflammatory and vasoactive mediators that may facilitate the emergence of cardiometabolic problems linked to obesity.Citation2,Citation66,Citation67 Individuals who are fat may acquire metabolic irregularities as a result of certain foods. For instance, in obese individuals, dietary fructose intake promotes de novo lipogenesis, encourages atherogenic dyslipidemia, exacerbates IR, and increases visceral adiposity.Citation70 Drinking sweetened drinks increases the risk of MAFLD in overweight or obese individualsCitation71 Cardio-respiratory fitness and MAFLD are connected to one another.Citation72 According to Argo et al,Citation149 patients with NASH have aerobic power and capacity comparable to that of sedentary control subjects.
Elevated serum uric acid (SUA) levels are a novel risk factor for MAFLD.Citation73 10,000 Chinese people participated in the study by Zhang et al showed that obesity and high SUA levels both enhance the risk of MAFLD and hypertriglyceridemia.Citation150 There is evidence that some genetic variants associated with MAFLD interact with obesity. In particular, a significant interaction effect between the polymorphism patatin-like phospholipase domain-containing protein 3 (PNPLA3) rs738409 and obesity was identified.Citation28,Citation74,Citation151 A worldwide cohort study by Stender et al revealed that PNPLA3 G/G genotype carriers had a risk of cirrhosis that was roughly six times greater if they were obese compared to PNPLA3 C/G or C/C genotypes.Citation74
MAFLD and the Dynamic Change from MHO to MUO
In MHO and MUO patients during a median follow-up of 7.7 years, Kim et al observed that BMI was independently associated with deteriorating liver fibrosis. They also discovered that 70% of those with MHO developed MUO during follow-up, indicating that their metabolic health status was not static.Citation152 NASH and severe fibrosis appear to be substantially less common in patients with MHO than in those with MAO.Citation153,Citation154 Collectively, the available data indicate that patients with MHO are more likely to develop MAFLD and liver disease than individuals with normal weight who are metabolically healthy(NWMH). However, this risk is often lower than that in MUO patients. A significant effort should be made to discover MAFLD in all obese individuals since MHO is not a stable condition, and MAFLD can predict the shift from MHO to MUO [].
The Sarcopenia and MAFLD
The prevalence of sarcopenia in individuals with MAFLD ranges from 12.2% to 43.6%, which is substantially greater than that in patients without MAFLD, which ranges from 8% to 9.7%.Citation75 Skeletal muscle mass was negatively linked with the occurrence of MAFLDCitation155,Citation156 and positively associated with MAFLD resolution in two retrospective cohort investigations.Citation156 The degree of steatosis or fibrosis associated with MAFLD and sarcopenia was independently correlated according to further cross-sectional and retrospective investigations.Citation157–159 A greater risk of developing severe MAFLD was linked to reduced muscle mass and grip strength in another prospective trial with a 10-year median follow-up period.Citation160
The SO and MAFLD have been used in several studies. In a retrospective multicenter study involving MAFLD participants, the frequency of SO was 5.4% (1297/23,889).Citation76 Compared to the two components (sarcopenia and obesity) alone, surrogate indicators of sarcopenic obesity were separately linked to a greater risk of NAFLD,Citation77–79 NASH,Citation77,Citation78 and severe fibrosis.Citation77,Citation79 Chun et alCitation76 validated a model of high-risk and low-risk SO, and discovered that high-risk individuals had a markedly increased risk of severe hepatic fibrosis or atherosclerotic cardiovascular disease (ASCVD). After a median follow-up of three years, high-risk individuals had significantly higher cumulative incidences of significant liver fibrosis, CVD, cirrhosis, and all-cause mortality.Citation76
Normal-Weight and MAFLD
A significant portion of all MAFLD cases worldwide, between 10% and 20%, occur in the normal-weight population.Citation161 The so-called “lean” form of MAFLD (BMI within the ethnicity limit of 25 kg/m2 for Caucasian individuals and 23 kg/m2 for Asian subjects) can manifest even in the absence of obesity. With the exception of liver steatosis caused by a monogenic illness, the majority of lean MAFLD patients have visceral adiposity and insulin resistance, but a normal BMI. These patients may be lean at BMI thresholds, but obese based on waist circumference or other body composition measurements.Citation162 This subtype of MAFLD is likely caused by high calorie consumption, mainly from single carbohydrates, and a sedentary lifestyle, which leads to liver steatosis and lipotoxicity. The pathophysiology of this MAFLD subtype is similar to that of overweight and obese individualsCitation163 [, ].
Epidemiology
According to epidemiological research, lean individuals with MAFLD are less likely to exhibit metabolic abnormalities than overweight or obese patients, and are more likely to be male, older, and have larger waist circumferences.Citation164 A prevalence of between 5% and 26% has been reported for MAFLD in individuals of normal weight, accounting for 15%-50% of all instances of the disease. For instance, a study of 810 Chinese adults with normal weight found that 17.5% had MAFLD.Citation165 Lean MAFLD was present in 5.1% of the general population and 19.2% of the world’s MAFLD population, according to a meta-analysis of 93 different studies.Citation80 Overall, MAFLD prevalence among the normal-weight population was 12% in Asia, 10.2% in the Middle East, and 9.2% in Europe.Citation16
Lean MAFLD Disease Severity and Long-Term Prognosis
Data on the long-term prognosis of MAFLD in a population with normal weight are scarce and conflicting. In comparison to obese and MAFLD patients, a study of Swedish MAFLD patients found a 2.69-fold increased chance of developing severe liver disease but no increase in mortality in MAFLD patients with normal weight.Citation166 Similar to other studies, which similarly found no difference in survival between patients with lean MAFLD and those without it.Citation167 According to a meta-analysis encompassing 35,707 individuals, patients with lean MAFLD had higher overall, liver-specific, and cardiovascular mortality than those with obesity and MAFLD.Citation80 Those with lean MAFLD had greater all-cause mortality than those with non-lean MAFLD.Citation81 According to a previous study, individuals with lean MAFLD had milder clinical events and prognoses than those with obesity and MAFLD, and they also experienced fewer cardiovascular events and fatalities.Citation82,Citation83 MAFLD patients with normal weights had considerably lower rates of metabolic abnormalities, cirrhosis, and cardiovascular disease than non-lean participants.Citation84,Citation85
Multiple extrahepatic symptoms are associated with an elevated risk in patients with normal-weight MAFLD. Lean MAFLD was a substantial risk factor for incident T2DM over a 6-year follow-up median, according to a longitudinal study of 14,482 Chinese people without T2DM (p= 0.001).Citation168 Lean MAFLD individuals may have higher 15-year cumulative all-cause mortality, but there was no difference in cardiovascular or cancer-related mortality compared to obese MAFLD patients according to a real-world study of 4,711 MAFLD patients.Citation169 Additionally, a subanalysis showed that although it was lower than that in patients with non-lean MAFLD, patients with lean MAFLD had significantly higher all-cause (p=0.0002) and cardiovascular mortality (p=0.0004) than those with normal weight and without MAFLD.Citation170 The frequency of T2DM, hypertension, dyslipidemia, and CVD was shown in some other reports to be lower in patients with lean MAFLD than in those with non-lean MAFLD.Citation85,Citation89 In conclusion, patients with lean MAFLD may have a similar prognosis to those with overweight or obese MAFLD in terms of long-term outcomes compared to healthy individuals.
Histological Characteristics
The metabolic profile of IR in the main target tissues (muscle, liver, and adipose tissue) was not different from that of the sample of 12 lean individuals with biopsy-proven MAFLD compared to obese patients.Citation171 The morphological characteristics of MAFLD in individuals of normal weight are thought to be indistinguishable from those of MAFLD.Citation89 Those with lean MAFLD had better histological and metabolic symptoms than those with obesity. According to a study of 1339 patients, patients with normal weight were histologically less severe. Compared to overweight individuals, obese patients had a reduced prevalence of T2DM (9.2% vs 31.4%), steatohepatitis (54.1% vs 71.2%), and advanced fibrosis (10.1% vs 25.2%).Citation167 According to a meta-analysis of eight studies involving 1,441 individuals, patients with lean MAFLD had significantly less pathological steatohepatitis (39% vs 52.9%) and significant fibrosis (29.2% vs 38.3%) than obese patients with MAFLD.Citation80 However, other cross-sectional investigations showed that patients with lean MAFLD had worse liver histology than those with non-lean MAFLD, with greater proportions of advanced fibrosis, blossoming, lobular inflammation, and steatohepatitis.Citation172,Citation173
The Risk Correlation to MUNW and Lean MAFLD
The proportion of patients with MUNW ranged from 21% to 43.6% in the multi-ethnic atherosclerotic investigation, while the prevalence of lean MAFLD (BMI<30 kg/m2), as determined by computed tomography, was 11%.Citation174 The significant incidence of metabolic problems among lean MAFLD patients indicates that independent determinants of metabolic health may include food quality, in addition to overall calorie intake.Citation86 Determining metabolic health, for instance involves pro-inflammatory foods or eating habits with a strong pro-inflammatory profile.Citation36 Another study found that compared to healthy individuals of normal weight, those with metabolic obesity ingested more total energy, less fiber, lower levels of antioxidant chemicals, and fewer servings of fruit, legumes, nuts, and seeds.Citation87 Notably, patients with lean MAFLD have been shown to consume more cholesterol than those with obesity and MAFLD.Citation88 Higher dietary inflammatory index scores have been linked to inflammatory indicators in the serum, namely C-responsive proteins, according to several studies.Citation175 According to a microbiome study, individuals with lean MAFLD may have a microbiota that is more enriched than that of patients with obesity and MAFLD (such as Erysipelotrichaceae and Clostridiales), which is thought to contribute to the development of hepatic steatosis. There was a decrease in the Marvinbryantia and Christensellenaceae R7 groups and an increase in the Dorea spp in the lean MAFLD group compared with healthy individuals of normal weight.Citation89
Total Fat Mass and Regional Fat Accumulation
According to new research, the formation of a metabolically healthy phenotype versus an unhealthy phenotype is influenced by differences in total body fat and regional fat accumulation. Peripheral fat has a limited ability to store fat such as SAT, which has little metabolic impact. In the context of overeating, ectopic fat deposits in tissues, including the liver and skeletal muscle, increase the risk of CVD.Citation92 Ectopic fat is thought to be essential for the development of IR and lipotoxicity in humans, and is thought to have a more direct impact on the metabolic effects of obesity.Citation92 The distribution of body fat is likely governed by genetic factors, and numerous studies have discovered variants such as dendrocyte expressed seven transmembrane protein domain-containing 2 (DCST2) rs905938 and the golgin RAB6-interacting (GORAB) rs10919388.Citation90,Citation91 When compared to the healthy range, genome-wide association studies (GWAS)of up to 188,577 individuals found 53 loci [such as lethal(3)malignant brain tumor-like protein 3 (L3MBTL3), dynein axonemal heavy chain 10 (DNAH10), and coiled-coil domain containing 92 (CCDC92)] that were associated with a higher risk of cardiometabolic disease, lower peripheral fat, and increased IR (higher fasting insulin, higher triglyceride levels, and lower HDL cholesterol levels).Citation92 Other research have identified advantageous fat genes [(such as peroxisome proliferator-activated receptor gamma (PPARG) and lysophospholipase-like 1 (LYPLAL1)] that are linked to increased SAT but a decreased risk of heart disease, T2DM, liver fat, and hypertension.Citation176,Citation177
Pathophysiology of Lean MAFLD Genetic Contribution
The factors associated with lean MAFLD remain unknown despite the effectiveness of GWAS in identifying genetic loci linked to the risk of MAFLD development and progression.Citation178 Another study using whole-exome sequencing connected lean MAFLD to a phosphatidylethanolamine N-methyltransferase variety.Citation179 Other studies have linked the PNPLA3 risk allele to higher levels of fibrosis (stage 2 or greater) and the onset of steatohepatitis in patients with MAFLD of normal weight.Citation93,Citation94 The transmembrane 6 superfamily member 2 (TM6SF2) gene showed increased rates of transport of rs58542926 C>T in studies comparing MAFLD patients with normal weight and obese patients.Citation95,Citation96 Patients with normal weight had a significant independent correlation between the Interferon lambda 4(IFNL4) genotype rs368234815 TT allele and severe fibrosis (P=0.02) but not in obese patients (P=0.15).Citation97 Although understudied, the human epigenome offers crucial information for understanding the fundamentals of gene-environment interactions to clarify the pathophysiology of MAFLD in individuals of normal weight.
MAFLD and T2DM
Two pathologic diseases, MAFLD and T2DM, typically coexist and work in concert to increase the risk of unfavorable hepatic and extrahepatic outcomesCitation180,Citation181 [, ]. According to a meta-analysis of observational research from 20 different countries, over 56% of individuals with T2DM worldwide have MAFLD.Citation98 T2DM is also known to increase the risk of NAFLD, progressing more quickly to cirrhosis, NASH, or hepatocellular carcinoma (HCC). However, the association between NAFLD and T2DM is more complicated than previously believed and appears to be bilateral.Citation66,Citation182 Indeed, increasing evidence suggests that NAFLD could lead to and/or encourage the development of T2DM and that the likelihood of acquiring T2DM correlates with the severity of NAFLD.Citation183 Given the same Pathobiology of T2DM and MAFLD, both conditions coexist in many patients and may worsen the outcomes of underlying diseases with accelerated development and higher comorbidities.Citation184
MAFLD is Predictive of T2DM
Numerous cohort studies conducted over the past ten years have consistently demonstrated that MAFLD can predict incident T2DM. A meta-analysis involving 501,022 people found that MAFLD was linked to a doubling of the chance of developing T2DM and that this risk seemed to increase as hepatic steatosis and fibrosis severity increased.Citation185 Morrison et al supported the idea that there is a causal relationship between MAFLD and T2DM by confirming that individuals with MAFLD have a higher chance of developing T2DM than those without it.Citation186
Is the increased risk of T2DM caused by MAFLD present only in patients with advanced MAFLD, or does it affect all MAFLD patients? The magnitude of T2DM risk is clearly correlated with the severity of MAFLD and specifically the severity of liver fibrosis, according to recent data from a complete meta-analysis.Citation185 In particular, a retrospective cohort analysis of 396 Swedish MAFLD patients found that during the course of a mean follow-up of 18.4 years, more patients with fibrosis stage 3 than those with fibrosis stages 0–2 (51% versus 31%) acquired incident T2DM.Citation99 It is important to note that a rising amount of steatosis was likewise connected to the T2DM event in patients with 0–2 fibrosis.Citation99 In a different cohort study of 129 Swedish individuals with MAFLD, Nasr et al Incident T2DM occurred in 69 patients (53.5% of the total), and this risk was significantly higher in those who developed fibrosis over time.Citation187 The authors demonstrated that liver fat levels predicted the likelihood of T2DM in the same group.Citation188 Additionally, there was a link between a lower risk of T2DM and decreased liver fat between the baseline and the first follow-up.Citation188 According to a number of observational cohort studies, temporal variations in MAFLD status of MAFLD were linked to significantly varied T2DM risks. More importantly, regardless of changes in body weight, the likelihood of a T2DM event seemed to diminish over time when MAFLD was improved or resolved.Citation189–191 For instance, over a five-year period, independent of known risk variables, a significant decline in the probability of T2DM was observed in subjects with reduced liver steatosis.Citation192
Cluster Analysis of the Genotyping Array Study
Cluster analysis of the German Diabetes Study revealed that the subtype of diabetes is more likely to be insulin-resistant, and to a lesser extent, that particular subtypes associated with obesity and age showed noticeably greater levels of liver fat and noninvasive fibrosis biomarkers upon diagnosis of T2DM.Citation193 This discovery emphasizes the significance of the interplay between IR and lipid metabolism in the liver during T2DM onset. The rs738409(G) polymorphism of PNPLA3 was more common in the severe insulin-resistant diabetes subtype, and this genetic variation was also associated with higher IR in adipose tissue.Citation193,Citation194 Mendelian randomization studies have provided additional evidence that genetically induced MAFLD increases the risk of developing insulin resistance and new-onset T2DM (PNPLA3, TM6SF2, and other MAFLD-related genetic variations).Citation28,Citation100,Citation101 Increased levels of hepatic fat are linked to an increased risk of incident T2DM according to a significant exam-based genotyping study.Citation151
Mechanisms Linking MAFLD and T2DM
The specific aspects of MAFLD that increase the risk of T2DM are unclear. However, it is widely known that lipid accumulation in the liver is associated with both hepatic IR and inflammation, both of which are important aspects of MAFLD. Therefore, interventions that improve IR and chronic inflammation in MAFLD may help to lower the risk of T2DM by reducing hepatic lipid accumulation. [] The most prevalent plasma lipid abnormality in MAFLD is atherogenic dyslipidemia, which is often characterized by elevated levels of very low-density lipoprotein (VLDL), small dense LDLs, and decreased levels of HDL cholesterol.Citation102 The levels of VLDL1-triglycerides and VLDL1-palmitic acid increased more in individuals whose diabetes (which had been put into remission by weight loss) relapsed than in those whose diabetes did not relapse, which is an intriguing finding that suggests that the increase in VLDL may further enhance the risk of T2DM with MAFLD.Citation195 Additionally, these individuals had higher intra-pancreatic fat levels and no longer responded to glucose challenge with first-phase insulin.Citation195 It is not certain, nevertheless, that variations in VLDLs linked to MAFLD directly affect the incidence of T2DM.
As previously mentioned, throughout the spectrum of liver disease in MAFLD, an elevated risk of T2DM appears to occur early (with fat accumulation in the liver) and late (with inflammation and liver fibrosis). Strong data to date point to an increased risk of T2DM and hepatic, adipose tissue, and muscle insulin resistance related to the accumulation of lipids in the liver [].Citation180,Citation192,Citation196 To further support this notion, a decline in liver lipid accumulation has also been linked to a lower likelihood of developing T2DM.Citation192 A number of mechanisms have been proposed, including those linked to increased liver lipid accumulation, such as dietary components (for example, saturated fat and carbohydrate intake), changes in gut microbiota, and elements related to intestinal function (for example, bile acid metabolism, levels of lipopolysaccharide and incretins, or altered intestinal permeability).Citation16 However, the precise factors of MAFLD that may increase the risk of T2DM remain unknown. Factors associated with the accumulation of lipid metabolites in the liver, mitochondrial oxidative capacity, and lipoprotein secretion are anticipated to negatively impact T2DM risk in the early stages of liver disease. However, variables including lipid metabolites, pro-inflammatory cytokines, and hepatochemicals are most likely to be important pathogenic factors in the latter stages of liver disease, when the liver has already begun to swell and develop fibrosisCitation196,Citation197 [].
The Treatment for Obesity and MAFLD
In general, attempts to reduce weight through food and exercise result in 30% of patients losing more than 5% of their total body weight loss (TBWL) within 6 to 12 months.Citation198 However, the effectiveness of intensive lifestyle interventions has been limited. The use of second-level therapies for treating obesity, such as anti-obesity pharmacotherapy, bariatric endoscopy, and surgery for all patients with obesity or obesity-related comorbidities who do not successfully lose weight through lifestyle changes alone, must be increased to achieve meaningful weight loss and prevent weight regainCitation199 [].
Lifestyle Modifications
Changes in lifestyle, including food intake, activity, exercise, and weight loss, are the main treatments for MAFLD.Citation200,Citation201 According to several studies, weight loss in MAFLD patients reduces liver triglyceride and NAS levels while lowering cardiovascular risk markers such as IR and serum lipid concentration.Citation104 Musso et al found that a weight loss of 7% resulted in significant improvements in histological results and cardiometabolic profile; therefore, it is advised that the weight loss objective for NASH should be close to 10%.Citation202 Vilar-Gomez et al also discovered that weight loss improved the NASH-related histological parameters and was an independent factor (p<0.01).Citation203 A Meta by Haigh found that the degree of calorie restriction had a dose-response relationship with favorable effects on liver function and weight reduction, indicating that this strategy should continue to be the cornerstone of MAFLD diet treatment.Citation103 Ryan et al investigated the impact of the Mediterranean diet (Med Diet) in comparison to a low-fat, high-carbohydrate diet (LF-HCD) and discovered that while there was no significant difference in weight reduction between the two dietary groups, there was a substantial difference in MAFLD and insulin sensitivity.Citation105
A thorough lifestyle intervention program should include increasing aerobic physical activity by 150 min each week, in addition to a diet with fewer calories. With a target of more than 10,000 steps per day (as fast walking for 30 min each day on most days of the week). In the long term (more than a year), higher amounts of physical activity between 200 and 300 minutes per week are advised to sustain weight loss or prevent weight gain.Citation106 Recommendations for diet and exercise offer well-structured behavioral techniques to help with diet and exercise compliance.Citation204
Anti-Obesity Pharmacotherapy
The Food and Drug Administration (FDA) approved Anti-obesity medications (AOMs) for use in patients with a body mass index (BMI) >30 kg/m2 or those with a BMI >27 kg/m2 and one or more obesity-related comorbidities such as T2DM, hypertension, and dyslipidemia as the next line of treatment for obesity or obesity-related liver diseases.Citation205 AMOs currently approved for long-term use include Bupropion/naltrexone, Orlistat, Liraglutide, Semaglutide, and Phentermine/topiramate.Citation206 However, only Orlistat and Liraglutide have been thoroughly investigated for liver illness. Clinical investigations have included a wide range of novel therapeutic targets and prospective medications for the treatment of obesity and MAFLD; however, the results and efficacy have not yet been validated.Citation207–210
Glucagon-like peptide-1 (GLP-1)is an incretin hormone that originates from the gut. Additionally, it reduces caloric intake and stomach emptying while increasing insulin release through beta cells. According to one study, liraglutide completely resolved NASH without increasing fibrosis, and improved steatosis.Citation107 GLP-1 analogs increase insulin sensitivity and decrease body weight. A similar long-acting GLP-1 receptor agonist, semiglutide, demonstrated a substantial decrease in average body weight and improvement in NASH in a double-blind randomized controlled trial (RCT); however, the improvement in fibrosis stage was not significant.Citation108 These results support the use of Liraglutide or other GLP-1 mimics as desirable medications for patients with NASH.
By establishing covalent connections with serum residues from active lipase sites, orlistat locally inhibits stomach and pancreatic lipases, rendering them inactive. The use of orlistat improved all liver enzymes (ALT and AST) and liver fat content (LFC) based on liver histology, according to numerous studies that examined the impact of orlistat use and dietary changes on MAFLD.Citation199 Both the absolute drop in LFC in the Orlistat group and the experimental diet group were higher than those in the control group (P<0.05), at 9.1% and 5.4%, respectively.Citation110 One review, which included 330 individuals with NAFLD or NASH, discovered that orlistat improved the levels of ALT, AST, gamma-glutamyl transferase (GGT), glucose, triglycerides, HOMA-IR, and BMI, but not the liver fibrosis score (SMD=−0.14; P=0.7). Patients with NASH showed no discernible changes in the subanalyses of various patient categories.Citation111 However, no statistically significant difference in weight loss was identified between the orlistat/diet/vitamin E group and diet/vitamin E group in a RCT conducted by Harrison et al to evaluate the effectiveness of 120 mg of orlistat three times a day (TID) in treating NASH in patients, which suggests that orlistat improvement in MAFLD could be a result of weight reduction.Citation109
Bariatric Endoscopic Interventions
Endoscopic bariatric therapy (EBT), a recently developed safe alternative to more invasive and traditional bariatric operations, helps patients lose weight, especially those with mild to moderate obesity (BMI of 30–40 kg/m2) who do not have comorbid conditions and have tried unsuccessfully to lose weight through lifestyle and medication changes.Citation211 Theoretically, EBT may be among the safest, least intrusive, and most efficient treatments for MAFLD.
Intragastric balloon (IGB) is an endoscopic space occupancy procedure that promotes weight loss by decreasing the feeling of hunger before meals, enhancing satiety in the stomach, and delaying gastric emptying to improve postprandial satiety.Citation112 Apollo Endosurgery was granted permission by the US FDA to utilize Orbera in the treatment of patients with a BMI between 30 and 40 kg/m2 and non-cirrhotic NASH with hepatic fibrosis in March 2021.Citation212 In 21 patients with MAFLD, Bazerbachi et al evaluated the impact of single-fluid-filled IGB on the metabolic and histological features of NASH. After six months of IGB, the median initial TBWL was 11.7%, and the mean NAFLD Activity Score (NAS) considerably improved in 90% of patients (18/20), with a median decrease of 3 points, and 15% of participants experienced liver regression in fibrosis at 1.17 stages.Citation113 Another meta-analysis also found an average reduction in participant body weight of 11.9 kg, with a significant histological improvement (p=0.03) in the NAS.Citation114 Additionally, a systematic study by Candlean et al revealed that 83.5% of patients’ NAS improved, while 79.2% of patients’ steatosis improved.Citation115 These findings point to a bright future for the use of IGB in the treatment of NASH and MAFLD.
Endoscopic gastroplasty (ESG) is a minimally invasive bariatric endoscopic technique that involves reshaping of the greater curvature without making an incision.Citation116 ESG not only achieves substantial weight loss but also has strong potential to improve MAFLD and steatohepatitis. In a study of 118 ESG patients with liver steatosis, Hajifathalian et al discovered a significant reduction in NAS (p=0.034), and 20% of the patients had liver fibrosis stage F3-F4 to F0-F2 (p=0.02).Citation117
The duodenum is considered the target organ for weight loss. By modifying, eliminating, or omitting duodenal exposure to intraluminal nutrients, favorable metabolic effects were observed.Citation118 The process known as endoscopic duodenal mucosal resurfacing (DMR) is regarded as being minimally invasive. Oliveira et al performed a meta-analysis to investigate the effectiveness of DMR in metabolic enhancement. After three months of DMR, Glycosylated Hemoglobin, Type A1C (HbA1C) levels dramatically decreased by 1.72% (p=0.02), and the average weight decreased by 3.1 kg (p<0.001). Liver enzymes (ALT) and liver steatosis visible on magnetic resonance imaging(MRI) were both dramatically decreased (p<0.001).Citation119 These results lend credence to the effectiveness of the DMR method in resolving MAFLD. This may indicate a different mechanism by which the duodenal mucosa and released incretine hormones contribute to the onset of MAFLD.
Bariatric Surgical Intervention
Many studies have been conducted on how bariatric and metabolic surgeries affect long-term weight loss and improve obesity-related comorbidities.Citation213 Patients with a severe BMI of 40 kg/m2 or 35 kg/m2 and obesity-related comorbidities should undergo these procedures.Citation199 According to a long-term prospective study, at the 5-year follow-up, fibrosis regressed in 70% of patients (p<0.001) and resolved in 84% of patients with NASH due to bariatric surgery (p<0.001).Citation214
Restrictive bariatric treatment, known as sleeve gastrectomy (SG), entails resecting two-thirds of the stomach’s larger curvature and creating a long tubular gastric duct along the lower curvature.Citation120 When compared with Roux-en-Y Gastric Bypass (RYGB), Baldwin et al discovered that patients who underwent SG showed a substantial reduction in NAS of 2.3 (p<0.00001). Regarding NAFLD Fibrosis Score (NFS) reduction, there was a statistically significant difference between the two methods (p<0.00001): LSG had a mean reduction of 0.7 (p=0.07), whereas RYGB had a decrease of 1.0.Citation121 In a prospective study of 94 obese participants, SG caused statistically significant weight loss and a substantial decrease in BMI from 44. 54±5. 45 kg/m2 to 34. 23±2. 66 kg/m2 (p< 0.001) in the first year following surgery. In addition, the NAS score dramatically decreased over the course of a year, falling from 5.2±1. 96 to 2.63±1.55.Citation122 These results confirmed the effectiveness of SG in managing obesity complicated by MAFLD.
Surgery called the Roux-en-Y Gastric Bypass (RYGB) has both malabsorptive and restrictive effects. By joining a section of the small intestine to a smaller stomach pouch in the shape of the letter “Y”, it is regarded as a gastrointestinal reconstruction technique.Citation123 According to Fakhry et al, 91, 60, and 31% of all RYGB candidates had improved or fully resolved steatosis, steatohepatitis, and fibrosis, respectively.Citation215 Baldwins et al’s meta-analysis revealed that after RYGB surgery, the NAS and NFS were significantly reduced by −2.8 (p<0.00001) and −1.0 (p<0.00001), respectively.Citation121 In contrast, there was little difference between RYGB and SG in the head-to-head comparison of NAS, preferring RYGB outcomes. Overall, their research found that there was no clear advantage between the two types of operations and that both RYGB and SG had a favorable effect on the liver profile.Citation121
Conclusion
Compared to MAFLD, which develops in the context of overweight and obesity, some individuals are normal weight, or have T2DM. There are many unknowns in our understanding of disease progression. The answers to these questions will inform the development and mapping of effective preventive and therapeutic approaches for people with metabolic comorbidities. Since obesity and IR are correlated with MAFLD, this review shows a wide range of metabolic phenotypes of obesity, which are more likely to distinguish different types of MAFLD depending on patient characteristics.
This review provides a systematic introduction to the treatment of obesity and MAFLD. It includes diet, exercise, drugs for obesity, and endoscopic and surgical bariatric procedures. Drugs and Bariatric procedures provide more lasting weight loss, which was evident in some improvements in the MAFLD activity score and fibrosis. Although further research is needed to draw a definitive conclusion, the data presented above have significant implications for public health and clinical practice decision-making. This highlights the urgent need to develop effective treatments for MAFLD to reduce the risk of comorbidities and extra-liver complications.
Disclosure
The authors declare that they have no conflict of interest.
Additional information
Funding
References
- Bray GA. Evaluation of obesity. Who are the obese? Postgrad Med. 2003;114(6):19–27, 38. doi:10.3810/pgm.2003.12.1544
- Gutiérrez-Cuevas J, Santos A, Armendariz-Borunda J. Pathophysiological molecular mechanisms of obesity: a link between MAFLD and NASH with cardiovascular diseases. Int J Mol Sci. 2021;22(21):11629. PMID: 34769060. doi:10.3390/ijms222111629
- Gutiérrez-Cuevas J, Sandoval-Rodriguez A, Meza-Rios A, et al. Molecular mechanisms of obesity-linked cardiac dysfunction: an up-date on current knowledge. Cells. 2021;10(3):629. PMID: 33809061. doi:10.3390/cells10030629
- Boccatonda A, Andreetto L, D’Ardes D, et al. From NAFLD to MAFLD: definition, pathophysiological basis and cardiovascular implications. Biomedicines. 2023;11(3):883. doi:10.3390/biomedicines11030883
- Physical status: the use and interpretation of anthropometry. Report of a WHO expert committee. World Health Organ Tech Rep Ser. 1995;854:1–452.
- Li Z, Daniel S, Fujioka K, et al. Obesity among Asian American people in the United States: a review. Obesity. 2023;31(2):316–328. doi:10.1002/oby.23639
- Mayoral L-C, Andrade G, Mayoral E-C, et al. Obesity subtypes, related biomarkers & heterogeneity. Indian J Med Res. 2020;151(1):11–21. doi:10.4103/ijmr.IJMR_1768_17
- Blüher M, Aras M, Aronne LJ, et al. New insights into the treatment of obesity. Diabetes Obes Metab. 2023;25(8):2058–2072. PMID: 37055715. doi:10.1111/dom.15077
- Tanase DM, Gosav EM, Costea CF, et al. The intricate relationship between type 2 diabetes mellitus (T2DM), insulin resistance (IR), and nonalcoholic fatty liver disease (NAFLD). J Diabetes Res. 2020;2020:3920196. PMID: 32832560. doi:10.1155/2020/3920196
- International Diabetes Federation. IDF DIABETES ATLAS −9th EDITION; April, 2020.
- Strey CBM, de Carli LA, Pioner SR, et al. Impact of diabetes mellitus and insulin on nonalcoholic fatty liver disease in the morbidly obese. Ann Hepatol. 2018;17(4):585–591. doi:10.5604/01.3001.0012.0922
- En Li Cho E, Ang CZ, Quek J, et al. Global prevalence of non-alcoholic fatty liver disease in type 2 diabetes mellitus: an updated systematic review and meta-analysis. Gut. 2023;72(11):2138–2148. PMID: 37491159. doi:10.1136/gutjnl-2023-330110
- Eslam M, Newsome PN, Sarin SK, et al. A new definition for metabolic dysfunction-associated fatty liver disease: an international expert consensus statement. J Hepatol. 2020;73:202–209. doi:10.1016/j.jhep.2020.03.039
- Eslam M, Sarin SK, Wong VW, et al. The Asian Pacific association for the study of the liver clinical practice guidelines for the diagnosis and management of metabolic associated fatty liver disease. Hepatol Int. 2020;14:889–919. doi:10.1007/s12072-020-10094-2
- Rinella ME, Lazarus JV, Ratziu V, et al. A multi-society Delphi consensus statement on new fatty liver disease nomenclature. Ann Hepatol. 2023;101133:101133. doi:10.1016/j.aohep.2023.101133
- Eslam M, El-Serag HB, Francque S, et al. Metabolic (dysfunction)-associated fatty liver disease in individuals of normal weight. Nat Rev Gastroenterol Hepatol. 2022;19(10):638–651. doi:10.1038/s41575-022-00635-5
- Chalasani N, Younossi Z, Lavine JE, et al. The diagnosis and management of nonalcoholic fatty liver disease: practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2018;67(1):328–357. doi:10.1002/hep.29367
- Targher G. Concordance of MAFLD and NAFLD diagnostic criteria in “real-world” data. Liver Int. 2020;40:2879–2880. doi:10.1111/liv.14623
- Ayada I, van Kleef LA, Alferink LJM, et al. Systematically comparing epidemiological and clinical features of MAFLD and NAFLD by meta‐analysis: focusing on the non‐overlap groups. Liver Int. 2021;42(2):277–287. doi:10.1111/liv.15139
- Li J, Zou B, Yeo YH, et al. Prevalence, incidence, and outcome of non-alcoholic fatty liver disease in Asia, 1999-2019: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2019;4(5):389–398. PMID: 30902670. doi:10.1016/S2468-1253(19)30039-1
- Liu J, Mu C, Li K, Luo H, Liu Y, Li Z. Estimating global prevalence of metabolic dysfunction-associated fatty liver disease in overweight or obese children and adolescents: systematic review and meta-analysis. Int J Public Health. 2021;66:1604371. PMID: 34690666. doi:10.3389/ijph.2021.1604371
- Liu J, Ayada I, Zhang X, et al. Estimating global prevalence of metabolic dysfunction-associated fatty liver disease in overweight or obese adults. Clin Gastroenterol Hepatol. 2022;20(3):e573–e582. PMID: 33618024. doi:10.1016/j.cgh.2021.02.030
- Wu X, Cheung CKY, Ye D, et al. Serum thrombospondin-2 levels are closely associated with the severity of metabolic syndrome and metabolic associated fatty liver disease. J Clin Endocrinol Metab. 2022;107(8):e3230–e3240. PMID: 35532410. doi:10.1210/clinem/dgac292
- Moreno-Vedia J, Girona J, Ibarretxe D, Masana L, Rodríguez-Calvo R. Unveiling the role of the fatty acid binding protein 4 in the metabolic-associated fatty liver disease. Biomedicines. 2022;10(1):197. PMID: 35052876. doi:10.3390/biomedicines10010197
- Yamamura S, Eslam M, Kawaguchi T, et al. MAFLD identifies patients with significant hepatic fibrosis better than NAFLD. Liver Int. 2020;40(12):3018–3030. PMID: 32997882. doi:10.1111/liv.14675
- Ciardullo S, Perseghin G. Prevalence of NAFLD, MAFLD and associated advanced fibrosis in the contemporary United States population. Liver Int. 2021;41(6):1290–1293. PMID: 33590934. doi:10.1111/liv.14828
- Tsutsumi T, Nakano D, Hashida R, et al. The inter-organ crosstalk reveals an inevitable link between MAFLD and extrahepatic diseases. Nutrients. 2023;15(5):1123. doi:10.3390/nu15051123
- Eslam M, George J. Genetic contributions to NAFLD: leveraging shared genetics to uncover systems biology. Nat Rev Gastroenterol Hepatol. 2020;17(1):40–52. doi:10.1038/s41575-019-0212-0
- Di Renzo L, Sarlo F, Petramala L, et al. Association between −308 G/A TNF- α polymorphism and appendicular skeletal muscle mass index as a marker of sarcopenia in normal weight obese syndrome. Dis Markers. 2013;35(6):615–623. doi:10.1155/2013/983424
- Jia A, Xu S, Xing Y, et al. Prevalence and cardiometabolic risks of normal weight obesity in Chinese population: a nationwide study. Nutr Metab Cardiovasc Dis. 2018;28(10):1045–1053. doi:10.1016/j.numecd.2018.06.015
- Lonardo A, Mantovani A, Lugari S, et al. Epidemiology and pathophysiology of the association between NAFLD and metabolically healthy or metabolically unhealthy obesity. Ann Hepatol. 2020;19(4):359–366. doi:10.1016/j.aohep.2020.03.001
- Di Renzo L, Galvano F, Orlandi C, et al. Oxidative stress in normal-weight obese syndrome. Obesity. 2010;18(11):2125–2130. doi:10.1038/oby.2010.50
- Di Renzo L, Del Gobbo V, Bigioni M, Premrov MG, Cianci R, De Lorenzo A. Body composition analyses in normal weight obese women. Eur Rev Med Pharmacol Sci. 2006;10(4):191–196.
- Mathew H, Farr OM, Mantzoros CS. Metabolic health and weight: understanding metabolically unhealthy normal weight or metabolically healthy obese patients. Metabolism. 2016;65(1):73–80. doi:10.1016/j.metabol.2015.10.019
- Højland Ipsen D, Tveden-Nyborg P, Lykkesfeldt J. Normal weight dyslipidemia: is it all about the liver? Obesity. 2016;24(3):556–567. doi:10.1002/oby.21443
- Mannisto S, Harald K, Kontto J, et al. Dietary and lifestyle characteristics associated with normal-weight obesity: the National FINRISK 2007 Study. Br J Nutr. 2014;111(5):887–894. doi:10.1017/S0007114513002742
- Ruderman N, Chisholm D, Pi-Sunyer X, et al. The metabolically obese, normal-weight individual revisited. Diabetes. 1998;47(5):699–713. doi:10.2337/diabetes.47.5.699
- De Lorenzo A, Del Gobbo V, Premrov MG, et al. Normal-weight obese syndrome: early inflammation? Am J Clin Nutr. 2007;85(1):40–45. doi:10.1093/ajcn/85.1.40
- Berg AH, Scherer PE. Adipose tissue, inflammation, and cardiovascular disease. Circ Res. 2005;96(9):939–949. doi:10.1161/01.RES.0000163635.62927.34
- Lee S-H, Han K, Yang HK, et al. A novel criterion for identifying metabolically obese but normal weight individuals using the product of triglycerides and glucose. Nutr Diabetes. 2015;5(4):e149. doi:10.1038/nutd.2014.46
- Brandao I, Martins MJ, Monteiro R. Metabolically healthy obesity-heterogeneity in definitions and unconventional factors. Metabolites. 2020;10(2):48. doi:10.3390/metabo10020048
- Shaharyar S, Roberson LL, Jamal O, et al. Obesity and metabolic phenotypes (metabolically healthy and unhealthy variants) are significantly associated with prevalence of elevated C-reactive protein and hepatic steatosis in a large healthy Brazilian population. J Obes. 2015;2015:178526. doi:10.1155/2015/178526
- Iacobini C, Pugliese G, Blasetti Fantauzzi C, et al. Metabolically healthy versus metabolically unhealthy obesity. Metabolism. 2019;92:51–60. doi:10.1016/j.metabol.2018.11.009
- Munoz-Garach A, Cornejo-Pareja I, Tinahones FJ. Does metabolically healthy obesity exist? Nutrients. 2016;8(6):320. doi:10.3390/nu8060320
- Lin H, Zhang L, Zheng R, et al. The prevalence, metabolic risk and effects of lifestyle intervention for metabolically healthy obesity: a systematic review and meta-analysis: a PRISMA-compliant article. Medicine (Baltimore). 2017;96(47):e8838. doi:10.1097/MD.0000000000008838
- Perreault M, Zulyniak MA, Badoud F, et al. A distinct fatty acid profile underlies the reduced inflammatory state of metabolically healthy obese individuals. PLoS One. 2014;9(2):e88539. doi:10.1371/journal.pone.0088539
- Ferrer R, Pardina E, Rossell J, et al. Morbidly “Healthy” obese are not metabolically healthy but less metabolically imbalanced than those with type 2 diabetes or dyslipidemia. Obes Surg. 2015;25(8):1380–1391. doi:10.1007/s11695-014-1528-z
- Doumatey AP, Zhou J, Zhou M, et al. pro-inflammatory and lipid biomarkers mediate metabolically healthy obesity: a proteomics study. Obesity. 2016;24(6):1257–1265. doi:10.1002/oby.21482
- Tanriover C, Copur S, Gaipov A, et al. Metabolically healthy obesity: misleading phrase or healthy phenotype? Eur J Intern Med. 2023;111:5–20. doi:10.1016/j.ejim.2023.02.025
- De Lorenzo A, Soldati L, Sarlo F, et al. New obesity classification criteria as a tool for bariatric surgery indication. World J Gastroenterol. 2016;22(2):681–703. doi:10.3748/wjg.v22.i2.681
- Dobson R, Burgess MI, Sprung VS, et al. Metabolically healthy and unhealthy obesity: differential effects on myocardial function according to metabolic syndrome, rather than obesity. Int J Obes. 2016;40(1):153–161. doi:10.1038/ijo.2015.151
- Marques-Vidal P, Bochud M, Bastardot F, et al. Association between inflammatory and obesity markers in a Swiss population-based sample (CoLaus Study). Obes Facts. 2012;5(5):734–744. doi:10.1159/000345045
- Mamtani M, Kulkarni H, Dyer TD, et al. Genome- and epigenome-wide association study of hypertriglyceridemic waist in Mexican American families. Clin Epigenetics. 2016;8(1):6. doi:10.1186/s13148-016-0173-x
- Tyrovolas S, Koyanagi A, Olaya B, et al. Factors associated with skeletal muscle mass, sarcopenia, and sarcopenic obesity in older adults: a multi-continent study. J Cachexia Sarcopenia Muscle. 2016;7(3):312–321. doi:10.1002/jcsm.12076
- Abellan van Kan G, Houles M, Vellas B. Identifying sarcopenia. Curr Opin Clin Nutr Metab Care. 2012;15(5):436–441. doi:10.1097/MCO.0b013e328356bbf4
- Perna S, Spadaccini D, Rondanelli M. Sarcopenic obesity: time to target the phenotypes. J Cachexia Sarcopenia Muscle. 2019;10(3):710–711. doi:10.1002/jcsm.12425
- Kim JH, Cho JJ, Park YS. Relationship between sarcopenic obesity and cardiovascular disease risk as estimated by the Framingham risk score. J Korean Med Sci. 2015;30(3):264–271. doi:10.3346/jkms.2015.30.3.264
- Yang CW, Li CI, Li TC, et al. Association of sarcopenic obesity with higher serum high-sensitivity C-reactive protein levels in Chinese older males--a community-based study (Taichung Community Health Study-Elderly, TCHS-E) [published correction appears in PLoS One. 2015;10(8):e0136069]. PLoS One. 2015;10(7):e0132908. PMID: 26177029. doi:10.1371/journal.pone.0132908
- Perna S, Spadaccini D, Nichetti M, Avanzato I, Faliva MA, Rondanelli M. Osteosarcopenic visceral obesity and osteosarcopenic subcutaneous obesity, two new phenotypes of sarcopenia: prevalence, metabolic profile, and risk factors. J Aging Res. 2018;2018:6147426. PMID: 29862078. doi:10.1155/2018/6147426
- Shimabukuro M. Leptin resistance and lipolysis of white adipose tissue: an implication to ectopic fat disposition and its consequences. J Atheroscler Thromb. 2017;24(11):1088–1089. doi:10.5551/jat.ED083
- Fan X, Yuan W, Huang W, et al. Recent progress in leptin signaling from a structural perspective and its implications for diseases. Biochimie. 2023;212:60–75. doi:10.1016/j.biochi.2023.04.011
- Haskins IN, Chang J, Nor Hanipah Z, et al. Patients with clinically metabolically healthy obesity are not necessarily healthy subclinically: further support for bariatric surgery in patients without metabolic disease? Surg Obes Relat Dis. 2018;14(3):342–346. doi:10.1016/j.soard.2017.11.032
- Sung KC, Cha S-C, Sung J-W, et al. Metabolically healthy obese subjects are at risk of fatty liver but not of pre-clinical atherosclerosis. Nutr Metab Cardiovasc Dis. 2014;24(3):256–262. doi:10.1016/j.numecd.2013.07.005
- Lonardo A, Bellentani S, Argo CK, et al. Epidemiological modifiers of non-alcoholic fatty liver disease: focus on high-risk groups. Dig Liver Dis. 2015;47(12):997–1006. doi:10.1016/j.dld.2015.08.004
- Rinaldi L, Nascimbeni F, Giordano M, et al. Clinical features and natural history of cryptogenic cirrhosis compared to hepatitis C virus-related cirrhosis. World J Gastroenterol. 2017;23(8):1458–1468. doi:10.3748/wjg.v23.i8.1458
- Lonardo A, Nascimbeni F, Mantovani A, et al. Hypertension, diabetes, atherosclerosis and NASH: cause or consequence? J Hepatol. 2018;68(2):335–352. doi:10.1016/j.jhep.2017.09.021
- Badmus OO, Hinds TD, Stec DE. Mechanisms linking metabolic-associated fatty liver disease (MAFLD) to cardiovascular disease [published online ahead of print, 2023 May 16]. Curr Hypertens Rep. 2023. doi:10.1007/s11906-023-01242-8
- Foster M, Pagliassotti M. Metabolic alterations following visceral fat removal and expansion: beyond anatomic location. Adipocyte. 2012;1(4):192–199. PMID: 23700533. doi:10.4161/adip.21756
- Hernández-Conde M, Llop E, Carrillo C, et al. Estimation of visceral fat is useful for the diagnosis of significant fibrosis in patients with non-alcoholic fatty liver disease. World J Gastroenterol. 2020;26(42):6658–6668. PMID: 33268953. doi:10.3748/wjg.v26.i42.6658
- Drozdz K, Nabrdalik K, Hajzler W, et al. Metabolic-Associated Fatty Liver Disease (MAFLD), diabetes, and cardiovascular disease: associations with fructose metabolism and gut microbiota. Nutrients. 2021;14(1):103. doi:10.3390/nu14010103
- Ma J, Fox CS, Jacques PF, et al. Sugar-sweetened beverage, diet soda, and fatty liver disease in the Framingham Heart Study cohorts. J Hepatol. 2015;63(2):462–469. doi:10.1016/j.jhep.2015.03.032
- Kantartzis K, Thamer C, Peter A, et al. High cardiorespiratory fitness is an independent predictor of the reduction in liver fat during a lifestyle intervention in non-alcoholic fatty liver disease. Gut. 2009;58(9):1281–1288. doi:10.1136/gut.2008.151977
- Lonardo A, Nascimbeni F, Maurantonio M, et al. Nonalcoholic fatty liver disease: evolving paradigms. World J Gastroenterol. 2017;23(36):6571–6592. doi:10.3748/wjg.v23.i36.6571
- Stender S, Kozlitina J, Nordestgaard BG, et al. Adiposity amplifies the genetic risk of fatty liver disease conferred by multiple loci. Nat Genet. 2017;49(6):842–847. doi:10.1038/ng.3855
- Musio A, Perazza F, Leoni L, et al. Osteosarcopenia in NAFLD/MAFLD: an underappreciated clinical problem in chronic liver disease. Int J Mol Sci. 2023;24(8). doi:10.3390/ijms24087517
- Chun HS, Lee M, Lee HA, et al. Risk stratification for sarcopenic obesity in subjects with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2023;21(9):2298–2307.e18. doi:10.1016/j.cgh.2022.11.031
- Choe EY, Lee Y-H, Choi YJ, et al. Waist-to-calf circumstance ratio is an independent predictor of hepatic steatosis and fibrosis in patients with type 2 diabetes. J Gastroenterol Hepatol. 2018;33(5):1082–1091. doi:10.1111/jgh.14011
- Shi Y-X, Chen X-Y, Qiu H-N, et al. Visceral fat area to appendicular muscle mass ratio as a predictor for nonalcoholic fatty liver disease independent of obesity. Scand J Gastroenterol. 2021;56(3):312–320. doi:10.1080/00365521.2021.1879244
- Gan D, Wang L, Jia M, et al. Low muscle mass and low muscle strength associate with nonalcoholic fatty liver disease. Clin Nutr. 2020;39(4):1124–1130. doi:10.1016/j.clnu.2019.04.023
- Ye Q, Zou B, Yeo YH, et al. Global prevalence, incidence, and outcomes of non-obese or lean non-alcoholic fatty liver disease: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2020;5(8):739–752. doi:10.1016/S2468-1253(20)30077-7
- Ito T, Ishigami M, Zou B, et al. The epidemiology of NAFLD and lean NAFLD in Japan: a meta-analysis with individual and forecasting analysis, 1995-2040. Hepatol Int. 2021;15(2):366–379. doi:10.1007/s12072-021-10143-4
- Leung JC, Loong TC-W, Wei JL, et al. Histological severity and clinical outcomes of nonalcoholic fatty liver disease in nonobese patients. Hepatology. 2017;65(1):54–64. doi:10.1002/hep.28697
- Fracanzani AL, Valenti L, Bugianesi E, et al. Risk of nonalcoholic steatohepatitis and fibrosis in patients with nonalcoholic fatty liver disease and low visceral adiposity. J Hepatol. 2011;54(6):1244–1249. doi:10.1016/j.jhep.2010.09.037
- Weinberg EM, Trinh HN, Firpi RJ, et al. Lean Americans with nonalcoholic fatty liver disease have lower rates of cirrhosis and comorbid diseases. Clin Gastroenterol Hepatol. 2021;19(5):996–1008.e6. doi:10.1016/j.cgh.2020.06.066
- Tang A, Ng CH, Phang PH, et al. Comparative burden of metabolic dysfunction in lean NAFLD vs non-lean NAFLD - a systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2023;21(7):1750–1760.e12. doi:10.1016/j.cgh.2022.06.029
- Xie X, Guo B, Xiao X, et al. Healthy dietary patterns and metabolic dysfunction-associated fatty liver disease in less-developed ethnic minority regions: a large cross-sectional study. BMC Public Health. 2022;22(1):118. doi:10.1186/s12889-021-12486-x
- Amani R, Parohan M, Jomehzadeh N, et al. Dietary and biochemical characteristics associated with normal-weight obesity. Int J Vitam Nutr Res. 2019;89(5–6):331–336. doi:10.1024/0300-9831/a000477
- Enjoji M, Yasutake K, Kohjima M, et al. Nutrition and nonalcoholic Fatty liver disease: the significance of cholesterol. Int J Hepatol. 2012;2012:925807. doi:10.1155/2012/925807
- Chen F, Esmaili S, Rogers GB, et al. Lean NAFLD: a Distinct Entity Shaped by Differential Metabolic Adaptation. Hepatology. 2020;71(4):1213–1227. doi:10.1002/hep.30908
- Shungin D, Winkler TW, Croteau-Chonka DC, et al. New genetic loci link adipose and insulin biology to body fat distribution. Nature. 2015;518(7538):187–196. doi:10.1038/nature14132
- Fehlert E, Wagner R, Ketterer C, et al. Genetic determination of body fat distribution and the attributive influence on metabolism. Obesity. 2017;25(7):1277–1283. doi:10.1002/oby.21874
- Lotta LA, Gulati P, Day FR, et al. Integrative genomic analysis implicates limited peripheral adipose storage capacity in the pathogenesis of human insulin resistance. Nat Genet. 2017;49(1):17–26. doi:10.1038/ng.3714
- Wei JL, Leung JC-F, Loong TC-W, et al. Prevalence and severity of nonalcoholic fatty liver disease in non-obese patients: a population study using proton-magnetic resonance spectroscopy. Am J Gastroenterol. 2015;110(9):1306–14; quiz 1315. doi:10.1038/ajg.2015.235
- Fracanzani AL, Petta S, Lombardi R, et al. Liver and cardiovascular damage in patients with lean nonalcoholic fatty liver disease, and association with visceral obesity. Clin Gastroenterol Hepatol. 2017;15(10):1604–1611.e1. doi:10.1016/j.cgh.2017.04.045
- Eslam M, Mangia A, Berg T, et al. Diverse impacts of the rs58542926 E167K variant in TM6SF2 on viral and metabolic liver disease phenotypes. Hepatology. 2016;64(1):34–46. doi:10.1002/hep.28475
- Meroni M, Longo M, Lombardi R, et al. Low Lipoprotein(a) levels predict hepatic fibrosis in patients with nonalcoholic fatty liver disease. Hepatol Commun. 2022;6(3):535–549. doi:10.1002/hep4.1830
- Petta S, Valenti L, Tuttolomondo A, et al. Interferon lambda 4 rs368234815 TT>δG variant is associated with liver damage in patients with nonalcoholic fatty liver disease. Hepatology. 2017;66(6):1885–1893. doi:10.1002/hep.29395
- Younossi ZM, Golabi P, de Avila L, et al. The global epidemiology of NAFLD and NASH in patients with type 2 diabetes: a systematic review and meta-analysis. J Hepatol. 2019;71(4):793–801. doi:10.1016/j.jhep.2019.06.021
- Bjorkstrom K, Stål P, Hultcrantz R, et al. Histologic scores for fat and fibrosis associate with development of type 2 diabetes in patients with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2017;15(9):1461–1468. doi:10.1016/j.cgh.2017.04.040
- Liu Z, Zhang Y, Graham S, et al. Causal relationships between NAFLD, T2D and obesity have implications for disease subphenotyping. J Hepatol. 2020;73(2):263–276. doi:10.1016/j.jhep.2020.03.006
- Dongiovanni P, Stender S, Pietrelli A, et al. Causal relationship of hepatic fat with liver damage and insulin resistance in nonalcoholic fatty liver. J Intern Med. 2018;283(4):356–370. doi:10.1111/joim.12719
- Katsiki N, Mikhailidis DP, Mantzoros CS. Non-alcoholic fatty liver disease and dyslipidemia: an update. Metabolism. 2016;65(8):1109–1123. doi:10.1016/j.metabol.2016.05.003
- Haigh L, Kirk C, El Gendy K, et al. The effectiveness and acceptability of Mediterranean diet and calorie restriction in non-alcoholic fatty liver disease (NAFLD): a systematic review and meta-analysis. Clin Nutr. 2022;41(9):1913–1931. doi:10.1016/j.clnu.2022.06.037
- Gepner Y, Shelef I, Komy O, et al. The beneficial effects of Mediterranean diet over low-fat diet may be mediated by decreasing hepatic fat content. J Hepatol. 2019;71(2):379–388. doi:10.1016/j.jhep.2019.04.013
- Ryan MC, Itsiopoulos C, Thodis T, et al. The Mediterranean diet improves hepatic steatosis and insulin sensitivity in individuals with non-alcoholic fatty liver disease. J Hepatol. 2013;59(1):475–494. doi:10.1016/j.jhep.2013.02.012
- Riebe D, Franklin BA, Thompson PD, et al. Updating ACSM’s recommendations for exercise preparticipation health screening. Med Sci Sports Exerc. 2015;47(11):2473–2479. doi:10.1249/MSS.0000000000000664
- Armstrong MJ, Gaunt P, Aithal GP, et al. Liraglutide safety and efficacy in patients with non-alcoholic steatohepatitis (LEAN): a multicentre, double-blind, randomised, placebo-controlled phase 2 study. Lancet. 2016;387(10019):679–690. doi:10.1016/S0140-6736(15)00803-X
- Newsome PN, Buchholtz K, Cusi K, et al. A placebo-controlled trial of subcutaneous semaglutide in nonalcoholic steatohepatitis. N Engl J Med. 2021;384(12):1113–1124. doi:10.1056/NEJMoa2028395
- Harrison SA, Fecht W, Brunt EM, et al. Orlistat for overweight subjects with nonalcoholic steatohepatitis: a randomized, prospective trial. Hepatology. 2009;49(1):80–86. doi:10.1002/hep.22575
- Feng X, Lin Y, Zhuo S, et al. Treatment of obesity and metabolic-associated fatty liver disease with a diet or orlistat: a randomized controlled trial. Am J Clin Nutr. 2023;117(4):691–700. doi:10.1016/j.ajcnut.2023.02.008
- Wang H, Wang L, Cheng Y, Xia Z, Liao Y, Cao J. Efficacy of orlistat in non-alcoholic fatty liver disease: a systematic review and meta-analysis. Biomed Rep. 2018;9(1):90–96. doi:10.3892/br.2018.1100
- Gaur S, Levy S, Mathus-Vliegen L, et al. Balancing risk and reward: a critical review of the intragastric balloon for weight loss. Gastrointest Endosc. 2015;81(6):1330–1336. doi:10.1016/j.gie.2015.01.054
- Bazerbachi F, Haffar S, Sawas T, et al. Fluid-filled versus gas-filled intragastric balloons as obesity interventions: a network meta-analysis of randomized trials. Obes Surg. 2018;28(9):2617–2625. doi:10.1007/s11695-018-3227-7
- Popov VB, Thompson CC, Kumar N, et al. Effect of intragastric balloons on liver enzymes: a systematic review and meta-analysis. Dig Dis Sci. 2016;61(9):2477–2487. doi:10.1007/s10620-016-4178-2
- Chandan S, Mohan BP, Khan SR, et al. Efficacy and Safety of Intragastric Balloon (IGB) in Non-alcoholic Fatty Liver Disease (NAFLD): a comprehensive review and meta-analysis. Obes Surg. 2021;31(3):1271–1279. doi:10.1007/s11695-020-05084-0
- Lopez-Nava G, Galvão MP, Bautista-Castaño I, et al. Endoscopic Sleeve gastroplasty: how I do it? Obes Surg. 2015;25(8):1534–1538. doi:10.1007/s11695-015-1714-7
- Hajifathalian K, Mehta A, Ang B, et al. Improvement in insulin resistance and estimated hepatic steatosis and fibrosis after endoscopic sleeve gastroplasty. Gastrointest Endosc. 2021;93(5):1110–1118. doi:10.1016/j.gie.2020.08.023
- van Baar A, Holleman F, Crenier L, et al. Endoscopic duodenal mucosal resurfacing for the treatment of type 2 diabetes mellitus: one year results from the first international, open-label, prospective, multicentre study. Gut. 2020;69(2):295–303. doi:10.1136/gutjnl-2019-318349
- de Oliveira G, de Moura DTH, Funari MP, et al. Metabolic effects of endoscopic duodenal mucosal resurfacing: a systematic review and meta-analysis. Obes Surg. 2021;31(3):1304–1312. doi:10.1007/s11695-020-05170-3
- Benaiges D, Más-Lorenzo A, Goday A, et al. Laparoscopic sleeve gastrectomy: more than a restrictive bariatric surgery procedure? World J Gastroenterol. 2015;21(41):11804–11814. doi:10.3748/wjg.v21.i41.11804
- Baldwin D, Chennakesavalu M, Gangemi A. Systematic review and meta-analysis of Roux-en-Y gastric bypass against laparoscopic sleeve gastrectomy for amelioration of NAFLD using four criteria. Surg Obes Relat Dis. 2019;15(12):2123–2130. doi:10.1016/j.soard.2019.09.060
- Salman MA, Salman AA, Abdelsalam A, et al. Laparoscopic sleeve gastrectomy on the horizon as a promising treatment modality for NAFLD. Obes Surg. 2020;30(1):87–95. doi:10.1007/s11695-019-04118-6
- Bjorklund P, Laurenius A, Een E, et al. Is the Roux limb a determinant for meal size after gastric bypass surgery? Obes Surg. 2010;20(10):1408–1414. doi:10.1007/s11695-010-0192-1
- Han SK, Baik SK, Kim MY. Non-alcoholic fatty liver disease: definition and subtypes. Clin Mol Hepatol. 2023;29(suppl):S5–S16. doi:10.3350/cmh.2022.0424
- Valenzuela PL, Carrera-Bastos P, Castillo-García A, Lieberman DE, Santos-Lozano A, Lucia A. Obesity and the risk of cardiometabolic diseases. Nat Rev Cardiol. 2023;20(7):475–494. doi:10.1038/s41569-023-00847-5
- Li C, Ford ES, Zhao G, et al. Estimates of body composition with dual-energy X-ray absorptiometry in adults. Am J Clin Nutr. 2009;90(6):1457–1465. doi:10.3945/ajcn.2009.28141
- Chackrewarthy S, Gunasekera D, Pathmeswaren A, et al. A comparison between revised NCEP ATP III and IDF definitions in diagnosing metabolic syndrome in an urban Sri Lankan population: the Ragama Health Study. ISRN Endocrinol. 2013;2013:320176. doi:10.1155/2013/320176
- Oliveros E, Somers VK, Sochor O, et al. The concept of normal weight obesity. Prog Cardiovasc Dis. 2014;56(4):426–433. doi:10.1016/j.pcad.2013.10.003
- Madeira FB, Silva AA, Veloso HF, et al. Normal weight obesity is associated with metabolic syndrome and insulin resistance in young adults from a middle-income country. PLoS One. 2013;8(3):e60673. doi:10.1371/journal.pone.0060673
- Sakuma K, Yamaguchi A. Sarcopenic obesity and endocrinal adaptation with age. Int J Endocrinol. 2013;2013:204164. doi:10.1155/2013/204164
- Kang S, Kyung C, Park JS, et al. Subclinical vascular inflammation in subjects with normal weight obesity and its association with body fat: an 18 F-FDG-PET/CT study. Cardiovasc Diabetol. 2014;13:70. doi:10.1186/1475-2840-13-70
- April-Sanders AK, Rodriguez CJ. Metabolically healthy obesity redefined. JAMA Netw Open. 2021;4(5):e218860. doi:10.1001/jamanetworkopen.2021.8860
- Bradshaw PT, Monda KL, Stevens J. Metabolic syndrome in healthy obese, overweight, and normal weight individuals: the atherosclerosis risk in communities study. Obesity. 2013;21(1):203–209. doi:10.1002/oby.20248
- Hoddy KK, Axelrod CL, Mey JT, et al. Insulin resistance persists despite a metabolically healthy obesity phenotype. Obesity. 2022;30(1):39–44. PMID: 34816598. doi:10.1002/oby.23312
- Li X, Ren Y, Chang K, et al. Adipose tissue macrophages as potential targets for obesity and metabolic diseases. Front Immunol. 2023;14:1153915. doi:10.3389/fimmu.2023.1153915
- Guria S, Hoory A, Das S, et al. Adipose tissue macrophages and their role in obesity-associated insulin resistance: an overview of the complex dynamics at play. Biosci Rep. 2023;43(3). doi:10.1042/BSR20220200
- Esser N, L’homme L, De Roover A, et al. Obesity phenotype is related to NLRP3 inflammasome activity and immunological profile of visceral adipose tissue. Diabetologia. 2013;56(11):2487–2497. doi:10.1007/s00125-013-3023-9
- Alalwan TA. Phenotypes of sarcopenic obesity: exploring the effects on peri-muscular fat, the obesity paradox, hormone-related responses and the clinical implications. Geriatrics (Basel). 2020;5(1):8. doi:10.3390/geriatrics5010008
- Batsis JA, Villareal DT. Sarcopenic obesity in older adults: aetiology, epidemiology and treatment strategies. Nat Rev Endocrinol. 2018;14(9):513–537. doi:10.1038/s41574-018-0062-9
- Hamaguchi Y, Kaido T, Okumura S, et al. Preoperative visceral adiposity and muscularity predict poor outcomes after hepatectomy for hepatocellular carcinoma. Liver Cancer. 2019;8(2):92–109. doi:10.1159/000488779
- Lee SH, Yang HK, Ha H-S, et al. Changes in metabolic health status over time and risk of developing type 2 diabetes: a prospective cohort study. Medicine (Baltimore). 2015;94(40):e1705. doi:10.1097/MD.0000000000001705
- Hamer M, Bell JA, Sabia S, et al. Stability of metabolically healthy obesity over 8 years: the English Longitudinal Study of Ageing. Eur J Endocrinol. 2015;173(5):703–708. doi:10.1530/EJE-15-0449
- Bell JA, Hamer M, Sabia S, et al. The natural course of healthy obesity over 20 years. J Am Coll Cardiol. 2015;65(1):101–102. doi:10.1016/j.jacc.2014.09.077
- Younossi ZM, Koenig AB, Abdelatif D, et al. Global epidemiology of nonalcoholic fatty liver disease-meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64(1):73–84. doi:10.1002/hep.28431
- Kushner RF, Kahan S. Introduction: the State of Obesity in 2017. Med Clin North Am. 2018;102(1):1–11. doi:10.1016/j.mcna.2017.08.003
- Pang Q, Zhang JY, Song SD, et al. Central obesity and nonalcoholic fatty liver disease risk after adjusting for body mass index. World J Gastroenterol. 2015;21(5):1650–1662. doi:10.3748/wjg.v21.i5.1650
- Chang Y, Jung H-S, Cho J, et al. Metabolically healthy obesity and the development of nonalcoholic fatty liver disease. Am J Gastroenterol. 2016;111(8):1133–1140. doi:10.1038/ajg.2016.178
- Bhaskaran K, dos-Santos-Silva I, Leon DA, et al. Association of BMI with overall and cause-specific mortality: a population-based cohort study of 3.6 million adults in the UK. Lancet Diabetes Endocrinol. 2018;6(12):944–953. doi:10.1016/S2213-8587(18)30288-2
- Argo CK, Stine JG, Henry ZH, et al. Physical deconditioning is the common denominator in both obese and overweight subjects with nonalcoholic steatohepatitis. Aliment Pharmacol Ther. 2018;48(3):290–299. doi:10.1111/apt.14803
- Zhang S, Du T, Li M, et al. Combined effect of obesity and uric acid on nonalcoholic fatty liver disease and hypertriglyceridemia. Medicine (Baltimore). 2017;96(12):e6381. doi:10.1097/MD.0000000000006381
- Liu DJ, Peloso GM, Yu H, et al. Exome-wide association study of plasma lipids in >300,000 individuals. Nat Genet. 2017;49(12):1758–1766. doi:10.1038/ng.3977
- Kim Y, Chang Y, Cho YK, et al. Metabolically healthy versus unhealthy obesity and risk of fibrosis progression in non-alcoholic fatty liver disease. Liver Int. 2019;39(10):1884–1894. doi:10.1111/liv.14184
- Gutierrez-Grobe Y, Juárez-Hernández E, Sánchez-Jiménez BA, et al. Less liver fibrosis in metabolically healthy compared with metabolically unhealthy obese patients with non-alcoholic fatty liver disease. Diabetes Metab. 2017;43(4):332–337. doi:10.1016/j.diabet.2017.02.007
- Ampuero J, Aller R, Gallego‐Durán R, et al. The effects of metabolic status on non-alcoholic fatty liver disease-related outcomes, beyond the presence of obesity. Aliment Pharmacol Ther. 2018;48(11–12):1260–1270. doi:10.1111/apt.15015
- Lee MJ, Kim E-H, Bae S-J, et al. Age-related decrease in skeletal muscle mass is an independent risk factor for incident nonalcoholic fatty liver disease: a 10-year retrospective cohort study. Gut Liver. 2019;13(1):67–76. doi:10.5009/gnl18070
- Kim G, Lee S-E, Lee Y-B, et al. Relationship between relative skeletal muscle mass and nonalcoholic fatty liver disease: a 7-year longitudinal study. Hepatology. 2018;68(5):1755–1768. doi:10.1002/hep.30049
- Hyun Kim K, Kyung Kim B, Yong Park J, et al. Sarcopenia assessed using bioimpedance analysis is associated independently with significant liver fibrosis in patients with chronic liver diseases. Eur J Gastroenterol Hepatol. 2020;32(1):58–65. doi:10.1097/MEG.0000000000001475
- Chun HS, Kim MN, Lee JS, et al. Risk stratification using sarcopenia status among subjects with metabolic dysfunction-associated fatty liver disease. J Cachexia Sarcopenia Muscle. 2021;12(5):1168–1178. doi:10.1002/jcsm.12754
- Rigor J, Vasconcelos R, Lopes R, Moreira T, Barata P, Martins-Mendes D. Associations between muscle mass, strength, and performance and non-alcoholic fatty liver disease [published online ahead of print, 2022 Mar 28]. Minerva Gastroenterol (Torino). 2022. doi:10.23736/S2724-5985.22.03097-2
- Petermann-Rocha F, Gray SR, Forrest E, et al. Associations of muscle mass and grip strength with severe NAFLD: a prospective study of 333,295 UK Biobank participants. J Hepatol. 2022;76(5):1021–1029. doi:10.1016/j.jhep.2022.01.010
- Younossi ZM, Stepanova M, Younossi Y, et al. Epidemiology of chronic liver diseases in the USA in the past three decades. Gut. 2020;69(3):564–568. doi:10.1136/gutjnl-2019-318813
- Golabi P, Paik JM, Arshad T, et al. Mortality of NAFLD according to the body composition and presence of metabolic abnormalities. Hepatol Commun. 2020;4(8):1136–1148. doi:10.1002/hep4.1534
- Li C, Guo P, Okekunle AP, et al. Lean non-alcoholic fatty liver disease patients had comparable total caloric, carbohydrate, protein, fat, iron, sleep duration and overtime work as obese non-alcoholic fatty liver disease patients. J Gastroenterol Hepatol. 2019;34(1):256–262. doi:10.1111/jgh.14360
- Sookoian S, Pirola CJ. Systematic review with meta-analysis: risk factors for non-alcoholic fatty liver disease suggest a shared altered metabolic and cardiovascular profile between lean and obese patients. Aliment Pharmacol Ther. 2017;46(2):85–95. doi:10.1111/apt.14112
- Zeng J, Yang R-X, Sun C, et al. Prevalence, clinical characteristics, risk factors, and indicators for lean Chinese adults with nonalcoholic fatty liver disease. World J Gastroenterol. 2020;26(15):1792–1804. doi:10.3748/wjg.v26.i15.1792
- Hagstrom H, Nasr P, Ekstedt M, et al. Risk for development of severe liver disease in lean patients with nonalcoholic fatty liver disease: a long-term follow-up study. Hepatol Commun. 2018;2(1):48–57. doi:10.1002/hep4.1124
- Younes R, Govaere O, Petta S, et al. Caucasian lean subjects with non-alcoholic fatty liver disease share long-term prognosis of non-lean: time for reappraisal of BMI-driven approach? Gut. 2022;71(2):382–390. doi:10.1136/gutjnl-2020-322564
- Wei L, Cheng X, Luo Y, et al. Lean non-alcoholic fatty liver disease and risk of incident diabetes in a euglycaemic population undergoing health check-ups: a cohort study. Diabetes Metab. 2021;47(3):101200. doi:10.1016/j.diabet.2020.08.008
- Zou B, Yeo YH, Nguyen VH, et al. Prevalence, characteristics and mortality outcomes of obese, nonobese and lean NAFLD in the United States, 1999-2016. J Intern Med. 2020;288(1):139–151. doi:10.1111/joim.13069
- Golabi P, Paik J, Fukui N, et al. Patients with lean nonalcoholic fatty liver disease are metabolically abnormal and have a higher risk for mortality. Clin Diabetes. 2019;37(1):65–72. doi:10.2337/cd18-0026
- Bugianesi E, Gastaldelli A, Vanni E, et al. Insulin resistance in non-diabetic patients with non-alcoholic fatty liver disease: sites and mechanisms. Diabetologia. 2005;48(4):634–642. doi:10.1007/s00125-005-1682-x
- Denkmayr L, Feldman A, Stechemesser L, et al. Lean patients with non-alcoholic fatty liver disease have a severe histological phenotype similar to obese patients. J Clin Med. 2018;7(12):562. doi:10.3390/jcm7120562
- Wang Q, You H, Ou X, et al. Non-obese histologically confirmed NASH patients with abnormal liver biochemistry have more advanced fibrosis. Hepatol Int. 2019;13(6):766–776. doi:10.1007/s12072-019-09982-z
- Gujral UP, Vittinghoff E, Mongraw-Chaffin M, et al. Cardiometabolic abnormalities among normal-weight persons from five racial/ethnic groups in the United States: a cross-sectional analysis of two cohort studies. Ann Intern Med. 2017;166(9):628–636. doi:10.7326/M16-1895
- Tabung FK, Steck SE, Zhang J, et al. Construct validation of the dietary inflammatory index among postmenopausal women. Ann Epidemiol. 2015;25(6):398–405. doi:10.1016/j.annepidem.2015.03.009
- Ji Y, Yiorkas AM, Frau F, et al. Genome-wide and abdominal MRI data provide evidence that a genetically determined favorable adiposity phenotype is characterized by lower ectopic liver fat and lower risk of type 2 diabetes, heart disease, and hypertension. Diabetes. 2019;68(1):207–219. doi:10.2337/db18-0708
- Yaghootkar H, Lotta LA, Tyrrell J, et al. Genetic evidence for a link between favorable adiposity and lower risk of type 2 diabetes, hypertension, and heart disease. Diabetes. 2016;65(8):2448–2460. doi:10.2337/db15-1671
- Eslam M, Valenti L, Romeo S. Genetics and epigenetics of NAFLD and NASH: clinical impact. J Hepatol. 2018;68(2):268–279. doi:10.1016/j.jhep.2017.09.003
- Bale G, Vishnubhotla RV, Mitnala S, et al. Whole-exome sequencing identifies a variant in phosphatidylethanolamine N-methyltransferase gene to be associated with lean-nonalcoholic fatty liver disease. J Clin Exp Hepatol. 2019;9(5):561–568. doi:10.1016/j.jceh.2019.02.001
- Targher G, Lonardo A, Byrne CD. Nonalcoholic fatty liver disease and chronic vascular complications of diabetes mellitus. Nat Rev Endocrinol. 2018;14(2):99–114. doi:10.1038/nrendo.2017.173
- Mantovani A, Scorletti E, Mosca A, Alisi A, Byrne CD, Targher G. Complications, morbidity and mortality of nonalcoholic fatty liver disease. Metabolism. 2020;111S:154170. doi:10.1016/j.metabol.2020.154170
- Lonardo A, Lugari S, Ballestri S, Nascimbeni F, Baldelli E, Maurantonio M. A round trip from nonalcoholic fatty liver disease to diabetes: molecular targets to the rescue? Acta Diabetol. 2019;56(4):385–396. doi:10.1007/s00592-018-1266-0
- Targher G, Corey KE, Byrne CD, Roden M. The complex link between NAFLD and type 2 diabetes mellitus - mechanisms and treatments. Nat Rev Gastroenterol Hepatol. 2021;18(9):599–612. doi:10.1038/s41575-021-00448-y
- Jeeyavudeen MS, Khan SK, Fouda S, et al. Management of metabolic-associated fatty liver disease: the diabetology perspective. World J Gastroenterol. 2023;29(1):126–143. doi:10.3748/wjg.v29.i1.126
- Mantovani A, Petracca G, Beatrice G, et al. Non-alcoholic fatty liver disease and risk of incident diabetes mellitus: an updated meta-analysis of 501 022 adult individuals. Gut. 2021;70(5):962–969. doi:10.1136/gutjnl-2020-322572
- Morrison AE, Zaccardi F, Khunti K, et al. Causality between non-alcoholic fatty liver disease and risk of cardiovascular disease and type 2 diabetes: a meta-analysis with bias analysis. Liver Int. 2019;39(3):557–567. doi:10.1111/liv.13994
- Nasr P, Ignatova S, Kechagias S, et al. Natural history of nonalcoholic fatty liver disease: a prospective follow-up study with serial biopsies. Hepatol Commun. 2018;2(2):199–210. doi:10.1002/hep4.1134
- Nasr P, Fredrikson M, Ekstedt M, et al. The amount of liver fat predicts mortality and development of type 2 diabetes in non-alcoholic fatty liver disease. Liver Int. 2020;40(5):1069–1078. doi:10.1111/liv.14414
- Bae JC, Han JM, Cho JH, et al. The persistence of fatty liver has a differential impact on the development of diabetes: the Kangbuk Samsung Health Study. Diabetes Res Clin Pract. 2018;135:1–6. doi:10.1016/j.diabres.2017.10.019
- Lee J, Cho YK, Kang YM, et al. The impact of NAFLD and waist circumference changes on diabetes development in prediabetes subjects. Sci Rep. 2019;9(1):17258. doi:10.1038/s41598-019-53947-z
- Cho HJ, Hwang S, Park JI, et al. Improvement of nonalcoholic fatty liver disease reduces the risk of type 2 diabetes mellitus. Gut Liver. 2019;13(4):440–449. doi:10.5009/gnl18382
- Sung KC, Wild SH, Byrne CD. Resolution of fatty liver and risk of incident diabetes. J Clin Endocrinol Metab. 2013;98(9):3637–3643. doi:10.1210/jc.2013-1519
- Zaharia OP, Strassburger K, Strom A, et al. Risk of diabetes-associated diseases in subgroups of patients with recent-onset diabetes: a 5-year follow-up study. Lancet Diabetes Endocrinol. 2019;7(9):684–694. doi:10.1016/S2213-8587(19)30187-1
- Zaharia OP, Strassburger K, Knebel B, et al. Role of patatin-like phospholipase domain-containing 3 gene for hepatic lipid content and insulin resistance in diabetes. Diabetes Care. 2020;43(9):2161–2168. doi:10.2337/dc20-0329
- Al-Mrabeh A, Zhyzhneuskaya SV, Peters C, et al. Hepatic lipoprotein export and remission of human type 2 diabetes after weight loss. Cell Metab. 2020;31(2):233–249.e4. doi:10.1016/j.cmet.2019.11.018
- Byrne CD, Targher G. NAFLD: a multisystem disease. J Hepatol. 2015;62(1 Suppl):S47–64. doi:10.1016/j.jhep.2014.12.012
- Tilg H, Moschen AR, Roden M. NAFLD and diabetes mellitus. Nat Rev Gastroenterol Hepatol. 2017;14(1):32–42. doi:10.1038/nrgastro.2016.147
- Heymsfield SB, Wadden TA, Longo DL. Mechanisms, pathophysiology, and management of obesity. N Engl J Med. 2017;376(15):1492. doi:10.1056/NEJMra1514009
- Hashem A, Khalouf A, Acosta A. Management of obesity and nonalcoholic fatty liver disease: a literature review. Semin Liver Dis. 2021;41(4):435–447. doi:10.1055/s-0041-1731704
- Stefano JT, Duarte SMB, Ribeiro Leite Altikes RG, et al. Non-pharmacological management options for MAFLD: a practical guide. Ther Adv Endocrinol Metab. 2023;14:20420188231160394. doi:10.1177/20420188231160394
- Chai XN, Zhou B-Q, Ning N, et al. Effects of lifestyle intervention on adults with metabolic associated fatty liver disease: a systematic review and meta-analysis. Front Endocrinol (Lausanne). 2023;14:1081096. doi:10.3389/fendo.2023.1081096
- Musso G, Cassader M, Rosina F, et al. Impact of current treatments on liver disease, glucose metabolism and cardiovascular risk in non-alcoholic fatty liver disease (NAFLD): a systematic review and meta-analysis of randomised trials. Diabetologia. 2012;55(4):885–904. doi:10.1007/s00125-011-2446-4
- Vilar-Gomez E, Martinez-Perez Y, Calzadilla-Bertot L, et al. Weight loss through lifestyle modification significantly reduces features of nonalcoholic steatohepatitis. Gastroenterology. 2015;149(2):367–78.e5; quiz e14–5. doi:10.1053/j.gastro.2015.04.005
- Jensen MD, Ryan DH, Apovian CM, et al. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Obesity Society. J Am Coll Cardiol. 2014;63(25 Pt B):2985–3023. doi:10.1016/j.jacc.2013.11.004
- Daneschvar HL, Aronson MD, Smetana GW. FDA-approved anti-obesity drugs in the United States. Am J Med. 2016;129(8):879.e1–6. doi:10.1016/j.amjmed.2016.02.009
- Chakhtoura M, Haber R, Ghezzawi M, Rhayem C, Tcheroyan R, Mantzoros CS. Pharmacotherapy of obesity: an update on the available medications and drugs under investigation. EClinicalMedicine. 2023;58:101882. doi:10.1016/j.eclinm.2023.101882
- Prikhodko VA, Bezborodkina NN, Okovityi SV. Pharmacotherapy for non-alcoholic fatty liver disease: emerging targets and drug candidates. Biomedicines. 2022;10(2):274. doi:10.3390/biomedicines10020274
- Sangro P, de la Torre Aláez M, Sangro B, D’Avola D. Metabolic dysfunction-associated fatty liver disease (MAFLD): an update of the recent advances in pharmacological treatment [published online ahead of print, 2023 Mar 28]. J Physiol Biochem. 2023. doi:10.1007/s13105-023-00954-4
- Rong L, Zou J, Ran W, et al. Advancements in the treatment of non-alcoholic fatty liver disease (NAFLD). Front Endocrinol (Lausanne). 2022;13:1087260. doi:10.3389/fendo.2022.1087260
- Maestri M, Santopaolo F, Pompili M, et al. Gut microbiota modulation in patients with non-alcoholic fatty liver disease: effects of current treatments and future strategies. Front Nutr. 2023;10:1110536. doi:10.3389/fnut.2023.1110536
- Ali MR, Moustarah F, Kim JJ. American society for metabolic and bariatric surgery position statement on intragastric balloon therapy endorsed by the Society of American Gastrointestinal and Endoscopic Surgeons. Surg Obes Relat Dis. 2016;12(3):462–467. doi:10.1016/j.soard.2015.12.026
- NASH News March. 2021—Recognizing NASH patient advocacy. Available from: https://www.globalliver.org/news/2021/nash-news-march. Accessed April 5, 2021
- Lazaridis II, Delko T. Bariatric surgery and metabolic dysfunction-associated fatty liver disease: a 2022 update. Praxis. 2023;112(2):97–102. doi:10.1024/1661-8157/a003969
- Lassailly G, Caiazzo R, Ntandja-Wandji L-C, et al. Bariatric surgery provides long-term resolution of nonalcoholic steatohepatitis and regression of fibrosis. Gastroenterology. 2020;159(4):1290–1301.e5. doi:10.1053/j.gastro.2020.06.006
- Fakhry TK, Mhaskar R, Schwitalla T, et al. Bariatric surgery improves nonalcoholic fatty liver disease: a contemporary systematic review and meta-analysis. Surg Obes Relat Dis. 2019;15(3):502–511. doi:10.1016/j.soard.2018.12.002
