Abstract
The incidences of thyroid cancer and diabetes are rapidly increasing worldwide. The relationship between thyroid cancer and diabetes is a popular topic in medicine. Increasing evidence has shown that diabetes increases the risk of thyroid cancer to a certain extent. This mechanism may be related to genetic factors, abnormal thyroid-stimulating hormone secretion, oxidative stress injury, hyperinsulinemia, elevated insulin-like growth factor-1 levels, abnormal secretion of adipocytokines, and increased secretion of inflammatory factors and chemokines. This article reviews the latest research progress on the relationship between thyroid cancer and diabetes, including the association between diabetes and the risk of developing thyroid cancer, its underlying mechanisms, and potential anti-thyroid cancer effects of hypoglycemic drugs. It providing novel strategies for the prevention, treatment, and improving the prognosis of thyroid cancer.
Introduction
Diabetes is a chronic metabolic disease characterized by elevated blood glucose levels and is the most common endocrine disease. The rapid development of the global economy has brought about lifestyle changes and accelerated aging population, resulting in an increasing prevalence of diabetes each year. About 537 million adults worldwide have diabetes, and type 2 diabetes accounts for nearly 90%.Citation1,Citation2 Thyroid cancer is the most common malignant tumor of the endocrine system, and it originates from the thyroid follicular epithelial cells. In recent years, the incidence of thyroid cancer has increased significantly, making it the fastest-growing tumor globally. It is expected to become the fourth most common type of malignant tumor worldwide.Citation3,Citation4 Thyroid carcinoma encompasses various subtypes, including papillary thyroid carcinoma (PTC), follicular thyroid carcinoma, medullary thyroid carcinoma, and anaplastic thyroid carcinoma according to the histological origin. PTC is the most common subtype of thyroid carcinoma.Citation5 The incidence of concurrent diabetes and malignant tumors is increasing worldwide. The correlation between diabetes and malignant tumors has always been a research hotspot in medicine. A century ago, some scholars have proposed that there may be a link between diabetes and malignant tumors.Citation6 Clinical studies have confirmed that diabetes is a high-risk factor for various malignant tumors. Notably, the occurrence and development of breast, gastric, colorectal, and pancreatic cancers are closely associated with diabetes.Citation7–10
Through an examination of the clinical data from patients with diabetes and thyroid cancer, we found that the risk of thyroid cancer was significantly increased in patients with diabetes. With the increasing incidence of thyroid cancer, it is necessary to analyze the correlation between thyroid cancer and diabetes to provide valuable insights for the prevention and improving prognosis of thyroid cancer. This article presents a comprehensive review of the latest research findings on the association between thyroid cancer and diabetes, including the risk factors associated with the diseases, their shared pathogenesis, and the potential impact of hypoglycemic medications on thyroid cancer.
Diabetes as a Risk Factor of Thyroid Cancer
More and more studies have shown that diabetes can affect the risk of thyroid cancer (). A 10-year prospective study reported a 25% increased risk of thyroid cancer in patients with diabetes; the study also revealed that the risk of thyroid cancer in females with diabetes was significantly higher than that in males with diabetes.Citation11 A retrospective cohort study demonstrated that although thyroid cancer is rare among men, those with diabetes had a 40% higher thyroid cancer risk than women, emphasizing that there is a significantly higher risk in patients with diabetes.Citation12 Yeo et al observed that in areas with a high incidence of thyroid cancer, the risk of thyroid cancer in female patients with diabetes increased by 30%, while the risk in male patients did not increase.Citation13 Zhan et al observed that patients with a history of diabetes and high fasting blood glucose levels have a significantly increased risk of thyroid cancer.Citation14
Table 1 Studies on the Relationship Between Diabetes and the Risk of Thyroid Cancer
Furthermore, a systematic review and meta-analysis of thyroid cancer revealed that patients with insulin resistance and elevated blood glucose levels have a significantly increased risk of thyroid cancer.Citation15 Seo et al reported that within a duration of up to 5 years, patients with type 2 diabetes have low risk of developing thyroid cancer, and this risk increased after receiving hypoglycemic drugs.Citation16 Carstensen et al observed that, compared with the general population, female patients with type 1 diabetes exhibited an elevated overall incidence and increased risk of thyroid cancer.Citation17 Furthermore, research has demonstrated that patients with diabetes not only have an increased risk of thyroid cancer but are also more likely to have lymph node and distant metastasis, which may lead to worse prognoses.Citation16,Citation18 Bezin et al observed that the use of a glucagon-like peptide-1 receptor agonist increased the risk of all thyroid cancers, especially within 1–3 years of the drug use.Citation22 The results of a meta-analysis of a cohort study showed that the risk of thyroid cancer increased in any type of diabetic patients compared with non-diabetic patients.Citation19 A 14-year cross-sectional study found that diabetes increases the risk of thyroid cancer in women.Citation20 A prospective study on the risk of new-onset type 2 diabetes and cancer found that the risk of thyroid cancer in patients with type 2 diabetes increased significantly.Citation21 Many studies have demonstrated an increased risk of thyroid cancer in patients with diabetes; however, prospective studies with large sample sizes are still needed to verify the correlation between diabetes and thyroid cancer risk and to explore the potential mechanism underlying their relationship.
The Underlying Mechanisms of Diabetes and Thyroid Cancer
Hereditary Factors
Uncoupling proteins (UCPs) are located in the inner mitochondrial membrane and belong to the mitochondrial transporter family. It is a homolog of proton transporters and an antioxidant that inhibits the production of reactive oxygen species (ROS) within the mitochondria. UCP-encoding gene is located on human chromosome 11.Citation23–25 Studies have revealed that UCP2 polymorphism may play a pathological role in diabetes and cancer.Citation26–28 Other studies have found a negative correlation between UCP2 and glucose-stimulated insulin secretion; UCP2 upregulation inhibits insulin secretion and increases the risk of type 2 diabetes.Citation29,Citation30 Studies have also reported that UCP2 promotes cell proliferation, which may be an important mechanism for promoting tumorigenesis. For instance, in a study on skin cancer, UCP2 deficiency was found to inhibit skin cell proliferation, and when UCP2 was downregulated by miR-214 in hepatocellular carcinoma, cell proliferation was inhibited.Citation31,Citation32 Studies have found that UCP2 is a new therapeutic target for HER2-positive breast cancer, and UCP2 inhibitors can improve the efficacy of trastuzumab in the treatment of breast cancer.Citation33 Sithul et al found that the level of oxidative stress in tumor cells was significantly reduced after knocking out the UCP2 gene of thyroid cancer cells, indicating that high expression of UCP2 in tumor cells may significantly increase the level of oxidative stress in cells, thereby promoting the proliferation of tumor cells.Citation34 Li et al described the role of UCP2 gene in diabetes and cancer in detail, and found that UCP2 gene plays an important pathological role in diabetes and cancer.Citation35 UCP2 gene expression activity is regulated by many factors, such as ROS, free fatty acids and other factors.Citation36 Excessive ROS accumulation in diabetic patients leads to an increase in UCP2 expression activity, which may eventually promote the carcinogenesis of thyroid cells. Since the role of UCP2 gene in cancer has only received attention in recent years, it is still necessary to further study how diabetes promotes the occurrence and progression of thyroid cancer through UCP2 gene. Thyroid cancer is the fourth most common type of cancer worldwide. Investigating the molecular-level correlation between thyroid cancer and diabetes at the molecular level, particularly the expression of UCP2, could provide insights into their relationship.
Abnormal Secretion of Thyroid-Stimulating Hormone
Thyroid-stimulating hormone (TSH) is a thyroid cell growth factor secreted by the adenohypophysis that plays a crucial role in the malignant transformation of thyroid follicular cells. It activates adenylate cyclase by binding to TSH receptors and increases cyclic adenosine monophosphate and protein kinase A levels, thereby regulating the growth and proliferation of thyroid cells.Citation37 Studies have demonstrated that patients with poorly controlled blood glucose in type 2 diabetes have a high risk of subclinical hypothyroidism, and this risk increases with the increase of glycosylated hemoglobin.Citation38 Adhami et al reported that the concentration of TSH in plasma is closely related to the occurrence and development of thyroid cancer and that high levels of TSH promote the proliferation, invasion, and metastasis of tumor cells.Citation39 A case-control study revealed that elevated or decreased TSH levels increased the risk of thyroid cancer, and the risk of cervical lymph node metastasis in thyroid cancer is closely related to high plasma TSH levels.Citation40 Since subclinical hypothyroidism is the most common thyroid dysfunction in patients with diabetes, the majority of patients with diabetes and thyroid dysfunction have elevated plasma TSH levels, and high levels of TSH may promote tumor cell growth and accelerate tumor metastasis. Therefore, diabetes may lead to carcinogenesis of the thyroid follicular epithelium by affecting the secretion of TSH, and high levels of TSH may affect the prognosis of thyroid cancer. Song et al revealed that the thyroid cancer cells stimulated by TSH activate protein kinase B (PKB) and extracellular signal-regulated kinase (ERK) signaling pathways to promote the secretion of vascular endothelial growth factor and CXC chemokine ligand 8, thereby accelerating vascular growth and ultimately accelerating the proliferation of tumor cells.Citation37 Zou et al observed that TSH downregulates the expression of p53 in thyroid cancer through the mitogen-activated protein kinases and phosphoinositide 3-kinases (PI3K)/PKB signaling pathways to overcome Braf (V600E)-induced apoptosis, thereby accelerating the progression of thyroid cancer.Citation41 A survey of patients with thyroid cancer found that 80.4% of doctors recommended TSH suppression therapy for patients with moderate-risk papillary thyroid cancer, 48.8% recommended TSH suppression therapy for patients with low-risk papillary thyroid cancer, and 29.7% recommended TSH suppression therapy for patients with very low-risk papillary thyroid cancer.Citation42 Therefore, abnormal TSH secretion may affect the correlation between diabetes and thyroid cancer, and it is extremely important to control the plasma concentration of TSH in patients with confirmed thyroid cancer.
Oxidative Stress Injury
Oxidative stress refers to an imbalance between the oxidation and antioxidant systems in cells and tissues, caused by the excessive production or insufficient clearance of ROS and other active free radicals from the body. Hyperglycemia in patients with diabetes can induce the accumulation of ROS through sugar autooxidation, activation of the polyol pathway, generation of angiotensin-converting enzymes (ACEs), activation of protein kinase C, and non-enzymatic glycosylation of proteins, thereby increasing the level of oxidative stress.Citation43,Citation44 Studies have reported that islet β-cells are directly or indirectly affected by oxidative stress, resulting in impaired cell function, which in turn leads to decreased sensitivity of peripheral tissues to insulin and ultimately accelerates the progression of diabetes.Citation45 Many studies have revealed that patients with diabetes have increased levels of ROS and oxidative stress, which in turn affect cell growth and proliferation and lead to cell DNA mutations, which may play a role in the initiation and progression of multistage carcinogenesisCitation46–48 (). Other studies have revealed that the accumulation of ROS can lead to BRAFV600E mutations, which have a great impact on the prognosis of thyroid cancer.Citation49–51 Zhang et al demonstrated that oxidative stress can induce the expression of protein tyrosine phosphatase non-receptor type 2 (PTPN2), one of the protein tyrosine phosphatases (PTPs), and the upregulated PTPN2 can promote thyroid cancer progression.Citation52 Tabur et al reported that patients with thyroid cancer had severe oxidative damage to their DNA and impaired antioxidant status. The levels of DNA oxidative damage and oxidative stress index in the thyroid cancer group before and after surgery were significantly higher than those in the healthy control group, and the levels of DNA oxidative damage and oxidative stress in the thyroid cancer group after surgery were lower than those in the same group before surgery.Citation53 Furthermore, Ziros et al observed that excess oxygen free radicals act as signal transduction molecules to activate the nuclear transcription-related factor 2-antioxidant response element system to regulate gene transcription, generate a variety of Phase II detoxification enzymes and antioxidants, and increase the incidence and treatment resistance of thyroid cancer.Citation54 Oxidative stress injury is believed to play a role in the pathogenesis of diabetes and thyroid cancer.
Figure 1 The mechanism of oxidative stress injury in diabetes and thyroid cancer.
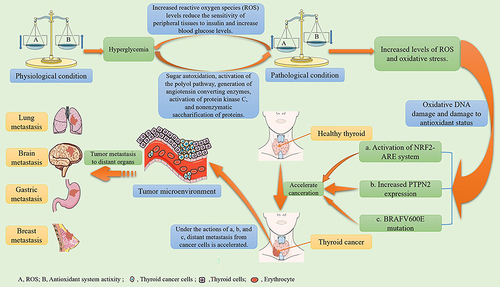
Hyperinsulinemia and Elevated Insulin-Like Growth Factor-1
In patients with type 2 diabetes, the efficiency of insulin in promoting glucose uptake and utilization is reduced, resulting in the compensatory secretion of large amounts of insulin leading to hyperinsulinemia. Insulin-like growth factors (IGFs) are mitogens that regulate cell proliferation, differentiation, and apoptosis. IGF-1 is a member of the IGF family of proteins. Studies have demonstrated that IGF-1 ligands and receptors are highly expressed in thyroid cancer cells.Citation55 IGF-binding protein-1 is inhibited in patients with hyperinsulinemia, thus the biological activity of IGF-1 increases, promoting tumor growth.Citation56 Studies have revealed that hyperinsulinemia caused by diabetes stimulates the secretion of IGF-1 in the liver and that insulin regulates the metabolism, accelerates the proliferation of S-phase cells, increases neovascularization, and inhibits apoptosis.Citation57,Citation58 Insulin and IGF-1 have long been known to induce protein and DNA synthesis in human thyroid cells.Citation59 Studies have shown that hyperinsulinemia may change the blood flow pattern of thyroid nodules, thereby altering the distribution, density, and structure of thyroid blood vessels, which may affect the growth and development of thyroid nodules.Citation60,Citation61 Moreover, studies have found that IGF-1 plays an important role in the development of cancer; high concentrations of IGF-1 in the plasma can significantly increase the risk of prostate, colorectal, and breast cancer.Citation62–64 A high concentration of IGF-1 in the plasma can significantly promote the proliferation of thyroid cells and the migration of tumor cells. The mechanism may be attributed to the activation of the insulin and IGF-1 receptors and the downstream PI3K/PKB signaling pathway, thereby inhibiting the activity of the tumor suppressor FoxO3a.Citation65–67 The PI3K/PKB signaling pathway promotes cell proliferation, accelerates cell metabolism, and repairs cells. Overexpression of the PI3K/PKB signaling pathway in patients with thyroid cancer indicates that hyperinsulinemia and elevated IGF-1 levels may play a role in the pathogenesis of thyroid cancer by affecting these signaling pathways.
Abnormal Secretion of Adipocytokines
Leptin, also known as the OB protein or obestatin, is a multifunctional polypeptide encoded by the obesity gene and secreted by adipocytes. It is a bioactive molecule that binds to receptors in the body and acts on the hypothalamus to inhibit appetite. Additionally, it regulates insulin secretion and promotes cell proliferation and angiogenesis.Citation68 A cross-sectional study demonstrated that leptin levels were significantly higher in patients with type 2 diabetes than in those without type 2 diabetes.Citation69 High expression of leptin receptors occurs in thyroid cancer cells, and high concentrations of leptin promote the proliferation of thyroid cancer cells and increase the risk of distant metastasis by activating important signaling pathways, such as PI3K/PKB and ERK/MAPK.Citation70–72 Many studies have shown that leptin is an adipocyte-derived cytokine that may be directly related to the occurrence and progression of thyroid, breast, and esophageal cancer.Citation73–75 Leptin interacts with many factors during the carcinogenic stage and participates in important signaling pathways involved in the occurrence and development of cancer. The relationship between leptin and thyroid, liver, breast, lung, prostate, pancreatic, and colorectal cancer has been confirmed in many studies.Citation76,Citation77 The leptin level in patients with diabetes is significantly higher than that in healthy individuals, and the leptin receptor is highly expressed in thyroid cancer cells. Therefore, high concentrations of leptin may promote the occurrence of thyroid cancer, indicating that an abnormal increase in leptin levels in patients with diabetes may be related to the occurrence and development of thyroid cancer.
Adiponectin (APN), also known as adipocyte complement-related protein, is a protein hormone secreted by adipocytes that has anti-diabetic, anti-vascular sclerosis, anti-inflammatory, and anti-tumor effects.Citation78 Bidulescu et al reported that APN levels in patients with type 2 diabetes were significantly lower than those in patients without type 2 diabetes.Citation79 The anti-tumor effect of APN may act directly on tumor cells through receptor-mediated pathways or indirectly on tumor cells by regulating insulin sensitivity and affecting tumor angiogenesis.Citation80 APN inhibits the proliferation of tumor cells in patients with breast, prostate, and colorectal cancers by affecting various intracellular signaling pathways. Additionally, APN levels are negatively correlated with thyroid cancer and play a protective role.Citation73,Citation81,Citation82 Furthermore, studies have revealed that patients with thyroid cancer who exhibit high expression of APN receptors have a better prognosis, Conversely, the level of APN in patients with diabetes is significantly decreased, which weakens the anti-tumor effect of APN and may lead to the occurrence and progression of thyroid cancer.Citation83–85 Therefore, a significant decrease in the APN levels in patients with diabetes may play a role in the pathogenesis of thyroid cancer.
Resistin is an adipokine produced by human adipocytes, monocytes, and macrophages that can promote cell proliferation, resist apoptosis, promote inflammation, and participate in angiogenesis.Citation86 It can affect the cell cycle and apoptosis through various mechanisms, thereby promoting tumor cell growth.Citation87,Citation88 Studies have found that resistin is abundantly expressed in breast cancer cells, and its expression level is positively correlated with tumor stage, size, and lymph node metastasis; similarly, patients with breast cancer with high resistin expression have a worse prognosis.Citation89,Citation90 Many studies have demonstrated that the concentration of resistin in patients with type 2 diabetes is higher than that in patients without type 2 diabetes, and the level of resistin in the plasma of patients with cancer is significantly higher than that in healthy controls, indicating that diabetic patients with high concentrations of resistin may be more prone to cancer than the healthy individuals.Citation91–93 Studies have revealed that resistin can stimulate the nuclear factor kB (NF-kB) signaling pathway by acting on Toll-like receptor 4 (TLR4), resulting in the production of interleukin-6 (IL-6) and tumor necrosis factor-α (TNF-α). However, IL-6 and TNF-α promote the proliferation of thyroid tumor cells, indicating that resistin can indirectly promote the development and progression of thyroid cancer.Citation94,Citation95 Many studies have demonstrated that resistin indirectly participates in cell proliferation and tumorigenesis by promoting the secretion of various inflammatory factors and the development of hyperinsulinemia.Citation96,Citation97 Abnormal resistin levels in patients with diabetes may accelerate the development of thyroid cancer. Therefore, neutralizing resistin may be a promising strategy for treating thyroid cancer.
Increased Secretion of Inflammatory Factors and Chemokines
Long-term hyperglycemia in patients with diabetes activates the growth factor cascade in the body through neurohumoral regulation, thereby increasing the production of inflammatory cytokines and chemokines. Studies have demonstrated that diabetes can promote the secretion of VEGF, IL, TNF, NF-kB, and other inflammatory factors and chemokines, and these are closely related to the occurrence of tumorsCitation98,Citation99 (). Additionally, NF-kB is a regulatory factor that inhibits apoptosis. A large increase in NF-kB is beneficial to the survival of thyroid tumor cells and upregulates VEGF expression, which in turn induces increased angiogenesis and lymph node metastasis in thyroid tumor tissues.Citation100 TNF-α is a cell-signaling protein mainly produced by T lymphocytes, natural killer (NK) cells, and activated macrophages. A meta-analysis revealed that the concentration of TNF-α in patients with thyroid cancer was significantly higher than that in the healthy control group. Overexpression of TNF-α in patients with thyroid cancer induces the excessive expression of TNF-α induced VEGF, promoting the proliferation of thyroid tumor cells and facilitating distant metastasis of tumor cells.Citation71 IL-6 is a pleiotropic cytokine that interferes with cell growth and differentiation. Studies have confirmed that IL-6 increases the risk of breast, liver, esophageal, and other cancers.Citation101 Other studies have demonstrated that IL-6 increases the invasiveness of thyroid tumor cells by activating the MAPK and JAK-STAT3 signaling pathways, resulting in the overexpression of the transcription factors p-c-Jun and p-stat3. These transcription factors bind to the promoter programmed cell death 1 ligand 1 (PD-L1) to enhance gene transcription.Citation95,Citation102,Citation103 IL-8 is a chemokine that promotes mitosis, inflammation and angiogenesis. Liotti et al reported that IL-8 was overexpressed in patients with thyroid cancer, and it directly and indirectly, participates in the growth and migration of thyroid tumor cells. Overexpression of IL-8 is significantly correlated with thyroid cancer prognosis.Citation104 Therefore, significantly increased levels of inflammatory factors and chemokines in patients with diabetes may be risk factors for the occurrence and progression of thyroid cancer.
Figure 2 Increased secretion of inflammatory factors and chemokines promotes the occurrence and progression of thyroid cancer.
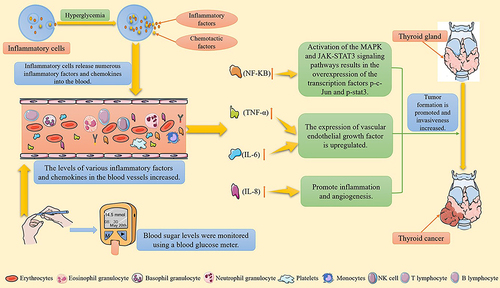
Anti-Thyroid Cancer Effects of Some Hypoglycemic Drugs
Thiazolidinediones
Thiazolidinediones (TZDs) are selective agonists of peroxisome proliferator-activated receptor γ (PPARγ), which improve glucose and lipid metabolism by increasing the sensitivity of target cells to insulin when activated.Citation105 It is worth noting that the use of thiazolidinediones in diabetic patients is declining significantly and is even almost completely removed from the market.Citation106,Citation107 Studies have revealed that activated PPARγ can inhibit tumorigenesis through anti-angiogenic and anti-inflammatory effects and can also control the tumor microenvironment by regulating the function of tumor cells, ultimately playing a role in resisting tumor metastasis.Citation108,Citation109 Lv et al reported that rosiglitazone and pioglitazone can inhibit the PI3K/PKB signaling pathway by activating PPARγ, thereby inducing cell cycle G2 arrest and apoptosis of the bladder cancer cells, ultimately inhibiting cell proliferation in vitro and tumor cell growth in vivo.Citation110 Kebebew et al observed that rosiglitazone treatment increased the uptake of radioactive iodine in patients with partially differentiated thyroid cancer and reduced serum thyroglobulin levels, which may assist in the treatment of patients with radioactive iodine-negative thyroid cancer.Citation111 Studies have demonstrated that the use of rosiglitazone can reduce the risk of thyroid cancer in patients with type 2 diabetes, particularly in patients older than 50 years.Citation112 Chen et al demonstrated that high expression of PPARγ in patients with thyroid cancer can significantly improve their recurrence-free survival. The study also revealed that rosiglitazone treatment could inhibit the growth of thyroid cancer cells and increase the expression of sodium/iodide cotransporter, thereby improving the prognosis of patients with thyroid cancer.Citation113 A basic study found that Lobeglitazone can inhibit the migration and invasion of thyroid cancer by inhibiting the P38 MAPK signaling pathway.Citation114 Tsubaki et al demonstrated that pioglitazone induces apoptosis through a PPARγ-independent pathway, thus describing pioglitazone as a potential therapeutic drug for controlling the progression of different cancers.Citation115 With an in-depth research on TZDs, these may potentially develop as chemotherapeutic drugs for thyroid cancer in the future.
Metformin
Metformin primarily increases glucose uptake in peripheral tissues, reduces liver glycogen output, inhibits gluconeogenesis, and reduces intestinal glucose absorption. It is the most commonly used first-line treatment for type 2 diabetes and is a potential anticancer drug.Citation116,Citation117 The viability of thyroid cancer cells decreased gradually with increasing metformin dose, and the apoptosis rate of cancer cells increased with increasing metformin concentration. This may be attributed to the downregulation of low-density lipoprotein receptor-related protein 2, which blocks the c-Jun N-terminal kinase signaling pathway to inhibit the proliferation of thyroid cancer cells and induce apoptosis.Citation118 Ye et al demonstrated that metformin inhibits the proliferation of thyroid cancer cells and induces apoptosis in a concentration- and time-dependent manner. The mechanism may involve increasing the apoptosis of thyroid cancer cells and inhibiting their proliferation by activating endoplasmic reticulum stress-related pathways.Citation119 Many studies have demonstrated that metformin can reduce the expression of the proto-oncogenes Cyclin D1 and c-Myc by inhibiting the mTOR signaling pathway, thereby inhibiting the proliferation, migration, and epithelial-mesenchymal transition of thyroid cancer cells.Citation117,Citation120,Citation121 Therefore, metformin and its molecular targets may contribute to the treatment of thyroid cancer. One study reported that the use of metformin in the treatment of patients with thyroid cancer can reduce tumor volume, and the lack of metformin treatment is an independent risk factor for reducing the complete remission rate and shortening progression-free survival in thyroid cancer.Citation122,Citation123 Cho et al observed no significant decrease in the risk of thyroid cancer in patients with early-stage diabetes receiving metformin; however, the effect of metformin on thyroid cancer was time- and concentration-dependent, leading to an overall significant reduction in the risk of thyroid cancer.Citation124 Therefore, metformin may be preferred as a treatment for patients with diabetes and thyroid cancer. The role and advantages of metformin in the treatment of thyroid cancer are becoming increasingly prominent, offering new perspectives for the clinical management of thyroid cancer in the future.
Alpha-Glucosidase Inhibitors
The hypoglycemic principle of α-glucosidase inhibitors is to inhibit glycosidase at the brush border of the small intestinal mucosa, thereby reducing the intestinal absorption of glucose by blocking the hydrolysis of complex carbohydrates.Citation125 Zhan et al conducted a study on studying mouse colon cancer and melanoma models and demonstrated that acarbose significantly inhibited tumor growth and further enhanced the therapeutic effect of anti-PD1, indicating that acarbose has the potential to be used in clinical tumor treatment.Citation126 Lai et al reported that the use of acarbose significantly reduced the risk of lung cancer in patients with diabetes in a randomized controlled trial.Citation127 Further, Tseng et al observed that acarbose dose-dependently reduced the risk of colorectal cancer by approximately 27% in patients with diabetes.Citation128 Acarbose, the most widely used α-glucosidase inhibitor in clinical practice, has been demonstrated to reduce the thyroid cancer metastasis risk; however, this risk is greater among diabetic patients with thyroid cancer compared to those without; nevertheless, acarbose use was demonstrated to significantly lower invasive tumor growth risk in patients with thyroid cancer compared to those who received other hypoglycemic drugs due to its intestinal flora interaction effects inhibiting thyroid cell proliferation.Citation129,Citation130 However, due to limited clinical trials and lack of understanding of the pathological mechanisms of acarbose, further large-sample, multicenter studies are required to verify the effects of acarbose on thyroid cancer cells growth and its potential to reduce the risk of distant metastasis in thyroid cancer.
Conclusions
A complex relationship exists between diabetes and thyroid cancer. Diabetes increases the risk of thyroid cancer, which may be associated with genetic factors, abnormal TSH secretion, oxidative stress injury, hyperinsulinemia, elevated IGF-1 levels, abnormal secretion of adipocytokines, and increased secretion of inflammatory factors and chemokines. Additionally, diabetes is closely associated with the occurrence, development, and prognosis of thyroid cancer. The correlation between thyroid cancer and diabetes still requires further investigation and improvement. In addition to the conducting more large-sample prospective studies to confirm the potential association between thyroid cancer and diabetes, we also need to focus on an in-depth exploration of preventive measures to effectively manage the risk of thyroid cancer in patients with diabetes. Furthermore, the potential common pathogenesis between thyroid cancer and diabetes suggests that there may be shared underlying mechanisms. The use of certain hypoglycemic drugs reduces the risk of thyroid cancer and distant metastasis. Therefore, conducting more large-sample multicenter clinical trials and mechanistic studies on hypoglycemic drugs may help identify novel targets for thyroid cancer treatment, providing new directions for the clinical prevention and treatment of thyroid cancer.
Data Sharing Statement
The raw data supporting the conclusions of this article will be made available by the authors without undue reservation.
Ethics Statement
This study does not involve human or animal research, and there is no ethical statement about the study.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We would like to thank Editage for English language editing.
Additional information
Funding
References
- Ahmad E, Lim S, Lamptey R, Webb DR, Davies MJ. Type 2 diabetes. Lancet. 2022;400(10365):1803–1820. doi:10.1016/S0140-6736(22)01655-5
- Quattrin T, Mastrandrea LD, Walker LSK. Type 1 diabetes. Lancet. 2023;401(10394):2149–2162. doi:10.1016/S0140-6736(23)00223-4
- Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022;72(1):7–33. doi:10.3322/caac.21708
- Lamartina L, Leboulleux S, Borget I, Schlumberger M. Global thyroid estimates in 2020. Lancet Diabetes Endocrinol. 2022;10(4):235–236. doi:10.1016/S2213-8587(22)00048-1
- Chen DW, Lang BHH, McLeod DSA, Newbold K, Haymart MR. Thyroid cancer. Lancet. 2023;401(10387):1531–1544. doi:10.1016/S0140-6736(23)00020-X
- Greenwood M, Wood F. The relation between the cancer and diabetes death-rates. J Hyg. 1914;14(1):83–118. doi:10.1017/S0022172400005702
- Popovic K, Smolovic B, Martinovic M, Vuckovic L. The relationship between diabetes mellitus and pancreatic cancer-diabetes mellitus as a red flag for pancreatic cancer. Cancer Epidemiol Biomarkers Prev. 2023;32(3):298–305. doi:10.1158/1055-9965.EPI-22-0951
- Guo J, Liu C, Pan J, Yang J. Relationship between diabetes and risk of gastric cancer: a systematic review and meta-analysis of cohort studies. Diabet Res Clin Pract. 2022;187:109866. doi:10.1016/j.diabres.2022.109866
- Sun X, Zhang Q, Kadier K, et al. Association between diabetes status and breast cancer in US adults: findings from the US National Health and Nutrition Examination Survey. Front Endocrinol. 2023;14:1059303. doi:10.3389/fendo.2023.1059303
- Khoa Ta HD, Nguyen NN, Ho DKN, et al. Association of diabetes mellitus with early-onset colorectal cancer: a systematic review and meta-analysis of 19 studies including 10 million individuals and 30,000 events. Diabetes Metab Syndr. 2023;17(8):102828. doi:10.1016/j.dsx.2023.102828
- Aschebrook-Kilfoy B, Sabra MM, Brenner A, et al. Diabetes and thyroid cancer risk in the National Institutes of Health-AARP Diet and Health Study. Thyroid. 2011;21(9):957–963. doi:10.1089/thy.2010.0396
- Linkeviciute-Ulinskiene D, Patasius A, Zabuliene L, Stukas R, Smailyte G. Increased risk of site-specific cancer in people with type 2 diabetes: a National Cohort Study. Int J Environ Res Public Health. 2019;17(1):246. doi:10.3390/ijerph17010246
- Yeo Y, Ma SH, Hwang Y, et al. Diabetes mellitus and risk of thyroid cancer: a meta-analysis. PLoS One. 2014;9(6):e98135. doi:10.1371/journal.pone.0098135
- Zhan YS, Feng L, Tang SH, et al. Glucose metabolism disorders in cancer patients in a Chinese population. Med Oncol. 2010;27(2):177–184. doi:10.1007/s12032-009-9189-9
- Yin DT, He H, Yu K, et al. The association between thyroid cancer and insulin resistance, metabolic syndrome and its components: a systematic review and meta-analysis. Int J Surg. 2018;57:66–75. doi:10.1016/j.ijsu.2018.07.013
- Seo YG, Choi HC, An AR, et al. The Association between Type 2 Diabetes Mellitus and Thyroid Cancer. J Diabetes Res. 2017;2017:5850879. doi:10.1155/2017/5850879
- Carstensen B, Read SH, Friis S, et al. Cancer incidence in persons with type 1 diabetes: a five-country study of 9000 cancers in type 1 diabetic individuals. Diabetologia. 2016;59(5):980–988. doi:10.1007/s00125-016-3884-9
- Li H, Qian J. Association of diabetes mellitus with thyroid cancer risk: a meta-analysis of cohort studies. Medicine. 2017;96(47):e8230.
- Dong WW, Zhang DL, Wang ZH, Lv CZ, Zhang P, Zhang H. Different types of diabetes mellitus and risk of thyroid cancer: a meta-analysis of cohort studies. Front Endocrinol. 2022;13:971213. doi:10.3389/fendo.2022.971213
- Saewai C, Fumaneeshoat O, Thongsuksai P, Ingviya T. Diabetes mellitus as cancer risk: a 14-year, cross-sectional analysis. Nutr Cancer. 2023;75(6):1454–1463. doi:10.1080/01635581.2023.2205054
- Hu Y, Zhang X, Ma Y, et al. Incident type 2 diabetes duration and cancer risk: a prospective study in two US cohorts. J Natl Cancer Inst. 2021;113(4):381–389. doi:10.1093/jnci/djaa141
- Bezin J, Gouverneur A, Penichon M, et al. GLP-1 receptor agonists and the risk of thyroid cancer. Diabetes Care. 2023;46(2):384–390. doi:10.2337/dc22-1148
- Cadenas S. Mitochondrial uncoupling, ROS generation and cardioprotection. Biochim Biophys Acta Bioenergy. 2018;1859(9):940–950. doi:10.1016/j.bbabio.2018.05.019
- Kunji ERS, King MS, Ruprecht JJ, Thangaratnarajah C. The SLC25 carrier family: important transport proteins in mitochondrial physiology and pathology. Physiology. 2020;35(5):302–327. doi:10.1152/physiol.00009.2020
- Huang R, Cai T, Zhou Y, et al. Ethnicity differences in the association of UCP1-3826A/G, UCP2-866G/A and Ala55Val, and UCP3-55C/T polymorphisms with type 2 diabetes mellitus susceptibility: an updated meta-analysis. Biomed Res Int. 2021;2021:3482879. doi:10.1155/2021/3482879
- Zhou M, He S, Ping F, et al. Uncoupling protein 2 and peroxisome proliferator-activated receptor gamma gene polymorphisms in association with diabetes susceptibility in Chinese Han Population with Variant Glucose Tolerance. Int J Endocrinol. 2018;2018:4636783. doi:10.1155/2018/4636783
- Zhou TC, Yang L, Liu YY, et al. Polymorphisms in the uncoupling protein 2 gene are associated with diabetic retinopathy in Han Chinese Patients with type 2 diabetes. Genet Test Mol Biomarkers. 2018;22(11):637–643. doi:10.1089/gtmb.2018.0115
- Marques D, Ferreira-Costa LR, Ferreira-Costa LL, et al. Association of insertion-deletions polymorphisms with colorectal cancer risk and clinical features. World J Gastroenterol. 2017;23(37):6854–6867. doi:10.3748/wjg.v23.i37.6854
- Seshadri N, Jonasson ME, Hunt KL, et al. Uncoupling protein 2 regulates daily rhythms of insulin secretion capacity in MIN6 cells and isolated islets from male mice. Mol Metab. 2017;6(7):760–769. doi:10.1016/j.molmet.2017.04.008
- Din I, Majid S, Rashid F, et al. Mitochondrial uncoupling protein 2 (UCP2) gene polymorphism - 866 G/A in the promoter region is associated with type 2 diabetes mellitus among Kashmiri population of Northern India. Mol Biol Rep. 2023;50(1):475–483. doi:10.1007/s11033-022-08055-z
- Li W, Zhang C, Jackson K, et al. UCP2 knockout suppresses mouse skin carcinogenesis. Cancer Prev Res. 2015;8(6):487–491. doi:10.1158/1940-6207.CAPR-14-0297-T
- Yu G, Wang J, Xu K, Dong J. Dynamic regulation of uncoupling protein 2 expression by microRNA-214 in hepatocellular carcinoma. Biosci Rep. 2016;36(3). doi:10.1042/BSR20160062
- Hua J, Zhang Z, Zhang L, Sun Y, Yuan Y. UCP-2 inhibitor enhanced the efficacy of trastuzumab against HER2 positive breast cancer cells. Cancer Chemother Pharmacol. 2021;88(4):633–642. doi:10.1007/s00280-021-04303-4
- Hima S, Sreeja S. Regulatory role of estrogen-induced reactive oxygen species in the modulatory function of UCP 2 in papillary thyroid cancer cells. IUBMB Life. 2015;67(11):837–846. doi:10.1002/iub.1440
- Li J, Jiang R, Cong X, Zhao Y. UCP2 gene polymorphisms in obesity and diabetes, and the role of UCP2 in cancer. FEBS Lett. 2019;593(18):2525–2534. doi:10.1002/1873-3468.13546
- Woyda-Ploszczyca AM, Jarmuszkiewicz W. The conserved regulation of mitochondrial uncoupling proteins: from unicellular eukaryotes to mammals. Biochim Biophys Acta Bioenergy. 2017;1858(1):21–33. doi:10.1016/j.bbabio.2016.10.003
- Song YS, Kim MJ, Sun HJ, et al. Aberrant thyroid-stimulating hormone receptor signaling increases VEGF-A and CXCL8 secretion of thyroid cancer cells, contributing to angiogenesis and tumor growth. Clin Cancer Res. 2019;25(1):414–425. doi:10.1158/1078-0432.CCR-18-0663
- Cho JH, Kim HJ, Lee JH, et al. Poor glycemic control is associated with the risk of subclinical hypothyroidism in patients with type 2 diabetes mellitus. Korean J Intern Med. 2016;31(4):703–711. doi:10.3904/kjim.2015.198
- Adhami M, Michail P, Rao A, et al. Anti-Thyroid Antibodies and TSH as potential markers of thyroid carcinoma and aggressive behavior in patients with indeterminate fine-needle aspiration cytology. World J Surg. 2020;44(2):363–370. doi:10.1007/s00268-019-05153-1
- Schiffmann L, Kostev K, Kalder M. Association between various thyroid gland diseases, TSH values and thyroid cancer: a case-control study. J Cancer Res Clin Oncol. 2020;146(11):2989–2994. doi:10.1007/s00432-020-03283-x
- Zou M, Baitei EY, Al-Rijjal RA, et al. TSH overcomes Braf(V600E)-induced senescence to promote tumor progression via downregulation of p53 expression in papillary thyroid cancer. Oncogene. 2016;35(15):1909–1918. doi:10.1038/onc.2015.253
- Papaleontiou M, Chen DW, Banerjee M, et al. Thyrotropin suppression for papillary thyroid cancer: a Physician Survey Study. Thyroid. 2021;31(9):1383–1390. doi:10.1089/thy.2021.0033
- Hasnain SZ, Borg DJ, Harcourt BE, et al. Glycemic control in diabetes is restored by therapeutic manipulation of cytokines that regulate beta cell stress. Nat Med. 2014;20(12):1417–1426. doi:10.1038/nm.3705
- Masenga SK, Kabwe LS, Chakulya M, Kirabo A. Mechanisms of oxidative stress in metabolic syndrome. Int J Mol Sci. 2023;24(9):7898. doi:10.3390/ijms24097898
- Yaribeygi H, Sathyapalan T, Atkin SL, Sahebkar A. Molecular mechanisms linking oxidative stress and diabetes mellitus. Oxid Med Cell Longev. 2020;2020:8609213. doi:10.1155/2020/8609213
- Thonsri U, Wongkham S, Wongkham C, et al. High glucose-ROS conditions enhance the progression in cholangiocarcinoma via upregulation of MAN2A2 and CHD8. Cancer Sci. 2021;112(1):254–264. doi:10.1111/cas.14719
- Ramteke P, Deb A, Shepal V, Bhat MK. Hyperglycemia associated metabolic and molecular alterations in cancer risk, progression, treatment, and mortality. Cancers. 2019;11(9):1402. doi:10.3390/cancers11091402
- Maradagi T, Kumar R, Ponesakki G. Hyperglycaemia-induced human hepatocellular carcinoma (HepG2) cell proliferation through ROS-mediated P38 activation is effectively inhibited by a xanthophyll carotenoid, lutein. Diabet Med. 2022;39(2):e14713. doi:10.1111/dme.14713
- Xing M, Alzahrani AS, Carson KA, et al. Association between BRAF V600E mutation and recurrence of papillary thyroid cancer. J Clin Oncol. 2015;33(1):42–50. doi:10.1200/JCO.2014.56.8253
- Wieczorek-Szukala K, Kopczynski J, Kowalska A, Lewinski A. Snail-1 overexpression correlates with metastatic phenotype in BRAF(V600E) positive papillary thyroid carcinoma. J Clin Med. 2020;9(9):2701. doi:10.3390/jcm9092701
- Han PA, Kim HS, Cho S, et al. Association of BRAF V600E mutation and MicroRNA expression with central lymph node metastases in papillary thyroid cancer: a prospective study from four endocrine surgery centers. Thyroid. 2016;26(4):532–542. doi:10.1089/thy.2015.0378
- Zhang Z, Xu T, Qin W, et al. Upregulated PTPN2 induced by inflammatory response or oxidative stress stimulates the progression of thyroid cancer. Biochem Biophys Res Commun. 2020;522(1):21–25. doi:10.1016/j.bbrc.2019.11.047
- Tabur S, Aksoy SN, Korkmaz H, Ozkaya M, Aksoy N, Akarsu E. Investigation of the role of 8-OHdG and oxidative stress in papillary thyroid carcinoma. Tumour Biol. 2015;36(4):2667–2674. doi:10.1007/s13277-014-2889-6
- Ziros PG, Manolakou SD, Habeos IG, et al. Nrf2 is commonly activated in papillary thyroid carcinoma, and it controls antioxidant transcriptional responses and viability of cancer cells. J Clin Endocrinol Metab. 2013;98(8):E1422–E1427. doi:10.1210/jc.2013-1510
- Manzella L, Massimino M, Stella S, et al. Activation of the IGF axis in thyroid cancer: implications for tumorigenesis and treatment. Int J Mol Sci. 2019;20(13):3258. doi:10.3390/ijms20133258
- Miller BS, Rogol AD, Rosenfeld RG. The history of the insulin-like growth factor system. Horm Res Paediatr. 2022;95(6):619–630. doi:10.1159/000527123
- Cheng HC, Chang TK, Su WC, Tsai HL, Wang JY. Narrative review of the influence of diabetes mellitus and hyperglycemia on colorectal cancer risk and oncological outcomes. Transl Oncol. 2021;14(7):101089. doi:10.1016/j.tranon.2021.101089
- Morrione A, Belfiore A. Obesity, diabetes, and cancer: the role of the Insulin/IGF axis; mechanisms and clinical implications. Biomolecules. 2022;12(5):612. doi:10.3390/biom12050612
- Deleu S, Pirson I, Coulonval K, et al. IGF-1 or insulin, and the TSH cyclic AMP cascade separately control dog and human thyroid cell growth and DNA synthesis, and complement each other in inducing mitogenesis. Mol Cell Endocrinol. 1999;149(1–2):41–51. doi:10.1016/S0303-7207(99)00005-2
- Wang K, Yang Y, Wu Y, et al. The association between insulin resistance and vascularization of thyroid nodules. J Clin Endocrinol Metab. 2015;100(1):184–192. doi:10.1210/jc.2014-2723
- Heidari Z, Mashhadi MA, Nosratzehi S. Insulin resistance in patients with benign thyroid nodules. Arch Iran Med. 2015;18(9):572–576.
- Murphy N, Carreras-Torres R, Song M, et al. Circulating levels of insulin-like growth factor 1 and insulin-like growth factor binding protein 3 associate with risk of colorectal cancer based on serologic and Mendelian randomization analyses. Gastroenterology. 2020;158(5):1300–12 e20. doi:10.1053/j.gastro.2019.12.020
- Murphy N, Knuppel A, Papadimitriou N, et al. Insulin-like growth factor-1, insulin-like growth factor-binding protein-3, and breast cancer risk: observational and Mendelian randomization analyses with approximately 430 000 women. Ann Oncol. 2020;31(5):641–649. doi:10.1016/j.annonc.2020.01.066
- Watts EL, Perez-Cornago A, Fensom GK, et al. Circulating insulin-like growth factors and risks of overall, aggressive and early-onset prostate cancer: a collaborative analysis of 20 prospective studies and Mendelian randomization analysis. Int J Epidemiol. 2023;52(1):71–86. doi:10.1093/ije/dyac124
- Zhang X, Sheng X, Miao T, Yao K, Yao D. Effect of insulin on thyroid cell proliferation, tumor cell migration, and potentially related mechanisms. Endocr Res. 2019;44(1–2):55–70. doi:10.1080/07435800.2018.1522641
- Pollak M. The insulin and insulin-like growth factor receptor family in neoplasia: an update. Nat Rev Cancer. 2012;12(3):159–169. doi:10.1038/nrc3215
- Nozhat Z, Hedayati M. PI3K/AKT Pathway and Its Mediators in Thyroid Carcinomas. Mol Diagn Ther. 2016;20(1):13–26. doi:10.1007/s40291-015-0175-y
- Ghasemi A, Saeidi J, Azimi-Nejad M, Hashemy SI. Leptin-induced signaling pathways in cancer cell migration and invasion. Cell Oncol. 2019;42(3):243–260. doi:10.1007/s13402-019-00428-0
- Mohammed Saeed W, Nasser Binjawhar D. Association of serum leptin and adiponectin concentrations with type 2 diabetes biomarkers and complications among Saudi women. Diabetes Metab Syndr Obes. 2023;16:2129–2140. doi:10.2147/DMSO.S405476
- Revilla G, Corcoy R, Moral A, Escola-Gil JC, Mato E. Cross-talk between inflammatory mediators and the epithelial mesenchymal transition process in the development of thyroid carcinoma. Int J Mol Sci. 2019;20(10):2466. doi:10.3390/ijms20102466
- Zhao J, Wen J, Wang S, Yao J, Liao L, Dong J. Association between adipokines and thyroid carcinoma: a meta-analysis of case-control studies. BMC Cancer. 2020;20(1):788. doi:10.1186/s12885-020-07299-x
- Celano M, Maggisano V, Lepore SM, et al. Expression of leptin receptor and effects of leptin on papillary thyroid carcinoma cells. Int J Endocrinol. 2019;2019:5031696. doi:10.1155/2019/5031696
- Nigro E, Orlandella FM, Polito R, et al. Adiponectin and leptin exert antagonizing effects on proliferation and motility of papillary thyroid cancer cell lines. J Physiol Biochem. 2021;77(2):237–248. doi:10.1007/s13105-021-00789-x
- Xie SH, Rabbani S, Ness-Jensen E, Lagergren J. Circulating levels of inflammatory and metabolic biomarkers and risk of esophageal adenocarcinoma and Barrett esophagus: systematic review and meta-analysis. Cancer Epidemiol Biomarkers Prev. 2020;29(11):2109–2118. doi:10.1158/1055-9965.EPI-20-0572
- Wang Y, Du L, Jing J, Zhao X, Wang X, Hou S. Leptin and leptin receptor expression as biomarkers for breast cancer: a retrospective study. BMC Cancer. 2023;23(1):260. doi:10.1186/s12885-023-10617-8
- Vansaun MN, Cheng H-C, Chang T-K, Su W-C, Tsai H-L, Wang J-Y. Molecular pathways: adiponectin and leptin signaling in cancer. Clin Cancer Res. 2013;19(8):1926–1932. doi:10.1158/1078-0432.CCR-12-0930
- Casado ME, Collado-Perez R, Frago LM, Barrios V. Recent advances in the knowledge of the mechanisms of leptin physiology and actions in neurological and metabolic pathologies. Int J Mol Sci. 2023;24(2):1422.
- Iwabu M, Yamauchi T, Okada-Iwabu M, et al. Adiponectin and AdipoR1 regulate PGC-1alpha and mitochondria by Ca(2+) and AMPK/SIRT1. Nature. 2010;464(7293):1313–1319. doi:10.1038/nature08991
- Bidulescu A, Dinh PC, Sarwary S, et al. Associations of leptin and adiponectin with incident type 2 diabetes and interactions among African Americans: the Jackson heart study. BMC Endocr Disord. 2020;20(1):31. doi:10.1186/s12902-020-0511-z
- Tsankof A, Tziomalos K. Adiponectin: a player in the pathogenesis of hormone-dependent cancers. Front Endocrinol. 2022;13:1018515. doi:10.3389/fendo.2022.1018515
- Hopkins BD, Goncalves MD, Cantley LC. Obesity and cancer mechanisms: cancer metabolism. J Clin Oncol. 2016;34(35):4277–4283. doi:10.1200/JCO.2016.67.9712
- Christodoulatos GS, Spyrou N, Kadillari J, Psallida S, Dalamaga M. The role of adipokines in breast cancer: current evidence and perspectives. Curr Obes Rep. 2019;8(4):413–433. doi:10.1007/s13679-019-00364-y
- Tumminia A, Vinciguerra F, Parisi M, et al. Adipose tissue, obesity and adiponectin: role in endocrine cancer risk. Int J Mol Sci. 2019;20(12):2863. doi:10.3390/ijms20122863
- Dossus L, Franceschi S, Biessy C, et al. Adipokines and inflammation markers and risk of differentiated thyroid carcinoma: the EPIC study. Int J Cancer. 2018;142(7):1332–1342. doi:10.1002/ijc.31172
- Maleki M, Karajibani M, Sarvani M, Montazerifar F, Salimi S, Heidari Z. Correlation between adiponectin rs2241766 and rs266729 polymorphisms and risk of papillary thyroid cancer. Mol Biol Res Commun. 2022;11(3):113–118. doi:10.22099/mbrc.2022.43012.1714
- Patel SD, Rajala MW, Rossetti L, Scherer PE, Shapiro L. Disulfide-dependent multimeric assembly of resistin family hormones. Science. 2004;304(5674):1154–1158. doi:10.1126/science.1093466
- Qiu L, Zhang GF, Yu L, Wang HY, Jia XJ, Wang TJ. Novel oncogenic and chemoresistance-inducing functions of resistin in ovarian cancer cells require miRNAs-mediated induction of epithelial-to-mesenchymal transition. Sci Rep. 2018;8(1):12522. doi:10.1038/s41598-018-30978-6
- Gao Y, Chen X, He Q, et al. Adipocytes promote breast tumorigenesis through TAZ-dependent secretion of Resistin. Proc Natl Acad Sci U S A. 2020;117(52):33295–33304. doi:10.1073/pnas.2005950117
- Rosendahl AH, Bergqvist M, Lettiero B, Kimbung S, Borgquist S. Adipocytes and obesity-related conditions jointly promote breast cancer cell growth and motility: associations with CAP1 for prognosis. Front Endocrinol. 2018;9:689. doi:10.3389/fendo.2018.00689
- Wang YY, Hung AC, Wu YC, et al. ADSCs stimulated by resistin promote breast cancer cell malignancy via CXCL5 in a breast cancer coculture model. Sci Rep. 2022;12(1):15437. doi:10.1038/s41598-022-19290-6
- Cobbold C. Type 2 diabetes mellitus risk and exercise: is resistin involved? J Sports Med Phys Fitness. 2019;59(2):290–297. doi:10.23736/S0022-4707.18.08258-0
- Peng X, Huang J, Zou H, et al. Roles of plasma leptin and resistin in novel subgroups of type 2 diabetes driven by cluster analysis. Lipids Health Dis. 2022;21(1):7. doi:10.1186/s12944-022-01623-z
- Gong WJ, Liu JY, Yin JY, et al. Resistin facilitates metastasis of lung adenocarcinoma through the TLR4/Src/EGFR/PI3K/NF-kappaB pathway. Cancer Sci. 2018;109(8):2391–2400. doi:10.1111/cas.13704
- Li B, Fang J, Zuo Z, et al. Activation of the porcine alveolar macrophages via toll-like receptor 4/NF-kappaB mediated pathway provides a mechanism of resistin leading to inflammation. Cytokine. 2018;110:357–366. doi:10.1016/j.cyto.2018.04.002
- Zhang GQ, Jiao Q, Shen CT, et al. Interleukin 6 regulates the expression of programmed cell death ligand 1 in thyroid cancer. Cancer Sci. 2021;112(3):997–1010. doi:10.1111/cas.14752
- Sudan SK, Deshmukh SK, Poosarla T, et al. Resistin: an inflammatory cytokine with multi-faceted roles in cancer. Biochim Biophys Acta Rev Cancer. 2020;1874(2):188419. doi:10.1016/j.bbcan.2020.188419
- Deshmukh SK, Srivastava SK, Bhardwaj A, et al. Resistin and interleukin-6 exhibit racially-disparate expression in breast cancer patients, display molecular association and promote growth and aggressiveness of tumor cells through STAT3 activation. Oncotarget. 2015;6(13):11231–11241. doi:10.18632/oncotarget.3591
- Dharshini LCP, Rasmi RR, Kathirvelan C, Kumar KM, Saradhadevi KM, Sakthivel KM. Regulatory components of oxidative stress and inflammation and their complex interplay in carcinogenesis. Appl Biochem Biotechnol. 2023;195(5):2893–2916. doi:10.1007/s12010-022-04266-z
- Peng Y, Wang P, Gong J, et al. Association between the Finnish Diabetes Risk Score and cancer in middle-aged and older adults: involvement of inflammation. Metabolism. 2023;144:155586. doi:10.1016/j.metabol.2023.155586
- Warakomski J, Romuk E, Jarzab B, Krajewska J, Sieminska L. Concentrations of selected adipokines, interleukin-6, and vitamin D in patients with papillary thyroid carcinoma in respect to thyroid cancer stages. Int J Endocrinol. 2018;2018:4921803. doi:10.1155/2018/4921803
- Mauer J, Denson JL, Bruning JC. Versatile functions for IL-6 in metabolism and cancer. Trends Immunol. 2015;36(2):92–101. doi:10.1016/j.it.2014.12.008
- Liang YN, Zhang Z, Song J, Yang F, Yang P, Wei X. Role of STAT3 expression in thyroid cancer: a meta-analysis and systematic review based on the Chinese Population. Evid Based Complement Alternat Med. 2022;2022:1116535. doi:10.1155/2022/1116535
- Zhang GQ, Xi C, Shen CT, Song HJ, Luo QY, Qiu ZL. Interleukin-6 promotes the dedifferentiation of papillary thyroid cancer cells. Endocr Relat Cancer. 2023;30(9). doi:10.1530/ERC-23-0130
- Liotti F, Collina F, Pone E, et al. Interleukin-8, but not the Related Chemokine CXCL1, sustains an autocrine circuit necessary for the properties and functions of thyroid cancer stem cells. Stem Cells. 2017;35(1):135–146. doi:10.1002/stem.2492
- Retnakaran R, Zinman B. Thiazolidinediones and clinical outcomes in type 2 diabetes. Lancet. 2009;373(9681):2088–2090. doi:10.1016/S0140-6736(09)61029-1
- Lipska KJ, Yao X, Herrin J, et al. Trends in drug utilization, glycemic control, and rates of severe hypoglycemia, 2006–2013. Diabetes Care. 2017;40(4):468–475. doi:10.2337/dc16-0985
- Greco A, Coperchini F, Croce L, Magri F, Teliti M, Rotondi M. Drug repositioning in thyroid cancer treatment: the intriguing case of anti-diabetic drugs. Front Pharmacol. 2023;14:1303844. doi:10.3389/fphar.2023.1303844
- Glazer RI. PPARdelta as a metabolic initiator of mammary neoplasia and immune tolerance. PPAR Res. 2016;2016:3082340. doi:10.1155/2016/3082340
- Lakshmi SP, Reddy AT, Banno A, Reddy RC. PPAR agonists for the prevention and treatment of lung cancer. PPAR Res. 2017;2017:8252796. doi:10.1155/2017/8252796
- Lv S, Wang W, Wang H, Zhu Y, Lei C. PPARgamma activation serves as therapeutic strategy against bladder cancer via inhibiting PI3K-Akt signaling pathway. BMC Cancer. 2019;19(1):204. doi:10.1186/s12885-019-5426-6
- Kebebew E, Lindsay S, Clark OH, Woeber KA, Hawkins R, Greenspan FS. Results of rosiglitazone therapy in patients with thyroglobulin-positive and radioiodine-negative advanced differentiated thyroid cancer. Thyroid. 2009;19(9):953–956. doi:10.1089/thy.2008.0371
- Tseng CH. Rosiglitazone may reduce thyroid cancer risk in patients with type 2 diabetes. Ann Med. 2013;45(8):539–544. doi:10.3109/07853890.2013.851865
- Chen JY, Wang JJ, Lee HC, Chi CW, Lee CH, Hsu YC. Combination of peroxisome proliferator-activated receptor gamma and retinoid X receptor agonists induces sodium/iodide symporter expression and inhibits cell growth of human thyroid cancer cells. J Chin Med Assoc. 2020;83(10):923–930. doi:10.1097/JCMA.0000000000000389
- Jin JQ, Han JS, Ha J, Baek HS, Lim DJ. Lobeglitazone, a peroxisome proliferator-activated receptor-gamma agonist, inhibits papillary thyroid cancer cell migration and invasion by suppressing p38 MAPK signaling pathway. Endocrinol Metab. 2021;36(5):1095–1110. doi:10.3803/EnM.2021.1155
- Tsubaki M, Takeda T, Tomonari Y, et al. Pioglitazone inhibits cancer cell growth through STAT3 inhibition and enhanced AIF expression via a PPARgamma-independent pathway. J Cell Physiol. 2018;233(4):3638–3647. doi:10.1002/jcp.26225
- Vancura A, Bu P, Bhagwat M, Zeng J, Vancurova I. Metformin as an anticancer agent. Trends Pharmacol Sci. 2018;39(10):867–878. doi:10.1016/j.tips.2018.07.006
- Garcia-Saenz M, Lobaton-Ginsberg M, Ferreira-Hermosillo A. Metformin in differentiated thyroid cancer: molecular pathways and its clinical implications. Biomolecules. 2022;12(4):574. doi:10.3390/biom12040574
- He Y, Cao L, Wang L, Liu L, Huang Y, Gong X. Metformin inhibits proliferation of human thyroid cancer TPC-1 cells by decreasing LRP2 to suppress the JNK pathway. Onco Targets Ther. 2020;13:45–50. doi:10.2147/OTT.S227915
- Ye J, Qi L, Chen K, et al. Metformin induces TPC-1 cell apoptosis through endoplasmic reticulum stress-associated pathways in vitro and in vivo. Int J Oncol. 2019;55(1):331–339. doi:10.3892/ijo.2019.4820
- Han B, Cui H, Kang L, et al. Metformin inhibits thyroid cancer cell growth, migration, and EMT through the mTOR pathway. Tumour Biol. 2015;36(8):6295–6304. doi:10.1007/s13277-015-3315-4
- Thakur S, Daley B, Klubo-Gwiezdzinska J. The role of an anti-diabetic drug metformin in the treatment of endocrine tumors. J Mol Endocrinol. 2019;63(2):R17–R35. doi:10.1530/JME-19-0083
- Klubo-Gwiezdzinska J, Costello J, Patel A, et al. Treatment with metformin is associated with higher remission rate in diabetic patients with thyroid cancer. J Clin Endocrinol Metab. 2013;98(8):3269–3279. doi:10.1210/jc.2012-3799
- Shin HS, Sun HJ, Whang YM, Park YJ, Park DJ, Cho SW. Metformin reduces thyroid cancer tumor growth in the metastatic niche of bone by inhibiting osteoblastic RANKL productions. Thyroid. 2021;31(5):760–771. doi:10.1089/thy.2019.0851
- Cho YY, Kang MJ, Kim SK, et al. Protective effect of metformin against thyroid cancer development: a population-based study in Korea. Thyroid. 2018;28(7):864–870. doi:10.1089/thy.2017.0550
- Mushtaq A, Azam U, Mehreen S, Naseer MM. Synthetic alpha-glucosidase inhibitors as promising anti-diabetic agents: recent developments and future challenges. Eur J Med Chem. 2023;249:115119. doi:10.1016/j.ejmech.2023.115119
- Zhan ZT, Liu L, Cheng MZ, Gao Y, Zhou WJ, Xu B. The effects of 6 common antidiabetic drugs on anti-PD1 immune checkpoint inhibitor in tumor treatment. J Immunol Res. 2022;2022:2651790. doi:10.1155/2022/2651790
- Lai SW, Liao KF, Chen PC, Tsai PY, Hsieh DP, Chen CC. Antidiabetes drugs correlate with decreased risk of lung cancer: a population-based observation in Taiwan. Clin Lung Cancer. 2012;13(2):143–148. doi:10.1016/j.cllc.2011.10.002
- Tseng YH, Tsan YT, Chan WC, Sheu WH, Chen PC. Use of an alpha-glucosidase inhibitor and the risk of colorectal cancer in patients with diabetes: a nationwide, population-based cohort study. Diabetes Care. 2015;38(11):2068–2074. doi:10.2337/dc15-0563
- Li C, Kuang J, Zhao Y, Sun H, Guan H. Effect of type 2 diabetes and antihyperglycemic drug therapy on signs of tumor invasion in papillary thyroid cancer. Endocrine. 2020;69(1):92–99. doi:10.1007/s12020-020-02291-8
- Chen J, Chen Z, Khan BA, Hou K. Editorial: role of gut microbiota in diabetes mellitus and tumor immunity. Front Immunol. 2023;14:1185080. doi:10.3389/fimmu.2023.1185080
