Abstract
Objective
Several oral antidiabetic regimens are available for treating type 2 diabetes mellitus (T2DM), dipeptidyl peptidase-4 inhibitors (DPP4i) being one of them. We conducted a network meta-analysis (NMA) comparing DPP4i plus metformin (Met) combination with other Met-based oral antidiabetic drug (OAD) combinations used in treating patients with T2DM.
Methods
We searched PubMed and Embase from inception until 19th April, 2022 for phase II and phase III trials in patients with T2DM on Met-based traditional OADs. The primary outcome was assessed by change in glycated hemoglobin (HbA1c), fasting plasma glucose (FPG), and 2-hour post-prandial blood glucose (2h-PPG). The secondary safety outcomes assessed were hypoglycemic events, serious adverse events (SAEs), cardiovascular (CV) events, and gastrointestinal (GI) events.
Results
Sixty-two trials were included in the analysis. The combination of DPP4i + Met revealed a comparable mean reduction in HbA1c levels to the glinides (Gli) + Met combination (mean difference [MD]: −0.03%, 95% CI: 0.69, −0.65), although the difference was not statistically significant. The mean HbA1c reduction with DPP4i + Met was greater than with sulfonylureas (SU) + Met (MD: −0.05, 95% CI: −0.29, 0.39), thiazolidinedione (TZD) + Met (MD: −0.69, 95% CI: −1.39, −0.02), and SU + TZD (MD: 0.21; 95% CI: −1.30, 1.71), with no statistical significance. DPP4i + Met demonstrated a non-significant lower incidence of CV events in comparison to TZD + Met (RR: 1.01, 95% CI: 0.46, 2.45) and SU + Met (RR: 1.06, 95% CI: 0.61, 2.06).
Conclusion
DPP4i in combination with Met was efficacious and had a well-tolerated safety profile compared with other traditional OADs. This combination can be considered as a suitable treatment option for patients with T2DM.
Introduction
Diabetes mellitus (DM) is an endocrine metabolic disease characterized by the presence of hyperglycemia.Citation1 About 537 million adults are currently living with the burden of diabetes, and this is expected to rise to 643 million by 2030 and 783 million subsequently.Citation2 In recent, the incidence of type 2 diabetes mellitus (T2DM) in developing countries has substantially escalated, posing a huge burden on the economy.Citation3 To reduce long-term complications, most patients are recommended glucose-lowering oral antidiabetic drugs (OADs).
The American Diabetes Association (ADA) states that first-line therapy for diabetes management usually involves metformin (Met), along with comprehensive lifestyle modification. Based upon a patient’s glycemic needs, agents such as sodium–glucose co-transporter 2 (SGLT2) inhibitor or a glucagon-like peptide-1 receptor agonist (GLP-1RA) with or without Met are recommended as initial therapy in patients with T2DM or who have a high risk for atherosclerotic cardiovascular (ASCV) disease, heart failure, and/or chronic kidney disease.Citation4 The efficacy of Met has been extensively studied, often in comparison with other drugs already in use. In short- and medium-term therapies, Met has shown efficacy comparable to sulfonylureas, mitigating the hypoglycemic risks. Moreover, it has demonstrated higher efficacy compared to dipeptidyl peptidase-4 inhibitors (DPP4i), but lower efficacy than that of glucagon-like peptide-1 (GLP-1) receptor agonists. In the context of long-term monotherapy, Met has shown increased efficacy compared to sulfonylureas.Citation5 Besides, SGLT2 inhibitors or GLP-1RA are also recommended in patients with established ASCV, kidney disease, or heart failure, considering patient-related factors and independent of glycemic levels.Citation4 However, many patients, particularly those with higher baseline glycated hemoglobin (HbA1c) values, may not achieve their glycemic goals on Met monotherapy and therefore require additional medication.Citation6 Although multiple classes of OADs are available, there remains a need for agents with different mechanisms of action that offer improved efficacy and/or can be used either as monotherapy or in combination with Met.
The US Food and Drug Administration (FDA) has approved several medications, such as the sulfonylureas (SU), meglitinides, biguanides, thiazolidinediones (TZDs), and alpha-glucosidase inhibitors (AGIs), for the treatment of adult patients with T2DM.Citation7 Dipeptidyl peptidase-4 inhibitors (DPP4i) are a class of OADs with a unique mechanism of action from other hypoglycemic agents, as they exert antihyperglycemic effects by inhibiting DPP-4 enzyme activity, thereby leading to an increase in the concentration of GLP-1 and glucose-dependent insulinotropic polypeptide (GIP) which, in turn, increase insulin secretion from β-cells and reduce blood glucose levels.Citation8 The foundation for the development of DPP4i relies on the augmentation of the incretin effect. The incretin effect is the increased insulin secretion that occurs after oral glucose delivery compared to intravenous glucose treatment. It is thought that gut hormones increase the amount of insulin secreted in response to glucose.Citation9 Moreover, DPP4i are weight-gain neutral and are available in combination with Met.Citation10 Hypoglycemia, weight gain, and edema are generally not associated with DPP4i; however, these adverse events (AEs) have been associated with other antidiabetic drug classes that are often used in conjunction with Met (eg, SUs, glinides, TZDs, and insulin).Citation11
The rationale for combining DPP4i in combination with Met has been outlined earlier. Met works primarily by lowering hepatic glucose production and enhancing muscle and liver insulin sensitivity, whereas DPP4i act by increasing GLP-1 levels, thus stimulating insulin secretion and inhibiting glucagon secretion. Met also increases GLP-1 expression and stimulates GLP-1 secretion from the gut.Citation12 Another fact to be noted is that the pharmacokinetics of Met and a DPP4i in combination therapy remains unaffected, which further indicates the feasibility of the combination.Citation13 It is therefore evident that the two drugs have the potential to act via different mechanisms in diabetes and provide an additive or synergistic action when used in combination. Also, in terms of AEs, a low propensity for both DPP4i and Met to cause hypoglycemia or weight gain has been observed, thus making this combination a suitable option for patients who fail to meet their glycemic goals.
Although the ADA does not prioritize any specific regimen for a patient’s therapy, the American Association of Clinical Endocrinologists recommends the use of SGLT2i, followed by DPP4i, TZD, α-glucosidase inhibitors, and SU among the OADs, mainly based on the weight-reducing effect.Citation14 To decide on the best treatment approach, clinicians must take into account various patient-centric factors, such as hypoglycemia, glucose-lowering efficacy, drug-to-drug interactions, and the AEs of each OAD. Currently, head-to-head direct comparison studies assessing the Met-based combination therapies are limited. Moreover, the existing studies have examined each drug pair separately, creating a research gap that remains unaddressed in a unified platform. Network meta-analysis (NMA) has become a powerful tool that can evaluate multiple direct or indirect interventions, and quantify and sort the efficacy and safety of each of these measures, so as to screen the most effective and tolerable interventions. Hence, this NMA aimed to comprehensively compare the key efficacy and safety outcomes of DPP4i versus other OADs as add-on therapy to Met in patients with T2DM. The primary objective was to assess the changes in levels of hemoglobin, fasting plasma glucose, and 2-hour post-prandial glucose (2h-PPG) from baseline.
Materials and Methods
Study Design
The NMA was conducted using a prespecified study protocol. The study was planned, conducted, and reported as per the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) extension statement for network meta-analysis,Citation15 and was registered in the PROSPERO International Prospective Register of Systematic Reviews (registration number: CRD42021288932). It is based on previously conducted studies and does not contain any studies with human participants or animals performed by any of the authors.
Data Sources and Search Strategy
A systematic literature search was performed in databases such as PubMed and Embase from inception until 19th April, 2022, with a predefined search strategy formulated using a population, intervention, comparator, outcome (PICO) framework. The search strategy included terms for the diabetes medications of interest and type 2 diabetes, and consisted of the following keywords: “type 2 diabetes mellitus”, “metformin”, “dipeptidyl peptidase-4 inhibitors”, “saxagliptin”, “sitagliptin”, “linagliptin”, “alogliptin”, “vildagliptin”, “anagliptin”, “gemigliptin”, “teneligliptin”, “omarigliptin”, “dutogliptin”, “gosogliptin”, “alpha-glucuronidase inhibitors”, “acarbose”, “migitol”, “voglibose”, “sulfonylureas”, “glimepiride”, “glibenclamide”, “chlorpropamide”, “glipizide”, “tolbutamide”, “tolazamide”, “glinides”, “meglitinides”, “nateglinide”, and “repaglinide”.
Study Selection Process
Studies were selected based on following inclusion criteria: 1) participants were adults (≥18 years) with T2DM, and no restrictions on participants’ gender, ethnicity, or other demographic characteristics were considered; 2) studies reporting the following key outcomes: change in HbA1c, fasting plasma glucose (FPG), and 2h-PPG from baseline; 3) randomized control studies (RCTs) published in the English language and evaluating the efficacy of various traditional OADs in combination with Met in dual therapy were included. Studies included either patients with uncontrolled T2DM after Met-based therapy or direct comparison between two different OADs in combination with Met. Studies were included irrespective of the duration and line of treatment. Studies focusing on children, participants with type 1 diabetes, gestational diabetes, or prediabetes were excluded. Articles with study designs other than RCTs (eg, case–control studies, cohort studies), reviews, meta-analyses, non-English-language articles, and articles without the desired outcomes of interest were excluded. Studies evaluating different doses and those evaluating the pharmacokinetics and pharmacodynamics (PK/PD) of Met-based combination therapy were not included in this meta-analysis. Also, studies on Met-based combination therapy that could not be accommodated in a completed network were excluded.
Data Extraction
The reviewers extracted data from each included study into a standardized MS Office Excel-based data extraction sheet, regarding author, trial ID/name, title, intervention, intervention drug class, comparator, comparator drug class, efficacy, and safety outcomes. In the first step, the relevant articles were independently screened based on their title and abstract by two researchers to identify potential trials. The reasons for exclusion of studies were documented as well. For secondary screening, full-text versions of selected papers were examined and assessed according to the inclusion criteria. Any differences in opinion were resolved through discussion until a consensus was reached. To minimize bias, a third reviewer was consulted, who double-checked the articles and confirmed their eligibility.
Methodological Assessment and Network Construction
The network was constructed at the level of study drug class for Met in combination with AGI, SUs, glinides (Gli), TZD, and DPP4i. Only Met in combination with DPP4i was considered at the level of the study drug, based on the purpose of the study.
Outcomes
The primary endpoint of the study was to assess change in HbA1c, FPG, and 2h-PPG from baseline; while the secondary outcomes were assessments of safety in terms of hypoglycemic events, serious adverse events (SAEs), cardiovascular (CV) events, and gastrointestinal (GI) events.
Statistical Analysis
The NMA was performed using the “Gemtc” 4.0.4 package (R Foundation for Statistical Computing, Vienna, Austria) using a Bayesian approach. The “gemtc” package provides a matrix of the treatment rank probabilities, as well as a plot of the rank probabilities.Citation16 Model fitting performed for data pooling used convergence between prior and posterior values with deviance information criteria (DIC). The I2 values and DIC assessed the heterogeneity among the studies. Statistically significant heterogeneity was defined as a χ2 P-value <0.1 or an I2 statistic >50%.Citation17 By plotting rankograms, the surface under the cumulative ranking curve (SUCRA) values were calculated. The SUCRA values represented the ranking probability of each treatment regimen. Mean differences with 95% confidence intervals (CIs) were used to represent the efficacy outcomes. For dichotomous scores (eg, AEs), the treatment effect was evaluated using the risk ratio (RR) with 95% CI.
Results
Study Selection
The initial electronic search yielded 2879 consolidated studies from the selected databases. Following the initial screening, 148 distinct studies were further scrutinized, and after careful assessment based on inclusion and exclusion criteria, only 62 studies were considered eligible for our analysis. A study flow diagram is presented in . The baseline characteristics of the included studies are provided in .
Table 1 Baseline Characteristics of the Included Studies
Change in HbA1c from Baseline
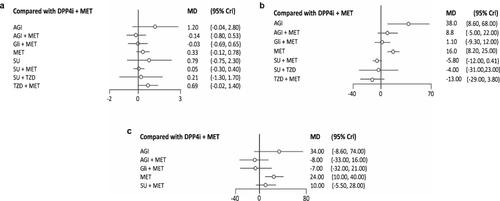
Forty-five articles reported efficacy outcomes related to change in HbA1c levels from baseline. The combination of DPP4i + Met revealed a comparable mean reduction in HbA1c levels to the Gli + Met combination (mean difference [MD]: −0.03%, 95% CI: 0.69, −0.65). In addition, DPP4i + Met showed a greater mean reduction in HbA1c than SU + Met (MD: 0.05, 95% CI: −0.30, 0.40), TZD + Met (MD: 0.69, 95% CI: −0.016, 1.4), and SU + TZD (MD: 0.21, 95% CI: −1.30, 1.71), and monotherapies of Met (MD: 0.33, 95% CI: −0.12, 0.78), AGI (MD: 1.21, 95% CI: −0.40, 2.85), and SU (MD: 0.79, 95% CI: −0.75, 2.35) (, , Table S1 and Figure S1).
Table 2 Effect Estimates for Efficacy Outcomes (Mean Difference and 95% CI)
Change in FPG
Evidence was available from 41 articles for mean changes in FPG reduction. The mean FPG reductions with DPP4i + Met and Gli + Met (MD: −1.09, 95% CI: −9.31, 11.77) were comparable, while the DPP4i + Met combination showed a greater reduction in FPG levels compared with AGI + Met (MD: 8.82, 95% CI: −4.97, 22.25), and monotherapies of Met (MD: 16.47, 95% CI: 8.24, 24.91) and AGI (MD: 38.48, 95% CI: 8.62, 67.98) (, , Table S2 and Figure S2).
Change in 2h-PPG
A total of 12 articles were considered for this analysis. Pairwise comparisons revealed that DPP4i + Met showed a greater mean reduction of 2h-PPG levels than SU + Met (MD: 10.1, 95% CI: −5.55, 27.66), Met (MD: 23.84, 95% CI: 10.34, 40.39), and AGI alone (MD: 33.79, 95% CI: −8.62, 73.86). Although AGI + Met (MD: −7.97, 95% CI: −33.17, 15.64) and Gli + Met (MD: −7.02, 95% CI: −32.26, 20.55) had better mean FPG reduction compared with DPP4i + Met, these differences were not significant (, , Table S3 and Figure S3).
Serious Adverse Events (SAEs)
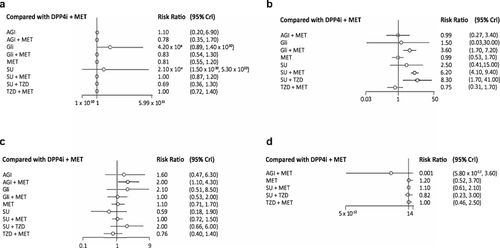
The network was constructed with 47 included articles. The incidence of SAEs with DPP4i + Met was lower and comparable to that of TZD + Met (RR: 1.02, 95% CI: 0.72, 1.36) and SU + Met (RR: 1.02, 95% CI: 0.87, 1.16). There were lower incidences of SAEs with SU + TZD (RR: 0.69, 95% CI: 0.36, 1.29), Met (RR: 0.81, 95% CI: 0.55, 1.16), AGI + Met (RR: 0.78 95% CI: 0.35, 1.70), Gli + Met (RR: 0.83, 95% CI: 0.54, 1.26), and AGI (RR: 1.11, 95% CI: 0.20, 6.91) when compared to DPP4i + Met, although these differences were not statistically different (, , Table S4 and Figure S4).
Table 3 Effect Estimates for Safety Outcomes (Risk Ratio and 95% CI)
Hypoglycemia
Forty-six articles were included in the analysis. The risk of hypoglycemia was lower with DPP4i + Met compared with Gli (RR: 1.47, 95% CI: 0.03, 30) and SU (RR: 2.54, 95% CI: 0.41, 15.31), while it was statistically significant compared with Gli + Met (RR: 3.61, 95% CI: 1.73, 7.20), SU + Met (RR: 6.21, 95% CI: 4.13, 9.37), and SU + TZD (RR: 8.29, 95% CI: 1.70, 40.64). DPP4i + Met showed comparable hypoglycemic risks when compared with AGI + Met (RR: 0.99, 95% CI: 0.27, 3.42) and Met (RR: 0.99, 95% CI: 0.53, 1.733) (, , Table S5 and Figure S5).
GI Events
The network was constructed with 33 included articles. The incidence of GI events was found to be lower for TZD + Met and SUs compared with DPP4i + Met (RR: 0.76, 95% CI: 0.40, 1.412) and (RR: 0.59, 95% CI: 0.17, 1.92), respectively. Further, GI events with DPP4i + Met were comparable to those for SU + Met (RR: 1.03, 95% CI: 0.72, 1.46), Gli + Met (RR: 1.04, 95% CI: 0.53, 2.00), and Met (RR: 1.08, 95% CI: 0.71, 1.65) while they were observed to be lower compared with SU + TZD (RR: 2.00, 95% CI: 0.66, 6.00), AGI + Met (RR: 2.04, 95% CI: 1.06, 4.33), AGI (RR: 1.64, 95% CI: 0.47, 6.29), and Gli (RR: 2.06, 95% CI: 0.51, 8.49) (, , Table S6 and Figure S6).
Cardiovascular Events
Eight articles were considered for the analysis. The incidence of CV events was lower with SU + TZD (RR: 0.82, 95% CI: 0.23, 3.03) and AGI + Met (RR: 0.01, 95% CI: 5.80×10−12, 3.57), although this difference was not statistically significant. DPP4i + Met showed lower incidences of CV events in comparison to TZD + Met (RR: 1.01, 95% CI: 0.46, 2.45), SU + Met (RR: 1.06, 95% CI: 0.61, 2.06), and Met monotherapy (RR: 1.23, 95% CI: 0.52, 3.69), although these differences were not significant (, , Table S7 and Figure S7).
Discussion
Despite advances in pharmacological management for T2DM, glycemic control remains frequently suboptimal, and hypoglycemia associated with uncontrolled or inadequate Met administration remains the most important concern. Clinicians often encounter challenges in comprehending the advantages and potential risks of emerging treatments. NMA offers a systematic means to visualize and comprehend a more comprehensive scope of evidence, enhancing the understanding of the real-world effectiveness and relative merits of these treatment regimens. Using the NMA tool, a recently published study considering a broader range of antidiabetic drugs discovered that SGLT-2i and GLP-1 RA medications were effective in reducing all-cause death, cardiovascular death, non-fatal myocardial infarction, hospitalization for heart failure, and end-stage kidney disease.Citation79
AACE/ACE guidelines have reported that DDP4i can reduce both FPG and 2h-PPG levels, both as monotherapy and in combination with Met. Especially in patients with HbA1c between 7.6% and 9.0%, DDP4i are recommended as part of double-combination therapy with Met and as a triple-combination therapy with Met and TZD for patients with HbA1c >9.0%.Citation80 We found that DPP4i in combination with Met effectively reduced the HbA1c and 2h-PPG levels, and improved various safety outcomes compared with SU and Met combinations. This combination was equally effective and well-tolerated in comparison to other Met-based OADs ().
Table 4 Summary Table of the Efficacy and Safety Outcomes of DPP4i + Met Combination with Other OAD-Based Met Combination Drug Classes
DPP4i in combination with Met was effective in reducing HbA1c levels compared with Met-based combinations with SU and TZD, while similar reductions were observed with glinides. This is in accordance with the findings observed in a previous meta-analysis that reported a greater reduction in HbA1c with DPP4i in comparison with SU and TZD.Citation81 Although both SU and DPP4i exert endogenous insulin secretion, DPP4i provide a more physiological meal-dependent action and help to improve beta- and alpha-cell function, which is speculated to be the reason behind the greater reduction. In an observational study evaluating sitagliptin and Met in combination, patients with T2DM had a significantly longer duration of treatment compared with an SU and Met combination owing to a comparable HbA1c reduction but a lower incidence of hypoglycemic events.Citation82
The ability of a therapy to maintain glycemic control must be weighed against its tendency to cause hypoglycemia. Prevention of hypoglycemia, even in a milder form, has a special place in the guidelines.Citation83 The common adverse reactions for DPP4i include hypoglycemia, GI problems, pancreatitis, upper respiratory tract infection, and urinary tract infection, while the symptoms of the side effects are mild.Citation84 In our analysis, the DPP4i and Met combination showed lower incidences of hypoglycemia compared with the SU and glinide-based Met combination, while the risk ratios were similar to the AGI and Met-based combination. The results were consistent with other published literature.Citation85 Further, a meta-analysis also reported that although there was no significant difference between DPP4i and SUs, when either was added to Met monotherapy, the safety analysis showed a significant decrease in the risk of hypoglycemic events in patients using DPP4i.Citation86,Citation87 Jeon et al also showed that SUs are especially associated with a higher rate of hypoglycemic events in comparison to DPP4i, indicating the good feasibility of DPP4i as an add-on therapy to Met.Citation88
The occurrence of GI events with the DPP4i and Met combination was comparable with that of OADs such as SU and Met, glinides and Met, and Met alone, whereas the incidence of GI events was lower with DPP4i plus Met compared with SU and TZD, glinides monotherapy, and especially AGI in monotherapy as well as in combination with Met. This was consistent with a previous meta-analysis that revealed a lower risk for DPP4i in combination with Met in comparison to AGI and Met.Citation89,Citation90 In that study, it was observed that the incidence of GI events after treatment with alogliptin, sitagliptin, and vildagliptin was significantly decreased, by 65%, 66%, and 62%, respectively, in comparison to AGI.Citation89 The combination of DPP4i and Met did not produce a statistically significant increase in the incidence of GI adverse events compared with Met alone.Citation91 A meta-analysis by Qian et al also revealed that no significant differences in the odds of diarrhea were observed when DPP4i or any other drug class was added to Met.Citation92 Also, in terms of SAEs, DPP4i were associated with lower SAEs compared with the SU-based Met combination but similar to the TZD-based Met combination.
Patients with T2DM usually have a very high risk for major adverse CV events. Previous studies have questioned the safety of traditional OADs in improving CV outcomes despite their ability to lower blood glucose levels. An NMA with 2967 patients consisting of 10 trials found no significant differences between patients taking DPP4i and those on placebo with regard to CV events.Citation93 Another observational study comparing DPP4i and Met combination with SU and Met combination reported lower risk of CV events with DPP4i. Moreover, DPP4i did not reduce myocardial infarction or admission for heart failure compared with SU.Citation87 In two meta-analyses,Citation94,Citation95 DPP4i reduced the risk for adverse CV effects and non-fatal myocardial infarction compared with placebo and other oral hypoglycemic agents. In a subgroup analysis, Engel et alCitation95 found that DPP4i were associated with a lower rate of CV events than SUs. In our study, DPP4i and Met was found to be better than SU in reducing CV events, and comparable to TZD-based Met combinations. A recent meta-analysis found no significant differences in CV events between SUs and DPP4i as add-on therapies to Met in adults diagnosed with T2DM.Citation88
The strengths of this NMA include the large sample size and the use of stringent inclusion and exclusion criteria. However, there were some limitations. The analyses were limited by the amount of data in the included studies. Also, moderate to substantial heterogeneity was observed in the studies included for the analysis. The RCTs included in this analysis involved a variety of study designs, including different patient populations and treatment durations. Also, our findings may have been impacted by variations in statistical analysis techniques and data quality between the RCTs. Further studies are needed to observe these results in a real-world clinical practice. Hence, observational studies are a prerequisite to address this evidence gap in effective patient management. Treatment for T2DM requires continuous management, which is often accompanied by adverse events. Although our findings suggest better safety outcomes in terms of hypoglycemia, GI events, and CV events from SU plus Met and a few other Met-based combinations, long-term safety assessments are needed to understand the chronic nature of diseases and uncover delayed adverse events. Finally, we did not distinguish among diverse drug doses or distinct medications within the same therapeutic class. As a consequence, our ability to ascertain whether observed outcomes were attributable to a class-wide effect or the specific impact of an individual drug was limited. Various medications in the same class or different doses of the same drug may have varying efficacy and safety. Owing to the significant level of inconsistency when the analysis was stratified by dosage, the impact of OAD dose variations on the therapeutic effect was not taken into consideration. So, when these drugs are used in clinical settings, it is important to consider the unique circumstances of each patient. Nevertheless, the results of the meta-analysis will assist in clinical decision making in treating patients with T2DM. The study will also assist in benchmarking the efficacies of OADs in T2DM and will provide insights for physicians to consider DPP4i-based Met combination as one of the suitable treatment options for their patients with T2DM. Future research should focus on implementing well-designed observational studies that could complement the findings of this meta-analysis and provide insights into the real-world effectiveness of these treatment regimens, including long-term evaluation of the outcomes and dose optimization.
Conclusion
Treatment with a combination of DPP4i and Met revealed comparable efficacy and safety compared to other traditional OADs, and thus can be considered as a viable option in treating patients with T2DM.
Data Sharing Statement
The data used to support the findings of this study are available from the corresponding author upon request.
Ethics Approval and Informed Consent
Not applicable: no ethical approval was required as this study is a meta-analysis and involves the collection of data from published literature.
Author Contributions
All authors contributed to data analysis, drafting or revising the article, have agreed on the journal to which the article will be submitted, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that they have no conflicts of interest in this work.
Acknowledgments
The authors would like to acknowledge Anwesha Mandal and Dr. Amit Bhat of Indegene Ltd, India, for their editorial and medical writing support. In addition, the authors extend their thanks to Ashwini Patil of Indegene Ltd, India, for statistical inputs and analysis support.
Additional information
Funding
References
- American Diabetes Association. Diagnosis and Classification of Diabetes Mellitus. Diabetes Care. 2009;32(Supplement_1):S62–7. doi:10.2337/dc09-S062
- Diabetes Facts & figures. Available from: https://idf.org/aboutdiabetes/what-is-diabetes/facts-figures.html. Accessed August 23, 2022.
- Misra A, Gopalan H, Jayawardena R, et al. Diabetes in developing countries. J Diabetes. 2019;11(7):522–539. doi:10.1111/1753-0407.12913
- American Diabetes Association. Standards of medical care in diabetes—2022 abridged for primary care providers. Clin Diabetes. 2022;40(1):10–38. doi:10.2337/cd22-as01
- Caturano A, Galiero R, Pafundi PC. Metformin for Type 2 Diabetes. JAMA. 2019;322(13):1312. doi:10.1001/jama.2019.11489
- Scheen AJ, Charpentier G, Östgren CJ, et al. Efficacy and safety of saxagliptin in combination with metformin compared with sitagliptin in combination with metformin in adult patients with type 2 diabetes mellitus. Diabetes Metab Res Rev. 2010;26(7):540–549. doi:10.1002/dmrr.1114
- Luna B, Feinglos MN. Oral Agents in the Management of Type 2 Diabetes Mellitus. AFP. 2001;63:1747.
- Nistala R, Savin V. Diabetes, hypertension, and chronic kidney disease progression: role of DPP4. Am J Physiol Renal Physiol. 2017;312:F661–70.
- Drucker DJ, Nauck MA. The incretin system: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet. 2006;368(9548):1696–1705. doi:10.1016/S0140-6736(06)69705-5
- Gallwitz B. Clinical Use of DPP-4 Inhibitors. Front Endocrinol. 2019;10:389. doi:10.3389/fendo.2019.00389
- Makrilakis K. The role of DPP-4 inhibitors in the treatment algorithm of type 2 diabetes mellitus: when to select, what to expect. Int J Environ Res Public Health. 2019;16:E2720.
- Ahrén B. Novel combination treatment of type 2 diabetes DPP-4 inhibition + metformin. Vasc Health Risk Manag. 2008;4:383–394. doi:10.2147/VHRM.S1944
- Herman GA, Bergman A, Yi B, et al. Tolerability and pharmacokinetics of metformin and the dipeptidyl peptidase-4 inhibitor sitagliptin when co-administered in patients with type 2 diabetes. Curr Med Res Opin. 2006;22(10):1939–1947. doi:10.1185/030079906X132587
- Garber AJ, Abrahamson MJ, Barzilay JI, et al. Consensus Statement By The American Association Of Clinical Endocrinologists And American College Of Endocrinology On The Comprehensive Type 2 Diabetes Management Algorithm - 2017 Executive Summary. Endocr Pract. 2017;23(2):207–238. doi:10.4158/EP161682.CS
- Palmer SC, Mavridis D, Nicolucci A, et al. Comparison of clinical outcomes and adverse events associated with glucose-lowering drugs in patients with type 2 diabetes: a meta-analysis. JAMA. 2016;316(3):313–324. doi:10.1001/jama.2016.9400
- Ge L, Tang Y, Zhang Q-N, et al. A network meta-analysis on the efficacy of targeted agents in combination with chemotherapy for treatment of advanced/metastatic triple-negative breast cancer. Oncotarget. 2017;8(35):59539–59551. doi:10.18632/oncotarget.19102
- Higgins JPT, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. doi:10.1136/bmj.327.7414.557
- Yang W, Pan CY, Tou C, et al. Efficacy and safety of saxagliptin added to metformin in Asian people with type 2 diabetes mellitus: a randomized controlled trial. Diabet Res Clin Pract. 2011;94(2):217–224. doi:10.1016/j.diabres.2011.07.035
- Pfützner A, Paz-Pacheco E, Allen E, et al. Initial combination therapy with saxagliptin and metformin provides sustained glycaemic control and is well tolerated for up to 76 weeks. Diabetes Obes Metab. 2011;13(6):567–576. doi:10.1111/j.1463-1326.2011.01385.x
- Phillips P, Karrasch J, Scott R, et al. Acarbose improves glycemic control in overweight type 2 diabetic patients insufficiently treated with metformin. Diabetes Care. 2003;26(2):269–273. doi:10.2337/diacare.26.2.269
- Chiasson JL, Naditch L; Miglitol Canadian University Investigator Group. The synergistic effect of miglitol plus metformin combination therapy in the treatment of type 2 diabetes. Diabetes Care. 2001;24(6):989–994. doi:10.2337/diacare.24.6.989
- Gu T, Ma J, Zhang Q, et al. Comparative effect of saxagliptin and glimepiride with a composite endpoint of adequate glycaemic control without hypoglycaemia and without weight gain in patients uncontrolled with metformin therapy: results from the SPECIFY study, a 48-week, multi-centre, randomized, controlled trial. Diabetes Obes Metab. 2019;21(4):939–948. doi:10.1111/dom.13605
- Du J, Liang L, Fang H, et al. Efficacy and safety of saxagliptin compared with acarbose in Chinese patients with type 2 diabetes mellitus uncontrolled on metformin monotherapy: results of a Phase IV open-label randomized controlled study (the SMART study). Diabetes Obes Metab. 2017;19(11):1513–1520. doi:10.1111/dom.12942
- Van Gaal L, Maislos M, Schernthaner G, et al. Miglitol combined with metformin improves glycaemic control in type 2 diabetes. Diabetes Obes Metab. 2001;3(5):326–331. doi:10.1046/j.1463-1326.2001.00141.x
- Dou J, Ma J, Liu J, et al. Efficacy and safety of saxagliptin in combination with metformin as initial therapy in Chinese patients with type 2 diabetes: results from the START study, a multicentre, randomized, double-blind, active-controlled, Phase 3 trial. Diabetes Obes Metab. 2018;20(3):590–598. doi:10.1111/dom.13117
- Hanefeld M, Brunetti P, Schernthaner GH, et al. One-year glycemic control with a sulfonylurea plus pioglitazone versus a sulfonylurea plus metformin in patients with type 2 diabetes. Diabetes Care. 2004;27(1):141–147. doi:10.2337/diacare.27.1.141
- Wang J-S, Huang C-N, Hung Y-J, et al. Acarbose plus metformin fixed-dose combination outperforms acarbose monotherapy for type 2 diabetes. Diabet Res Clin Pract. 2013;102(1):16–24. doi:10.1016/j.diabres.2013.08.001
- Cai X-L, Chen Y-L, Zhao -J-J, et al. Efficacy and safety of avandamet or uptitrated metformin treatment in patients with type 2 diabetes inadequately controlled with metformin alone: a multicenter, randomized, controlled trial. Chin Med J. 2015;128(10):1279–1287. doi:10.4103/0366-6999.156735
- Rosenstock J, Rood J, Cobitz A, et al. Initial treatment with rosiglitazone/metformin fixed-dose combination therapy compared with monotherapy with either rosiglitazone or metformin in patients with uncontrolled type 2 diabetes. Diabetes Obes Metab. 2006;8(6):650–660. doi:10.1111/j.1463-1326.2006.00659.x
- Oh TJ, Yu JM, Min KW, et al. Efficacy and safety of voglibose plus metformin in patients with type 2 diabetes mellitus: a randomized controlled trial. Diabetes Metab J. 2019;43(3):276–286. doi:10.4093/dmj.2018.0051
- Moses R, Slobodniuk R, Boyages S, et al. Effect of repaglinide addition to metformin monotherapy on glycemic control in patients with type 2 diabetes. Diabetes Care. 1999;22(1):119–124. doi:10.2337/diacare.22.1.119
- Marre M, Van Gaal L, Usadel K-H, et al. Nateglinide improves glycaemic control when added to metformin monotherapy: results of a randomized trial with type 2 diabetes patients. Diabetes Obes Metab. 2002;4(3):177–186. doi:10.1046/j.1463-1326.2002.00196.x
- Horton ES, Clinkingbeard C, Gatlin M, et al. Nateglinide alone and in combination with metformin improves glycemic control by reducing mealtime glucose levels in type 2 diabetes. Diabetes Care. 2000;23(11):1660–1665. doi:10.2337/diacare.23.11.1660
- Raskin P, Lewin A, Reinhardt R, et al. Twice-daily dosing of a repaglinide/metformin fixed-dose combination tablet provides glycaemic control comparable to rosiglitazone/metformin tablet. Diabetes Obes Metab. 2009;11(9):865–873. doi:10.1111/j.1463-1326.2009.01062.x
- Lewin A, Lipetz R, Wu J, et al. Comparison of extended-release metformin in combination with a sulfonylurea (glyburide) to sulfonylurea monotherapy in adult patients with type 2 diabetes: a multicenter, double-blind, randomized, controlled, phase III study. Clin Ther. 2007;29(5):844–855. doi:10.1016/j.clinthera.2007.05.013
- Jin S-M, Park C-Y, Cho YM, et al. Lobeglitazone and pioglitazone as add-ons to metformin for patients with type 2 diabetes: a 24-week, multicentre, randomized, double-blind, parallel-group, active-controlled, phase III clinical trial with a 28-week extension. Diabetes Obes Metab. 2015;17(6):599–602. doi:10.1111/dom.12435
- Wang J-S, Lee I-T, Lee W-J, et al. Glycemic excursions are positively associated with changes in duration of asymptomatic hypoglycemia after treatment intensification in patients with type 2 diabetes. Diabet Res Clin Pract. 2016;113:108–115. doi:10.1016/j.diabres.2015.12.010
- Perez A, Zhao Z, Jacks R, et al. Efficacy and safety of pioglitazone/metformin fixed-dose combination therapy compared with pioglitazone and metformin monotherapy in treating patients with T2DM. Curr Med Res Opin. 2009;25(12):2915–2923. doi:10.1185/03007990903350011
- Kawamori R, Kaku K, Hanafusa T, et al. Effect of combination therapy with repaglinide and metformin hydrochloride on glycemic control in Japanese patients with type 2 diabetes mellitus. J Diabetes Investig. 2014;5(1):72–79. doi:10.1111/jdi.12121
- Wang W, Bu R, Su Q, et al. Randomized study of repaglinide alone and in combination with metformin in Chinese subjects with type 2 diabetes naive to oral antidiabetes therapy. Expert Opin Pharmacother. 2011;12(18):2791–2799. doi:10.1517/14656566.2011.602341
- Raskin P, Klaff L, McGill J, et al. Efficacy and safety of combination therapy: repaglinide plus metformin versus nateglinide plus metformin. Diabetes Care. 2003;26(7):2063–2068. doi:10.2337/diacare.26.7.2063
- Moses R. Repaglinide in combination therapy with metformin in Type 2 diabetes. Exp Clin Endocrinol Diabetes. 1999;107(Suppl 4):S136–139. doi:10.1055/s-0029-1212169
- Vaccaro O, Masulli M, Nicolucci A, et al. Effects on the incidence of cardiovascular events of the addition of pioglitazone versus sulfonylureas in patients with type 2 diabetes inadequately controlled with metformin (TOSCA.IT): a randomised, multicentre trial. Lancet Diabetes Endocrinol. 2017;5(11):887–897. doi:10.1016/S2213-8587(17)30317-0
- Handelsman Y, Lauring B, Gantz I, et al. A randomized, double-blind, non-inferiority trial evaluating the efficacy and safety of omarigliptin, a once-weekly DPP-4 inhibitor, or glimepiride in patients with type 2 diabetes inadequately controlled on metformin monotherapy. Curr Med Res Opin. 2017;33(10):1861–1868. doi:10.1080/03007995.2017.1335638
- Derosa G, Bonaventura A, Bianchi L, et al. Vildagliptin compared to glimepiride on post-prandial lipemia and on insulin resistance in type 2 diabetic patients. Metabolism. 2014;63(7):957–967. doi:10.1016/j.metabol.2014.04.008
- Kim G, Oh S, Jin S-M, et al. The efficacy and safety of adding either vildagliptin or glimepiride to ongoing metformin therapy in patients with type 2 diabetes mellitus. Expert Opin Pharmacother. 2017;18(12):1179–1186. doi:10.1080/14656566.2017.1353080
- Derosa G, D’Angelo A, Fogari E, et al. Effects of nateglinide and glibenclamide on prothrombotic factors in naïve type 2 diabetic patients treated with metformin: a 1-year, double-blind, randomized clinical trial. Intern Med. 2007;46(22):1837–1846. doi:10.2169/internalmedicine.46.0320
- González-Ortiz M, Guerrero-Romero JF, Violante-Ortiz R, et al. Efficacy of glimepiride/metformin combination versus glibenclamide/metformin in patients with uncontrolled type 2 diabetes mellitus. J Diabetes Complications. 2009;23(6):376–379. doi:10.1016/j.jdiacomp.2008.09.002
- Chien -H-H, Chang C-T, Chu N-F, et al. Effect of glyburide-metformin combination tablet in patients with type 2 diabetes. J Chin Med Assoc. 2007;70(11):473–480. doi:10.1016/S1726-4901(08)70044-3
- Garber A, Klein E, Bruce S, et al. Metformin-glibenclamide versus metformin plus rosiglitazone in patients with type 2 diabetes inadequately controlled on metformin monotherapy. Diabetes Obes Metab. 2006;8(2):156–163. doi:10.1111/j.1463-1326.2005.00570.x
- Kim H-S, Shin J-A, Lee S-H, et al. A comparative study of the effects of a dipeptidyl peptidase-IV inhibitor and sulfonylurea on glucose variability in patients with type 2 diabetes with inadequate glycemic control on metformin. Diabetes Technol Ther. 2013;15(10):810–816. doi:10.1089/dia.2013.0038
- Schernthaner G, Durán-Garcia S, Hanefeld M, et al. Efficacy and tolerability of saxagliptin compared with glimepiride in elderly patients with type 2 diabetes: a randomized, controlled study (GENERATION). Diabetes Obes Metab. 2015;17(7):630–638. doi:10.1111/dom.12461
- Garber AJ, Donovan DS, Dandona P, et al. Efficacy of glyburide/metformin tablets compared with initial monotherapy in type 2 diabetes. J Clin Endocrinol Metab. 2003;88(8):3598–3604. doi:10.1210/jc.2002-021225
- Del Prato S, Camisasca R, Wilson C, et al. Durability of the efficacy and safety of alogliptin compared with glipizide in type 2 diabetes mellitus: a 2-year study. Diabetes Obes Metab. 2014;16(12):1239–1246. doi:10.1111/dom.12377
- Schwarz SL, Gerich JE, Marcellari A, et al. Nateglinide, alone or in combination with metformin, is effective and well tolerated in treatment-naïve elderly patients with type 2 diabetes. Diabetes Obes Metab. 2008;10(8):652–660. doi:10.1111/j.1463-1326.2007.00792.x
- Umpierrez G, Issa M, Vlajnic A. Glimepiride versus pioglitazone combination therapy in subjects with type 2 diabetes inadequately controlled on metformin monotherapy: results of a randomized clinical trial. Curr Med Res Opin. 2006;22(4):751–759. doi:10.1185/030079906X104786
- Göke B, Gallwitz B, Eriksson JG, et al. Saxagliptin vs. glipizide as add-on therapy in patients with type 2 diabetes mellitus inadequately controlled on metformin alone: long-term (52-week) extension of a 52-week randomised controlled trial. Int J Clin Pract. 2013;67(4):307–316. doi:10.1111/ijcp.12119
- Gerich J, Raskin P, Jean-Louis L, et al. PRESERVE-beta: two-year efficacy and safety of initial combination therapy with nateglinide or glyburide plus metformin. Diabetes Care. 2005;28:2093–2099.
- Forst T, Anastassiadis E, Diessel S, et al. Effect of linagliptin compared with glimepiride on postprandial glucose metabolism, islet cell function and vascular function parameters in patients with type 2 diabetes mellitus receiving ongoing metformin treatment. Diabetes Metab Res Rev. 2014;30(7):582–589. doi:10.1002/dmrr.2525
- Seck T, Nauck M, Sheng D, et al. Safety and efficacy of treatment with sitagliptin or glipizide in patients with type 2 diabetes inadequately controlled on metformin: a 2-year study. Int J Clin Pract. 2010;64(5):562–576. doi:10.1111/j.1742-1241.2010.02353.x
- Ristic S, Collober‐Maugeais C, Pecher E, et al. Comparison of nateglinide and gliclazide in combination with metformin, for treatment of patients with Type 2 diabetes mellitus inadequately controlled on maximum doses of metformin alone. Diabet Med. 2006;23(7):757–762. doi:10.1111/j.1464-5491.2006.01914.x
- Göke B, Gallwitz B, Eriksson J, et al. Saxagliptin is non-inferior to glipizide in patients with type 2 diabetes mellitus inadequately controlled on metformin alone: a 52-week randomised controlled trial. Int J Clin Pract. 2010;64(12):1619–1631. doi:10.1111/j.1742-1241.2010.02510.x
- Matthews DR, Dejager S, Ahren B, et al. Vildagliptin add-on to metformin produces similar efficacy and reduced hypoglycaemic risk compared with glimepiride, with no weight gain: results from a 2-year study. Diabetes Obes Metab. 2010;12(9):780–789. doi:10.1111/j.1463-1326.2010.01233.x
- Arechavaleta R, Seck T, Chen Y, et al. Efficacy and safety of treatment with sitagliptin or glimepiride in patients with type 2 diabetes inadequately controlled on metformin monotherapy: a randomized, double-blind, non-inferiority trial. Diabetes Obes Metab. 2011;13(2):160–168. doi:10.1111/j.1463-1326.2010.01334.x
- Nauck MA, Meininger G, Sheng D, et al. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor, sitagliptin, compared with the sulfonylurea, glipizide, in patients with type 2 diabetes inadequately controlled on metformin alone: a randomized, double-blind, non-inferiority trial. Diabetes Obes Metab. 2007;9(2):194–205. doi:10.1111/j.1463-1326.2006.00704.x
- Ferrannini E, Fonseca V, Zinman B, et al. Fifty-two-week efficacy and safety of vildagliptin vs. glimepiride in patients with type 2 diabetes mellitus inadequately controlled on metformin monotherapy. Diabetes Obes Metab. 2009;11(2):157–166. doi:10.1111/j.1463-1326.2008.00994.x
- Ristic S, Collober-Maugeais C, Cressier F, et al. Nateglinide or gliclazide in combination with metformin for treatment of patients with type 2 diabetes mellitus inadequately controlled on maximum doses of metformin alone: 1-year trial results. Diabetes Obes Metab. 2007;9(4):506–511. doi:10.1111/j.1463-1326.2006.00632.x
- Derosa G, Bonaventura A, Bianchi L, et al. Retracted: comparison of vildagliptin and glimepiride: effects on glycaemic control, fat tolerance and inflammatory markers in people with Type 2 diabetes. Diabet Med. 2014;31(12):1515–1523. doi:10.1111/dme.12499
- Charpentier G, Fleury F, Kabir M, et al. Improved glycaemic control by addition of glimepiride to metformin monotherapy in type 2 diabetic patients. Diabet Med. 2001;18(10):828–834. doi:10.1046/j.1464-5491.2001.00582.x
- Feinglos M, Dailey G, Cefalu W, et al. Effect on glycemic control of the addition of 2.5 mg glipizide GITS to metformin in patients with T2DM. Diabet Res Clin Pract. 2005;68(2):167–175. doi:10.1016/j.diabres.2004.09.002
- Lin S-D, Wang J-S, Hsu S-R, et al. The beneficial effect of α-glucosidase inhibitor on glucose variability compared with sulfonylurea in Taiwanese type 2 diabetic patients inadequately controlled with metformin: preliminary data. J Diabetes Complications. 2011;25(5):332–338. doi:10.1016/j.jdiacomp.2011.06.004
- Garber AJ, Larsen J, Schneider SH, et al. Simultaneous glyburide/metformin therapy is superior to component monotherapy as an initial pharmacological treatment for type 2 diabetes. Diabetes Obes Metab. 2002;4(3):201–208. doi:10.1046/j.1463-1326.2002.00211.x
- Home PD, Jones NP, Pocock SJ, et al. Rosiglitazone RECORD study: glucose control outcomes at 18 months. Diabet Med. 2007;24(6):626–634. doi:10.1111/j.1464-5491.2007.02160.x
- Wang M, Lin S, Chen Y, et al. Saxagliptin is similar in glycaemic variability more effective in metabolic control than acarbose in aged type 2 diabetes inadequately controlled with metformin. Diabet Res Clin Pract. 2015;108(3):e67–70. doi:10.1016/j.diabres.2015.02.022
- Rosenstock J, Lewin AJ, Norwood P, et al. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor PF-734200 added to metformin in Type 2 diabetes. Diabet Med. 2011;28(4):464–469. doi:10.1111/j.1464-5491.2010.03181.x
- Shankar RR, Inzucchi SE, Scarabello V, et al. A randomized clinical trial evaluating the efficacy and safety of the once-weekly dipeptidyl peptidase-4 inhibitor omarigliptin in patients with type 2 diabetes inadequately controlled on metformin monotherapy. Curr Med Res Opin. 2017;33(10):1853–1860. doi:10.1080/03007995.2017.1335637
- Filozof C, Gautier J-F. A comparison of efficacy and safety of vildagliptin and gliclazide in combination with metformin in patients with Type 2 diabetes inadequately controlled with metformin alone: a 52-week, randomized study. Diabet Med. 2010;27(8):318–326. doi:10.1111/j.1464-5491.2010.02938.x
- Forst T, Uhlig-Laske B, Ring A, et al. Linagliptin (BI 1356), a potent and selective DPP-4 inhibitor, is safe and efficacious in combination with metformin in patients with inadequately controlled Type 2 diabetes. Diabet Med. 2010;27(12):1409–1419. doi:10.1111/j.1464-5491.2010.03131.x
- Ceriello A, Rodbard HW, Battelino T, et al. Data from network meta-analyses can inform clinical practice guidelines and decision-making in diabetes management: perspectives of the taskforce of the guideline workshop. Cardiovasc Diabetol. 2023;22(1):277. doi:10.1186/s12933-023-01993-3
- DeFronzo RA, Hissa MN, Garber AJ, et al. The efficacy and safety of saxagliptin when added to metformin therapy in patients with inadequately controlled type 2 diabetes with metformin alone. Diabetes Care. 2009;32(9):1649–1655. doi:10.2337/dc08-1984
- Oh S, Purja S, Shin H, et al. Hypoglycemic agents and glycemic variability in individuals with type 2 diabetes: a systematic review and network meta-analysis. Diabetes Vasc Dis Res. 2022;19(3):14791641221106866. doi:10.1177/14791641221106866
- Valensi P, de Pouvourville G, Benard N, et al. Treatment maintenance duration of dual therapy with metformin and sitagliptin in type 2 diabetes: the ODYSSEE observational study. Diabetes Metab. 2015;41(3):231–238. doi:10.1016/j.diabet.2015.03.007
- Mearns ES, Sobieraj DM, White CM, et al. Comparative efficacy and safety of antidiabetic drug regimens added to metformin monotherapy in patients with type 2 diabetes: a network meta-analysis. PLoS One. 2015;10:e0125879.
- Wang X, Li X, Qie S, et al. The efficacy and safety of once-weekly DPP-4 inhibitor omarigliptin in patients with type 2 diabetes mellitus: a systemic review and meta-analysis. Medicine. 2018;97(34):e11946. doi:10.1097/MD.0000000000011946
- Amblee A, Lious D, Fogelfeld L. Combination of saxagliptin and metformin is effective as initial therapy in new-onset type 2 diabetes mellitus with severe hyperglycemia. J Clin Endocrinol Metab. 2016;101(6):2528–2535. doi:10.1210/jc.2015-4097
- Foroutan N, Muratov S, Levine M. Safety and efficacy of dipeptidyl peptidase-4 inhibitors vs sulfonylurea in metformin-based combination therapy for type 2 diabetes mellitus: systematic review and meta-analysis. Clin Invest Med. 2016;39(2):E48–E62. doi:10.25011/cim.v39i2.26481
- Ou S-M, Shih C-J, Chao P-W, et al. Effects on clinical outcomes of adding dipeptidyl peptidase-4 inhibitors versus sulfonylureas to metformin therapy in patients with type 2 diabetes mellitus. Ann Intern Med. 2015;163(9):663–672. doi:10.7326/M15-0308
- Jeon WK, Kang J, Kim H-S, et al. Correction to: “cardiovascular outcomes comparison of dipeptidyl peptidase-4 inhibitors versus sulfonylurea as add-on therapy for type 2 diabetes mellitus: a meta-analysis. J Lipid Atheroscler. 2022;11(1):89–101. doi:10.12997/jla.2022.11.1.89
- Wu S, Chai S, Yang J, et al. Gastrointestinal adverse events of dipeptidyl peptidase 4 inhibitors in type 2 diabetes: a systematic review and network meta-analysis. Clin Ther. 2017;39(9):1780–1789.e33. doi:10.1016/j.clinthera.2017.07.036
- Wu D, Li L, Liu C. Efficacy and safety of dipeptidyl peptidase-4 inhibitors and metformin as initial combination therapy and as monotherapy in patients with type 2 diabetes mellitus: a meta-analysis. Diabetes Obes Metab. 2014;16(1):30–37. doi:10.1111/dom.12174
- Goldstein BJ, Feinglos MN, Lunceford JK, et al. Effect of initial combination therapy with sitagliptin, a dipeptidyl peptidase-4 inhibitor, and metformin on glycemic control in patients with type 2 diabetes. Diabetes Care. 2007;30(8):1979–1987. doi:10.2337/dc07-0627
- Qian D, Zhang T, Zheng P, et al. Comparison of oral antidiabetic drugs as add-on treatments in patients with type 2 diabetes uncontrolled on metformin: a network meta-analysis. Diabetes Ther. 2018;9(5):1945–1958. doi:10.1007/s13300-018-0482-5
- Tricco AC, Antony J, Khan PA, et al. Safety and effectiveness of dipeptidyl peptidase-4 inhibitors versus intermediate-acting insulin or placebo for patients with type 2 diabetes failing two oral antihyperglycaemic agents: a systematic review and network meta-analysis. BMJ Open. 2014;4(12):e005752. doi:10.1136/bmjopen-2014-005752
- Patil HR, Al Badarin FJ, Al Shami HA, et al. Meta-analysis of effect of dipeptidyl peptidase-4 inhibitors on cardiovascular risk in type 2 diabetes mellitus. Am J Cardiol. 2012;110(6):826–833. doi:10.1016/j.amjcard.2012.04.061
- Engel SS, Golm GT, Shapiro D, et al. Cardiovascular safety of sitagliptin in patients with type 2 diabetes mellitus: a pooled analysis. Cardiovasc Diabetol. 2013;12(1):3. doi:10.1186/1475-2840-12-3