Abstract
Objective
Gestational diabetes mellitus (GDM) is a condition of glucose intolerance, which may be accompanied with inflammation. The levels of hematological parameters during pregnancy can reflect inflammatory conditions in pregnant women. This study aims to describe the dynamic change of blood cell parameters from the first trimester (6–12 weeks of gestation) to the second trimester (24–28 weeks of gestation) and to investigate the associations of these biomarkers with the risk of GDM.
Methods
This study was a prospective double-center study conducted in Beijing, China (clinical trial number: NCT03246295). Hematological parameters were tested four times during the follow-up. Logistic regression analysis and Receiver Operating Characteristic (ROC) curve analysis were used to explore the association and predictive ability of hematological parameters for GDM.
Results
There were 258 of 1027 pregnant women in our study developed GDM. Among the 1027 pregnant women, white blood cells (WBC) gradually increased, and red blood cells (RBC), hemoglobin (HGB), and platelet (PLT) tended to decrease from the first trimester to second trimester. After adjusting for confounding factors, higher levels of RBC, HGB, and PLT in both early and middle pregnancy were positively associated with GDM risk, whereas the level of WBC was associated with GDM risk only in early pregnancy. WBC, RBC, HGB, and PLT in early and middle pregnancy were all correlated with fasting insulin (FINS) in early pregnancy.
Conclusion
Higher levels of hematological parameters in early and middle pregnancy were associated with glucose metabolism in early pregnancy and the subsequent risk of GDM.
Introduction
Gestational diabetes mellitus (GDM), defined as glucose intolerance first recognized during pregnancy, is one of the most common complications of pregnancy, with an increasing prevalence in recent years.Citation1 As the prevalence of GDM varies between different regions, the prevalence of GDM in China is about 17.7–25%, which is higher than that in other countries.Citation2–4 GDM is associated with maternal and fetal complications including preeclampsia, stillbirth, and large for gestational age (LGA) and can also have long-term consequences including type 2 diabetes mellitus (T2DM), obesity, and other metabolic syndromes.Citation5–7 The gold standard for the diagnosis of GDM is a 2-hr, 75-g oral glucose tolerance test (OGTT) conducted at 24 to 28 weeks of gestation, whereas studies have observed that GDM women have mild hyperglycemia and insulin resistance in the first trimester, indicating an abnormal glucose metabolism of GDM in early pregnancy.Citation8
Accumulating evidence suggests that inflammatory response plays an important role in the development of GDM and insulin resistance, which reveals a similar pathogenesis between GDM and T2DM.Citation9,Citation10 Several studies have explored the association between GDM and various inflammatory parameters, including white blood cells (WBC), neutrophils, tumor necrosis factor-ɑ (TNF-ɑ), and interleukin-6 (IL-6).Citation11–13 However, TNF-ɑ and IL-6 are not routine laboratory tests during pregnancy in most areas of China, which limits the application of these inflammatory factors in the clinic. Although there were studies explored the association between hematological parameters (white blood cell [WBC], red blood cell [RBC], hemoglobin [HGB], and platelet [PLT]) and GDM, these studies did not concentrate on the dynamic changes of these parameters during pregnancy.Citation14–16 Zhang et al described the distribution of complete blood counts at different phases of pregnancy in GDM and normal pregnant women, but the association between complete blood counts and the risk of GDM was not assessed.Citation17 Moreover, only a few studies concentrated on the correlation between erythrocyte or hemoglobin and GDM, and the results were inconsistent.Citation18,Citation19 To the best of our knowledge, there is a lack of studies to explore the dynamic changes of hematological parameters at different gestational stages and their associations with GDM.
Thus, this study aims to investigate the changes in hematological parameters as the progress of pregnancy in GDM and non-GDM women and to evaluate the associations of these parameters with glucose metabolism and the risk of GDM.
Materials and Methods
Study Design and Participants
This prospective double-center cohort study was started in 2019 and was conducted in Haidian District Maternal and Child Health Care Hospital and Chaoyang District Maternal and Child Health Care Hospital (Beijing, China) (clinical trial number: NCT03246295). The details regarding the trial design and baseline characteristics of the participants have been published previously.Citation20 Ethics approval was granted by the ethics review committee of the National Center for Women and Children’s Health, Chinese Center for Disease Control and Prevention in Beijing, China (Approval number FY2019-01). All participants gave written informed consent. The study was performed in accordance with the Declaration of Helsinki as revised in 2013.
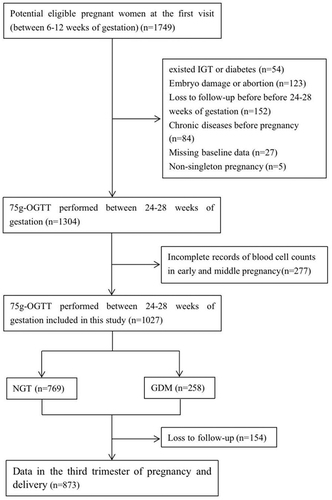
Singleton pregnant women over 18 years old were recruited in this cohort if their first prenatal visit was in the first trimester of pregnancy (between 6 and 12 weeks). The exclusion criteria of pregnant women were as follows: 1) with known impaired glucose tolerance or diabetes mellitus before pregnancy, or fasting plasma glucose (FPG) ≥6.1mmol/L at the first visit; 2) severe chronic diseases or infectious diseases (eg, liver disease, urinary system disease, cardiovascular disease, autoimmune disease, hematological disease, AIDS and other diseases before pregnancy); 3) medications other than vitamins used before or during pregnancy; and 4) inability to understand and follow-up regularly. Hematological samples were collected at the following four periods during pregnancy: gestational age 1 (6–10 weeks of gestation), gestational age 2 (12–14 weeks of gestation), gestational age 3 (16–20 weeks of gestation), and gestational age 4 (24–28 weeks of gestation). For the current analyses, 277 participants were excluded because of incomplete blood cell counts in early and middle pregnancy. Finally, a total of 1027 pregnant women were included in this analysis. The enrollment flow chart is shown in .
Data Collection and Laboratory Measurements
Clinical data and anthropometric parameters were recorded at the first visit, including maternal age, parity, family history of diabetes mellitus, history of adverse pregnancy, pre-pregnancy height and weight, gestational age, and blood pressure in the clinic. Gestational age was calculated based on the last menstrual period and confirmed by ultrasound. Pre-pregnancy BMI (preBMI) was calculated as pre-pregnancy weight (kg)/height (m)2. Maternal body weight, systolic blood pressure (SBP) and diastolic blood pressure (DBP) were measured at each follow-up visit. The BP was measured twice at 5-min intervals using an automatic BP monitor.
Laboratory measurements of fasting blood samples were collected at the first visit, and hematological parameters (WBC, RBC, HGB, and PLT) were tested for the above four gestational ages. The normal reference ranges of WBC, RBC, HGB, and PLT were 4.00–10.00×109/L, 3.50–5.00×1012/L, 110–150g/L, and 100–350×109/L, respectively. All available data were recorded and verified by two researchers at the same time.
Definition of Pregnancy Outcomes
All of the participants were screened for GDM by a 2-hr, 75-g oral glucose tolerance test (OGTT) during 24 to 28 weeks of gestation. GDM was diagnosed according to the International Association of Diabetes and Pregnancy Study Groups (IADPSG) criterion in 2010: fasting plasma glucose ≥5.1mmol/L, and/or 1-hr blood glucose ≥10.0mmol/L, and/or 2-hr blood glucose ≥8.5mmol/L.Citation21 Preterm delivery was defined as delivery before gestational week 37.Citation22 LGA and small for gestational age (SGA) were defined as birth weight above the 90th percentile and below the 10th percentile of the mean weight for gestational age and sex, respectively.Citation23 Delivery data were available for 873 of the 1027 pregnant women in the present study.
Statistical Analysis
Continuous variables were presented as the mean±SD if normally distributed and as medians (interquartile range) otherwise, and categorical variables were presented as percentages. The χ2 test (or Fisher’s exact test in case of small frequencies) was used for categorical variables. The quartiles of hematological parameters were calculated, and the significance of P value for linear trend over quartiles (from the lowest to the highest) was tested.
Pearson correlation was used to evaluate the association between hematological parameters and glucose metabolism indices in the first trimester. Logistic regression analyses were performed to evaluate the associations between hematological parameters and the risk of GDM. We performed a restricted cubic spline analysis with three knots at the 10th, 50th, and 90th percentiles to analyze the non-linear associations between hematological parameters and the risk of GDM. Receiver operating characteristic (ROC) curve analysis was used to explore the predictive ability of hematological parameters for GDM.
Statistical analyses were performed using the software IBM SPSS statistical program (version 26.0; IBM Corporation, Armonk, N.Y., USA), GraphPad Prism (version 9.5.1, GraphPad Software, San Diego, California, USA), and R statistical software (version 4.3.1). A P value of less than 0.05 (two-tailed) was considered statistically significant.
Results
Characteristics of Participants with and without GDM
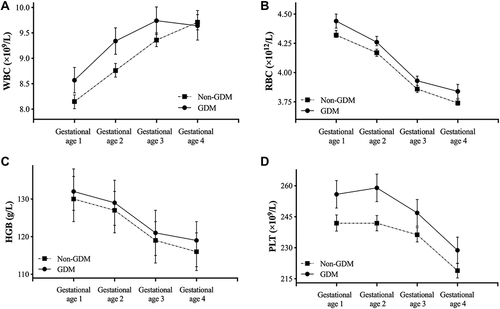
Of the 1027 pregnant women, 258 (25.12%) women were diagnosed with GDM (). Compared with participants without GDM, those with GDM tended to be older and heavier, and were more likely to have a history of adverse pregnancy. The four gestational ages of blood samples were 7.71 (6.86, 8.71) weeks, 12.57 (12.14, 13.24) weeks, 17.43 (16.57, 19.43) weeks, and 26.57 (23.68, 29.57) weeks, respectively. As shown in , the levels of WBC, RBC, HGB, and PLT in GDM pregnant women at different gestational ages were significantly higher than those in non-GDM group except for WBC at gestational age 4, and GDM women had markedly higher levels of FPG and FINS in the first trimester (both P value <0.001). From early to middle pregnancy, the levels of WBC gradually increased in both GDM and non-GDM pregnant women, and WBC counts became similar between the two groups at gestational age 4, while the levels of RBC, HGB, and PLT gradually decreased regardless of GDM status. Changes in hematological parameters from early to middle pregnancy are presented in . Regarding pregnancy outcomes, the delivery time between non-GDM and GDM groups showed significant differences, whereas the incidence of preterm delivery did not differ significantly between the two groups. In addition, the proportion of large for gestational age (LGA) in GDM group was significantly higher than that in non-GDM group (13.2% vs 6.3%, P=0.001).
Table 1 Characteristics and Laboratory Data of the Study Participants
Associations of Hematological Parameters with the Risk of GDM
The associations of WBC, RBC, HGB, and PLT at different gestational ages with the risk of GDM are presented in . At gestational age 1, the incidence of GDM gradually increased with increasing quartile levels of hematological parameters, and all the P values for trends were statistically significant (P for trend <0.01). Compared with women in the lowest quartiles, those in the highest quartiles had a significantly higher risk of GDM. After adjusted for maternal age, preBMI, family history of diabetes, and history of adverse pregnancy, the ORs for GDM in women from the highest quartiles of WBC, RBC, HGB, and PLT were 1.61 (95% CI 1.05–2.47), 1.79 (95% CI 1.18–2.71), 1.67 (1.09, 2.55), and 1.77 (95% CI 1.16–2.69), respectively. Similar trends were observed between these parameters at gestational age 2 and GDM risk. At gestational ages 3 and 4, the associations of WBC with GDM risk were attenuated, while RBC, HGB, and PLT remained as independent risk factors for GDM after adjusted for the above confounding factors.
Table 2 The Odds Ratios (ORs) of GDM Across Increased Quartiles of Hematological Parameters During Different Gestational Ages
Restricted cubic splines were used to visualize the non-linear associations between hematological parameters and GDM. After adjusted for maternal age, preBMI, family history of diabetes, and history of adverse pregnancy, only the association for RBC at gestational week 3 with GDM was non-linear (P for non-linear=0.049), whereas there was a lack of evidence for non-linear associations for other parameters (P for non-linear >0.05) (Supplemental Figure 1).
Sensitivity Analyses
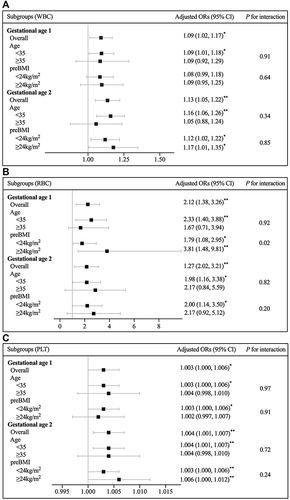
The associations of hematological parameters at gestational ages 1 and 2 with the risk of GDM were further analyzed in subgroups by different maternal age and preBMI (). Maternal age was stratified by <35 and ≥35 years old according to the criteria for advanced maternal age on perinatal outcomes, and preBMI was stratified by <24kg/m2 and ≥24kg/m2 according to Chinese criteria for overweight.Citation24,Citation25 The interaction between RBC at gestational age 1 and preBMI for GDM risk was significant (P for interaction=0.02), while there were absences of effect modifications for other parameters (P for interaction>0.05). Several adjusted ORs in the subgroups of maternal age≥35 years old and preBMI≥24kg/m2 were not statistically significant (P>0.05), perhaps because of the relatively small sample size of the two subgroups.
Figure 3 The forest plots of subgroup analyses of WBC (a), RBC (b), and PLT (c) at gestational age 1 and 2 by maternal age and preBMI. The ORs were adjusted for maternal age, preBMI, family history of diabetes, and history of adverse pregnancy (defined as embryo damage, spontaneous abortion or preterm delivery). *P<0.05 and **P<0.01.
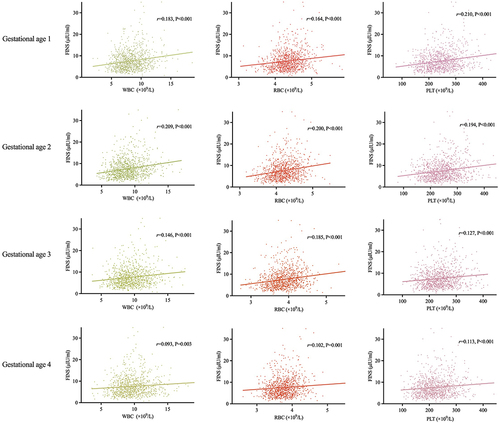
Associations Between Hematological Parameters and Glucose Metabolism in Early Pregnancy
There were no associations between blood cell counts at any gestational age and FPG in the first trimester. However, Pearson's analysis found positive correlations between WBC, RBC, and PLT at the four gestational ages and FINS in the first trimester (). These parameters in early pregnancy (gestational ages 1 and 2) showed relatively stronger correlations with FINS than those in middle pregnancy (gestational ages 3 and 4). The strongest correlation was observed between WBC at gestational age 2 and FINS in the first trimester (r=0.209, P<0.001).
The Predictive Ability of Hematological Parameters for GDM
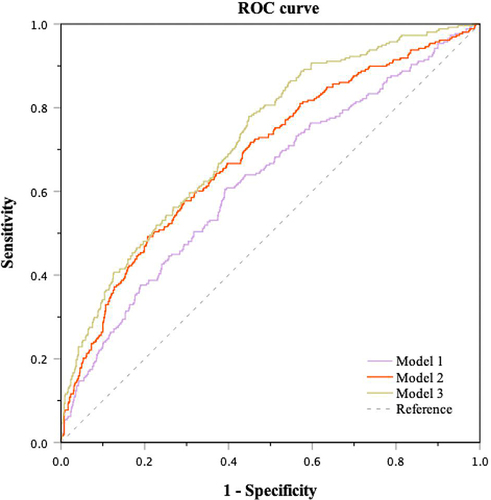
To identify the risk of GDM in early pregnancy, ROC curve analysis was used to explore the ability of hematological parameters at gestational ages 1 and 2 for the prediction of GDM risk (). Because of the similar composition of RBC and HGB, only RBC was included for analysis. When WBC, RBC, and PLT at gestational ages 1 and 2 were included as predictors for GDM, the area under the curve (AUC) of the screening model was 0.624 (95% CI 0.584–0.664, P<0.001). The predictive ability improved after adding clinical characteristics (maternal age, preBMI, family history of diabetes, history of adverse pregnancy), with an ROC-AUC of 0.684 (95% CI 0.646–0.722, P<0.001). When FPG and FINS in the first trimester were also included in the screening model, the ROC-AUC increased to 0.724 (95% CI 0.689–0.759, P<0.001). The pairwise comparisons showed statistically significant differences among these screening models (P<0.01) (Supplemental Table 1).
Figure 5 The ability of blood cell counts in early pregnancy combined with other parameters for the prediction of GDM. Model 1: WBC, RBC, and PLT at gestational age 1 and 2; Model 2: Model 1 + maternal age, preBMI, family history of diabetes, and history of adverse pregnancy; Model 3: Model 2 + FBG and FINS in the first trimester.
Discussion
In this prospective study, we described the dynamic change of hematological parameters from early to middle pregnancy, and found significant differences in these parameters between GDM and non-GDM pregnant women. Elevated levels of RBC, HGB, and PLT in both early and middle pregnancy were associated with the risk of GDM, whereas WBC levels were associated with GDM risk only in early pregnancy.
The relationship between GDM and several inflammatory biomarkers has been explored in previous studies, but the conclusions were inconsistent. A retrospective study included 1418 pregnant Chinese women found that decreased monocyte count in the first trimester was positively related to the development of GDM and the levels of IL-10 and CD206, indicated the association between GDM and the chronic inflammatory state.Citation11 A case–control study reported a slight increase in TNF-ɑ in the GDM group compared with the normal group, but the levels of serum TNF-ɑ were not useful in the first-trimester screening for GDM.Citation26 The above inflammatory biomarkers were not routinely tested during pregnancy, and thus the associations between blood cell parameters and the risk of GDM were investigated. Lyx et al conducted a retrospective study in Beijing, China, and reported that elevated levels of WBC, neutrophils, RBC, and HGB in the first trimester were significant predictors of the risk of GDM.Citation15 Similarly, another case–control study observed that WBC, PLT, mean platelet volume (MPV), plateletcrit (PCT), and other indicators in middle pregnancy were significantly higher in the GDM group than in the control group.Citation16 These inflammatory biomarkers change dynamically throughout pregnancy, and two other previous studies reported the associations of blood cell parameters from early to middle pregnancy with GDM risk.Citation27,Citation28 Compared with previous studies, our study was the first to explore the levels of hematological parameters in four different gestational ages during early and middle pregnancy, which could comprehensively describe the changes in these biomarkers.
Strict regulation of maternal immune function, in addition to components of inflammation, was paramount to successful pregnancy, and any imbalance between pro-inflammatory and anti-inflammatory cytokines could lead to the occurrence of pregnancy complications.Citation29 Cinkajzlová et al observed that the expression of inflammatory factors (IL-6, IL-8, and TNF-ɑ) in subcutaneous (SAT) and visceral adipose tissue (VAT) increased significantly in pregnant women regardless of the presence of GDM compared with age and BMI matched non-pregnant individuals, suggesting subclinical low-grade inflammation during pregnancy.Citation30 The dynamic changes in hematological parameters during normal pregnancy were the results of inflammatory response combined with increased plasma volume and hemodilution, leading to decreased HGB and PLT levels and increased WBC and neutrophil levels from early to middle pregnancy, which was consistent with our results.Citation31 Although the levels of RBC, HGB, and PLT were significantly different between the GDM and non-GDM groups in our study both in early and middle pregnancy, the differences in WBC levels between the two groups gradually attenuated during pregnancy, and WBC levels in middle pregnancy (gestational age 3 and 4) were not associated with the risk of GDM. A longitudinal study among 1860 pregnant Chinese women found approximately equivalent levels in WBC between the GDM and non-GDM groups during gestational weeks 25–28, and then WBC in the GDM group decreased until delivery, whereas WBC levels in the non-GDM group continued to increase during pregnancy and peaked at week 1 postpartum.Citation17 Another study conducted in Turkey enrolled 269 pregnant women found no significant difference in WBC levels between the GDM and non-GDM group during 24–28 weeks of gestation.Citation32 However, the mechanism of the slight decrease of WBC in GDM women has not been elucidated, and it may be related to the inflammation and insulin resistance in middle pregnancy. Recent studies have found that the expression of some micro RNAs (miRs) changed substantially in the total WBC of pregnant women with GDM.Citation33 miR21 and miR-155-5p were both found to be downregulated in patients with GDM, and these two miRs were associated with insulin sensitivity and the function of pancreatic β-cells.Citation34,Citation35 Given the limited data on hematological parameters in GDM women, especially in middle and late pregnancy, the associations of WBC and other parameters with GDM need to be explored by other studies.
Our studies found close associations of RBCs from gestational ages 1 to 4 with the risk of GDM. However, few studies concentrated on the relationship between RBC and GDM, and the limited conclusions were inconsistent. A retrospective study conducted by Lyx et al discovered elevated RBC level in the first trimester was associated with an increased risk of GDM.Citation15 However, another study among 600 pregnant women in Iran failed to find a significant difference of RBC between GDM and non-GDM groups.Citation28 Zhang et al described the distribution of complete blood counts during pregnancy and found that RBC differed in the two groups after 13 weeks of gestation and remained until delivery.Citation17 Positive correlations between biomarkers of oxidative stress in RBC with GDM were discovered by previous study, suggesting the potential relationship between RBC and inflammatory response.Citation36 In addition, higher levels of RBC and HGB were accompanied with higher blood viscosity, which was reported to be a risk factor for insulin resistance.Citation37 The associations of RBC with insulin resistance and GDM need to be further confirmed.
The correlations between hematological parameters and glycometabolism in early pregnancy were explored in our study. Although no associations were observed between these parameters and FPG in the first trimester, strong positive correlations were found between WBC, RBC, PLT at all the four gestational ages and FINS in the first trimester. These evidences indicated an underlying link between inflammatory response and glucose metabolism and also suggested that abnormal glucose metabolism in GDM appeared since early pregnancy. Our study proposed a feasible tool to screen for GDM risk in early pregnancy, which could be applicable in most areas of China.
Several limitations of this study should be acknowledged. First, all participants were recruited from Beijing, China, which could limit the generalizability of our findings to other regions. Second, participants with incomplete hematological parameters during pregnancy were excluded from the analysis, which could cause a selected bias. Third, confounding factors associated with inflammation including smoking and chronic infection failed to be analyzed in this study. However, pregnant Chinese women were strictly recommended to stop smoking, and very few pregnant women in China were smokers. In addition, pregnant women with severe chronic diseases or medication use were not enrolled in this study, which minimized the interference factors that could affect hematological parameters. Fourth, other inflammatory factors including neutrophils, monocytes, MPV, etc. were not recorded in this study. Fifth, hematological parameters after 28 weeks of gestation were not available.
Conclusions
In conclusion, hematological parameters changed dynamically during early and middle pregnancy, with increased WBC levels and decreased RBC, HGB, and PLT levels. Elevated levels of RBC, HGB, and PLT in both early and middle pregnancy were associated with higher risk of GDM, and higher WBC levels were associated with GDM risk only in early pregnancy. Our findings indicate the relationship between inflammatory blood cell parameters and glucose metabolism during pregnancy and provide a novelty perspective for screening GDM risk in early pregnancy.
Data Sharing Statement
The datasets generated during and analyzed during the current study are available from the corresponding author on reasonable request.
Ethics Approval
The study was performed in accordance with the Declaration of Helsinki as revised in 2013. This study was part of an ongoing prospective double-center observational cohort study started in 2019, which was conducted at Haidian District Maternal and Child Health Care Hospital and Chaoyang District Maternal and Child Health Care Hospital (Beijing, China) (clinical trial number: NCT03246295). The Ethical Review Committee of National Center for Women and Children’s Health, Chinese Center for Disease Control and Prevention in Beijing, China, approved this study on Apr 3, 2019 (Approval number FY2019-01).
Consent to Participant
Written informed consent was obtained from all participants.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising, or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors have nothing to disclose for this work.
Additional information
Funding
References
- American Diabetes Association. Classification and diagnosis of diabetes: standards of medical care in diabetes-2019. Diabetes Care. 2019;42(Suppl 1):S13–S28. doi:10.2337/dc19-S002
- Wang C, Jin L, Tong M, et al. Prevalence of gestational diabetes mellitus and its determinants among pregnant women in Beijing. J Matern Neonatal Med. 2020;21:1–7. doi:10.1080/14767058.2020.1754395
- Guariguata L, Linnenkamp U, Beagley J, Whiting DR, Cho NH. Global estimates of the prevalence of hyperglycaemia in pregnancy. Diabet Res Clin Pract. 2014;103:176–185. doi:10.1016/j.diabres.2013.11.003
- Schwartz N, Nachum Z, Green MS. The prevalence of gestational diabetes mellitus recurrence--effect of ethnicity and parity: a meta analysis. Am J Obstet Gynecol. 2015;213(3):310–317. doi:10.1016/j.ajog.2015.03.011
- Bianco ME, Josefson JL. Hyperglycemia during pregnancy and long-term offspring outcomes. Curr Diab Rep. 2019;19:143. doi:10.1007/s11892-019-1267-6
- Damm P, Houshmand-Oeregaard A, Kelstrup L, Lauenborg J, Mathiesen ER, Clausen TD. Gestational diabetes mellitus and long-term consequences for mother and offspring: a view from Denmark. Diabetologia. 2016;59(7):1396–1399. doi:10.1007/s00125-016-3985-5
- Clausen TD, Mathiesen ER, Hansen T, et al. High prevalence of type 2 diabetes and pre-diabetes in adult offspring of women with gestational diabetes mellitus or type 1 diabetes: the role of intrauterine hyperglycemia. Diabetes Care. 2008;31(2):340–346. doi:10.2337/dc07-1596
- Chiefari E, Arcidiacono B, Foti D, Brunetti A. Gestational diabetes mellitus: an updated overview. J Endocrinol Invest. 2017;40(9):899–909. 10.1007/s40618-016-0607-5
- Pantham P, Aye IL, Powell TL. Inflammation in maternal obesity and gestational diabetes mellitus. Placenta. 2015;36(7):709–715. doi:10.1016/j.placenta.2015.04.006
- De Luccia TPB, Pendeloski KPT, Ono E, et al. Unveiling the pathophysiology of gestational diabetes: studies on local and peripheral immune cells. Scand J Immunol. 2020;91(4):e12860. doi:10.1111/sji.12860
- Huang X, Zha B, Zhang M, et al. Decreased monocyte count is associated with gestational diabetes mellitus development, macrosomia, and inflammation. J Clin Endocrinol Metab. 2022;107(1):192–204. doi:10.1210/clinem/dgab657
- Hassiakos D, Eleftheriades M, Papastefanou I, et al. Increased maternal serum interleukin-6 concentrations at 11 to 14 weeks of gestation in low risk pregnancies complicated with gestational diabetes mellitus: development of a prediction model. Horm Metab Res. 2016;48(1):35–41. doi:10.1055/s-0034-1395659
- Guillemette L, Lacroix M, Battista MC, et al. TNFα dynamics during the oral glucose tolerance test vary according to the level of insulin resistance in pregnant women. J Clin Endocrinol Metab. 2014;99(5):1862–1869. doi:10.1210/jc.2013-4016
- Wang L, Yao H, Shen W, et al. Gestational diabetes mellitus is associated with blood inflammatory indicators in a Chinese pregnant women population. Gynecol Endocrinol. 2022;38(2):153–157. doi:10.1080/09513590.2021.2015762
- Lyu X, Jia J, Yang H, et al. Hematological parameters in the first trimester and the risk of gestational diabetes Mellitus - Beijing, China, 2017–2020. China CDC Wkly. 2023;5(9):194–200. doi:10.46234/ccdcw2023.035
- Fashami MA, Hajian S, Afrakhteh M, Khoob MK. Is there an association between platelet and blood inflammatory indices and the risk of gestational diabetes mellitus? Obstet Gynecol Sci. 2020;63(2):133–140. doi:10.5468/ogs.2020.63.2.133
- Zhang Y, Zhang Y, Zhao L, Shang Y, He D, Chen J. Distribution of complete blood count constituents in gestational diabetes mellitus. Medicine. 2021;100(23):e26301. doi:10.1097/MD.0000000000026301
- Tan PC, Chai JN, Ling LP, Omar SZ. Maternal hemoglobin level and red cell indices as predictors of gestational diabetes in a multi-ethnic Asian population. Clin Exp Obstet Gynecol. 2011;38(2):150–154.
- Hassan B, Rayis DA, Musa IR, Eltayeb R, ALhabardi N, Adam I. Blood groups and hematological parameters do not associate with first trimester gestational diabetes mellitus (Institutional Experience). Ann Clin Lab Sci. 2021;51(1):97–101.
- Duo Y, Song S, Zhang Y, et al. Relationship between serum uric acid in early pregnancy and gestational diabetes mellitus: a prospective cohort study. Endocrine. 2023. doi:10.1007/s12020-023-03544-y
- International Association of Diabetes and Pregnancy Study Groups Consensus Panel. International association of diabetes and pregnancy study groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care. 2010;33:676–682. 16. doi:10.2337/dc09-1848
- Goldstein RF, Abell SK, Ranasinha S, et al. Association of gestational weight gain with maternal and infant outcomes: a systematic review and meta-analysis. JAMA. 2017;317:2207–2225. doi:10.1001/jama.2017.3635
- Dai L, Deng C, Li Y, et al. Birth weight reference percentiles for Chinese. PLoS One. 2014;9(8):e104779. doi:10.1371/journal.pone.0104779
- Kahveci B, Melekoglu R, Evruke IC, Cetin C. The effect of advanced maternal age on perinatal outcomes in nulliparous singleton pregnancies. BMC Pregnancy Childbirth. 2018;18(1):343. doi:10.1186/s12884-018-1984-x
- Pan XF, Wang L, Pan A. Epidemiology and determinants of obesity in China. Lancet Diabetes Endocrinol. 2021;9(6):373–392. doi:10.1016/S2213-8587(21)00045-0
- Syngelaki A, Visser GH, Krithinakis K, Wright A, Nicolaides KH. First trimester screening for gestational diabetes mellitus by maternal factors and markers of inflammation. Metabolism. 2016;65(3):131–137. doi:10.1016/j.metabol.2015.10.029
- Ye YX, Wang Y, Wu P, et al. Blood cell parameters from early to middle pregnancy and risk of gestational diabetes mellitus. J Clin Endocrinol Metab. 2023;dgad336. doi:10.1210/clinem/dgad336
- Shaarbaf Eidgahi E, Nasiri M, Kariman N, et al. Diagnostic accuracy of first and early second trimester multiple biomarkers for prediction of gestational diabetes mellitus: a multivariate longitudinal approach. BMC Pregnancy Childbirth. 2022;22(1):13. doi:10.1186/s12884-021-04348-6
- Kalagiri RR, Carder T, Choudhury S, et al. Inflammation in complicated pregnancy and its outcome. Am J Perinatol. 2016;33(14):1337–1356. doi:10.1055/s-0036-1582397
- Cinkajzlová A, Anderlová K, Šimják P, et al. Subclinical inflammation and adipose tissue lymphocytes in pregnant females with gestational diabetes mellitus. J Clin Endocrinol Metab. 2020;105(11):dgaa528. doi:10.1210/clinem/dgaa528
- Li A, Yang S, Zhang J, Qiao R. Establishment of reference intervals for complete blood count parameters during normal pregnancy in Beijing. J Clin Lab Anal. 2017;31(6):e22150. doi:10.1002/jcla.22150
- Gorar S, Abanonu GB, Uysal A, et al. Comparison of thyroid function tests and blood count in pregnant women with versus without gestational diabetes mellitus. J Obstet Gynaecol Res. 2017;43(5):848–854. doi:10.1111/jog.13280
- Sun X, Sun H, Li P. Association of circulating inflammatory cells and platelets with gestational diabetes and pregnancy outcomes. Clin Chim Acta. 2021;523:87–96. doi:10.1016/j.cca.2021.09.004
- Hocaoglu M, Demirer S, Senturk H, Turgut A, Komurcu-Bayrak E. Differential expression of candidate circulating microRNAs in maternal blood leukocytes of the patients with preeclampsia and gestational diabetes mellitus. Pregnancy Hypertens. 2019;17:5–11. doi:10.1016/j.preghy.2019.04.004
- Hocaoglu M, Demirer S, Loclar Karaalp I, et al. Identification of miR-16-5p and miR-155-5p microRNAs differentially expressed in circulating leukocytes of pregnant women with polycystic ovary syndrome and gestational diabetes. Gynecol Endocrinol. 2021;37(3):216–220. doi:10.1080/09513590.2020.1843620
- León-Reyes G, Guzmán-Grenfell AM, Medina-Navarro R, et al. Is gestational diabetes mellitus in obese women predicted by oxidative damage in red blood cells? Gynecol Endocrinol. 2018;34(11):995–1000. doi:10.1080/09513590.2018.1473360
- Tamariz LJ, Young JH, Pankow JS, et al. Blood viscosity and hematocrit as risk factors for type 2 diabetes mellitus: the atherosclerosis risk in communities (ARIC) study. Am J Epidemiol. 2008;168(10):1153–1160. doi:10.1093/aje/kwn243