Abstract
Purpose
Polycystic ovary syndrome (PCOS) is a frequent cause of infertility in reproductive-age women. Our work aims to evaluate the effects of glucagon-like peptide-1 receptor agonists (GLP-1RAs) on gut microbiota, with metabolic parameters including body weight and the hormone profile in PCOS.
Patients and Methods
Dehydroepiandrosterone (DHEA)-induced PCOS mice were established and then treated with two GLP-1RAs: liraglutide and novel form semaglutide for four weeks. Changes in body weight and metabolic parameters were measured. Fecal samples were collected and analyzed using metagenomic sequencing.
Results
Liraglutide and semaglutide modulated both alpha and beta diversity of the gut microbiota in PCOS. Liraglutide increased the Bacillota-to-Bacteroidota ratio through up-regulating the abundance of butyrate-producing members of Bacillota like Lachnospiraceae. Moreover, liraglutide showed the ability to reverse the altered microbial composition and the disrupted microbiota functions caused by PCOS. Semaglutide increased the abundance of Helicobacter in PCOS mice (p < 0.01) which was the only bacteria found negatively correlated with body weight. Moreover, pathways involving porphyrin and flavonoids were increased after semaglutide intervention.
Conclusion
Liraglutide and semaglutide improved reproductive and metabolic disorders by modulating the whole structure of gut microbiota in PCOS. The greater efficacy in weight loss compared with liraglutide observed after semaglutide intervention was positively related with Helicobacter. The study may provide new ideas in the treatment and the underlying mechanisms of GLP-1RAs to improve PCOS.
Introduction
Polycystic ovary syndrome (PCOS) is a common endocrine and metabolic disorder in reproductive-aged women, with a worldwide prevalence of up to 18%.Citation1 The pathophysiological mechanism of PCOS is not yet fully elucidated but might be related to abdominal obesity, insulin resistance, hyperandrogenemia, and low-grade metabolic inflammation.Citation2 The high prevalence as well as the heterogeneous character increases the risk of infertility, endometrial carcinoma, and cardiovascular disease (CVD), which poses a huge challenge to public health and contributes to the lack of effective clinical treatments.Citation3
A type of antihyperglycemic medicines collectively known as glucagon-like peptide-1 receptor agonists (GLP-1RAs) was reported to have a significant weight-sparing effect in patients with type 2 diabetes (T2D), as well as in obese or overweight individuals.Citation4,Citation5 Moreover, they also showed the ability to improve hyperandrogenism and insulin resistance.Citation6 Due to their beneficial effects on weight loss and metabolic disorders, GLP-1RAs have been recently studied for the clinical treatment of polycystic ovary syndrome.Citation7 As reported, GLP-1RAs could significantly lose weight and improve insulin sensitivity and other glycemic parameters, as well as reduce the risk of CVD.Citation8–10 However, the underlying mechanisms of GLP-1 RAs in ameliorating PCOS still remain to be verified.
Liraglutide has been one of the most applied GLP-1RAs in the clinical study of PCOS women, with a half-life of 13 hours.Citation11 Semaglutide, on the other hand, is a longer-acting GLP-1RA with a half-life of 168–184 hours, which was recently approved by the United States Food and Drug Administration (FDA) in 2021.Citation12 Our previous study found that both liraglutide and semaglutide had beneficial effects on reproductive and metabolic disturbances in DHEA-induced PCOS model mice, as well as the ability of anti-inflammation and promoting the browning of white adipose tissue.Citation13 Nevertheless, the potential mechanisms of these two GLP-1RAs interventions in improving PCOS have not been further explored.
Recent studies have shown that the imbalance of gut microbiota has a role in the development of PCOS and may be associated with other metabolic disorders.Citation14–17 For example, Qi et alCitation14 found that both alpha and beta diversity of gut flora were decreased in PCOS model mice with a reduced abundance of Lactobacillus compared with controls. A positive relationship between the species Bacteroides fragilis and metabolic parameters like BMI and serum LH levels was observed in human studies.Citation17–20 On the other hand, GLP-1 RAs (mainly liraglutide) were recently reported to lower weight and regulate insulin secretion via modulating the structure of gut microbiota in obese patients/mice.Citation21,Citation22 These results implied that the modulated gut microbiota might play an important role in the possible mechanisms of GLP-1 RAs in improving metabolic disorders. Therefore, this current study is a following research on the underlying mechanisms of liraglutide and semaglutide in alleviating PCOS and further compares the evaluation of liraglutide and semaglutide intervention based on the modulation of gut microbiota.
Materials and Methods
Animals and Treatments
Female C57BL/6J mice (21 days old) were purchased from Shanghai Laboratory Animal Center (SLAC). All mice had unconstrained access to drinking water and food with standard laboratory conditions: temperature (22°C ± 3°C), humidity (45–55%), and lighting (12 h light/12 h dark cycle). Body weight was measured weekly during the whole procedure. All animal studies were approved by the Institutional Animal Care and Use Committee (IACUC) of Shanghai Jiao Tong University School of Medicine and conducted according to the guidelines for the ethical review of laboratory animal welfare People’s Republic of China National Standard GB/T 35892–2018.
Twenty-four mice (25 days old) were randomly divided into four groups: control, PCOS, PCOS+Liraglutide and PCOS+Semaglutide (n = 6). To induce the PCOS model, the mice in the PCOS, PCOS+Liraglutide, and PCOS+Semaglutide groups were subcutaneously injected with DHEA (6 mg/100 g body weight; dissolved in 0.1 mL of sesame oil: D4000-10 g, Sigma Aldrich, USA), while the controls were injected with the same dose of sesame oil for 3 weeks as previously described.Citation23 After 3 consecutive weeks of induction, the PCOS+Liraglutide group and the PCOS+Semaglutide group were intraperitoneally injected with liraglutide (0.3 mg/kg body weight; daily) and semaglutide (0.1 mg/kg body weight; 3 times a week) for 4 weeks, respectively. After 3 weeks of modeling and subsequent 4 weeks of interventions, all mice were sacrificed, and blood samples were collected to measure metabolic parameters.Citation13
Fecal Samples Collection and DNA Extraction
Fresh fecal samples were taken from each mouse before sacrifice and were snap-frozen at −80°C. Bacterial DNA from each fecal sample was extracted using the E.Z.N.A.® Soil DNA Kit (Omega Bio-Tek, Norcross, GA, USA) according to the standard protocols. In more detail, 1 mL of SLX-Mlus Buffer was added to each 50 mg of fecal samples in a Disruptor Tube pre-filled with glass beads. Bead beating was performed with a homogeneous instrument (FastPrep-24; MP Biomedicals) at 5.0 ms−1 for 40s. We subsequently determined the DNA concentration and purity using NanoDrop2000 (Thermo Fisher Scientific, USA).
Library Preparation and Shotgun Sequencing
For paired-end library construction, we used Covaris M220 to fragment genomic DNA to about 300 bp. Illumina paired-end libraries were then generated using the NovaSeq6000 S4 Reagent Kit (Illumina, San Diego, CA, USA). Next, we performed shallow shotgun sequencing on the Illumina NovaSeq platform at Honsunbio Technology Co., Ltd. (Shanghai, China) using NovaSeq Reagent Kits (https://www.illumina.com).
Processing of Sequencing Data
Quality control and preprocessing of raw FASTQ reads were performed using fastp version 0.23.2. More specifically, low-quality bases (quality score <Q20) or fragmented short reads (<50 bp) or reads with ambiguous nucleotides were removed.Citation24 Next, we used Megahit version 1.2.9 to assemble reads that were from the metagenomic dataset.Citation25 In the end, contiguous sequences of at least 300 bp in length could be used for further gene annotations. Then, Prodigal version 2.6.3 was used to predict open reading frames (ORFs) from the assembled contigs.Citation26 A catalog with non-redundant genes was constructed by using MMseqs2 version 14.7e284 to remove redundant genes (95% sequence identity and 90% coverage).Citation27 The representative sequences were aligned using DIAMOND version 2.0.15.153 to assign and identify taxonomy.Citation28 Overall, a total of 164 gut microbiota at the phylum level, 842 at the family level, 2353 at the genus level, and 9662 at the species level were identified in 24 fecal samples after removing unannotated bacteria. For the KEGG annotation, we used KofamScan version 1.3.0 against the KEGG database (https://www.genome.jp/kegg/).Citation29
Statistical Analysis
All data were processed using GraphPad Prism version 9.0.0 and R version 3.6.3. The statistical differences of the microbial community and functional modules among the four groups were analyzed using the non-parametric Kruskal–Wallis test. The LEfSe (linear discriminant analysis (LDA) effect size) analysis was performed to identify features (species and functional genes) differently expressed in each group.Citation30 Spearman correlations were used for correlation analysis of gut microbiota and metabolic parameters. As for continuous variables, the comparisons among the four groups were tested by one-way ANOVA, and the data in each group is reported as the mean ± SEM. P <0.05 was considered statistically significant.
Results
Liraglutide and Semaglutide Could Alter the Gut Microbiota Diversity of DHEA-Induced PCOS Mice
To determine the microbial community structure variations among the four groups, we performed the principal coordinate analysis (PCoA) at the species level with Bray-Curtis distance (). The distance of PCoA spots in the control, PCOS, PCOS+Liraglutide, and PCOS+Semaglutide groups was apparently separated, indicating that the four groups had different bacterial community structures. According to the analysis of similarities (ANOSIM), the beta diversity of PCOS microbiomes was significantly decreased compared with controls (R= 0.702, p = 0.003), while liraglutide and semaglutide intervention both could reverse this alteration (). The alpha-diversity analysis was conducted by ACE and Simpson indexes ( and ). ACE and Shannon indexes represented the microflora community richness and diversity, respectively. Both of them were markedly decreased in the PCOS+Liraglutide and PCOS+Semaglutide group compared with the PCOS group, which demonstrated the effects of GLP-1RAs liraglutide and semaglutide on the alpha diversity of gut microbiota. However, the differences between the controls and the PCOS group were not statistically significant.
Figure 1 Liraglutide and semaglutide changed both the α and β diversity of gut microbiota in DHEA-induced PCOS mice. (A)Principal coordinate analysis (PCoA) with Bray-Curtis distance among four groups at the species level. (B) Analysis of similarities(ANOSIM) with Bray-Curtis distance among four groups, between the control group and the PCOS group, between the PCOS group and the PCOS+Liraglutide group, and between the PCOS group and the PCOS+Semaglutide group at the species level, respectively. (C) The ACE index shows the community richness of each group. (D) The Simpson index shows the community diversity of each group. *P <0.05.
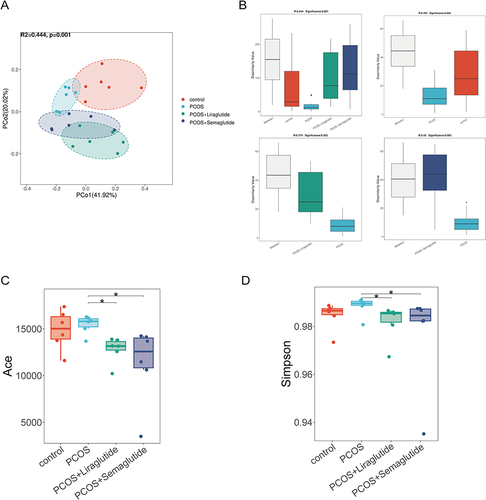
Taken together, the beta diversity was significantly decreased in DHEA-induced PCOS model mice, while there was no alteration in the microflora community richness and diversity based on ACE and Simpson indexes compared with controls. Moreover, liraglutide and semaglutide could change both alpha and beta diversity of the gut microbiota structure in PCOS model mice, and the effects of the two agents were identical.
Liraglutide and Semaglutide Could Recover the Imbalanced Gut Microbiota Community of DHEA-Induced PCOS Mice
To find out the bacterial profile difference between the PCOS group and the healthy control group, as well as determine the changes in the gut microbiota community after liraglutide and semaglutide intervention, we assessed the variety and relative abundance of bacteria at different taxonomic levels among four groups.
As observed in the gut microbiota community structure histogram at the phylum level (), the top nine florae were Bacteroidota, Bacillota (also known as Firmicutes),Citation31 Unassigned Bacteria, Verrucomicrobiota, Uroviricota, Campylobacterota, Actinomycetota, Pseudomonadota, and Chlamydiota. The relative abundance of the two largest phyla, Bacteroidota and Bacillota, was markedly altered in PCOS model mice. To be more specific, PCOS model mice had a higher relative abundance of Bacteroidota but a lower relative abundance of Bacillota compared with controls (P < 0.05), which resulted in a decreased ratio of Bacillota to Bacteroidota than that in the control group (). Interestingly, as observed in , liraglutide treatment could restore the ratio of Bacillota to Bacteroidota by enriching the phylum Bacillota and inhibiting the phylum Bacteroidota. However, Semaglutide showed no significant effects on the ratio of Bacillota to Bacteroidota. This longer-acting GLP-1RA mainly increased the relative abundance of Campylobacterota at the phylum level.
Figure 2 Effects of liraglutide and semaglutide on the composition of gut microbiota DHEA-induced PCOS model mice.(A) Microbiota compositions at the phylum level. (B) The relative abundance of Bacillota, Bacteroidota, the ratio of Bacillota to Bacteroidota and Campylobacterota among the four groups. Data are presented as means ± SEM. (C) Microbiota compositions at the family level and the relative abundance of Muribaculaceae, Bacteroidales, Lachnospiraceae, Oscillospiraceae, and Helicobacteraceae among the four groups. (D) Microbiota compositions at the genus level and the relative abundance of Lepagella, Bacteroides, Bifidobacterium, Ileibacterium, and Helicobacter among the four groups. *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001.
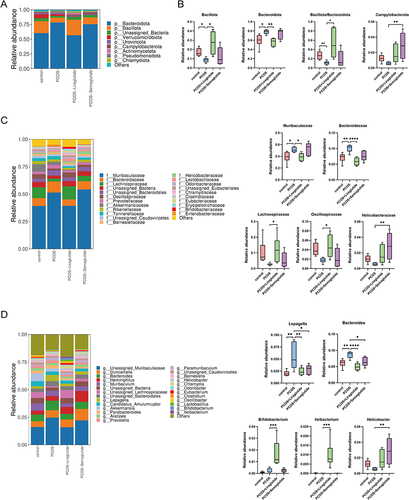
At the family level, Muribaculaceae and Bacteroidales were enriched in the PCOS group, while the relative abundance of Lachnospiraceae significantly decreased compared with controls (). Liraglutide intervention reversed the increased bacteria in the PCOS group and markedly up-regulated the gut microbiota members belonging to the phylum Bacillota: Lachnospiraceae and Oscillospiraceae. Only the family Helicobacteraceae was found to be enriched in the PCOS+Semaglutide group.
As observed at the genus level (), the two genera belonging to the phylum Bacteroidota (Lepagella and Bacteroides) were all increased in the PCOS model mice compared with the controls, while liraglutide lowered the relative abundance of the genera increased in PCOS model mice and enriched the beneficial genera Bifidobacterium and Ileibacterium. Notably, semaglutide also elevated the relative abundance of the genus Helicobacter.
The Venn diagrams showed the numbers of species in different groups (). The control group contained 1523 specific species; however, only 505 specific species were found in the samples from the PCOS group. As compared with those in the PCOS group, the total number of specific species after liraglutide intervention decreased by 150. On the other hand, the number of specific species in the PCOS+semaglutide group was much larger than that in the PCOS group. Next, we used the LDA effect size (LEfSe) method to further determine the core bacterial species contributing to the alterations in the gut microbiota composition. Interestingly, all 17 dominant species in the PCOS group compared with the control include 15 species from the phylum Bacteroidota and 11 species from the family Muribaculaceae (). This enrichment may explain the markedly reduced numbers of bacteria found in the PCOS group.
Figure 3 The differences in microbiota compositions at the species level made by liraglutide and semaglutide. (A) Venn diagrams showing the numbers of species between the control group and the PCOS group, between the PCOS group and the PCOS+Liraglutide group, and between the PCOS group and the PCOS+Semaglutide group, respectively. (B) LDA (linear discriminant analysis) scores for the species abundance in the control group and the PCOS group. Positive and negative LDA scores indicate the dominant species in the control group and the PCOS group, respectively. Only the species with LDA scores > 3 are shown. (C) LDA scores for the species abundance in the PCOS group and the PCOS+Liraglutide group. (D) LDA scores for the species abundance in the PCOS group and the PCOS+Semaglutide group. (E) The relative abundance of the species Lepagella muris, Bacteroides acidifaciens, Muribaculaceae bacterium Isolate-037 Harlan, and Alistipes muris among the four groups. Data are presented as means ± SEM.*P < 0.05; **P < 0.01; ***P < 0.001.
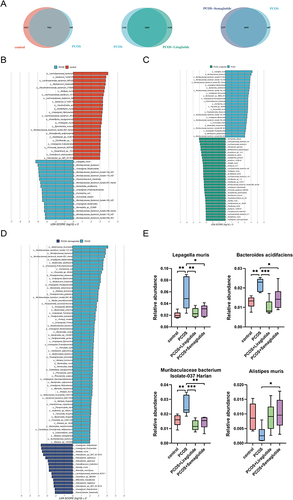
Another LEfSe analysis showed that 25 of the 31 dominant species in the PCOS+liraglutide group compared with the PCOS group belong to the phylum Bacillota and most of them belong to the family Lachnospiraceae (), while the majority of the dominant species in the PCOS+semaglutide group belong to the genera Helicobacter (). To be more specific, the species Lepagella muris and Bacteroides acidifaciens which were enriched in the PCOS group were inhibited after liraglutide intervention, and the relative abundance of Muribaculaceae bacterium Isolate-037 Harlan was also significantly lower in the PCOS+liraglutide group (). Semaglutide could also down-regulate the relative abundance of these three species. In addition, showed that semaglutide intervention enriched the specific species Alistipes muris.
Overall, there were remarkable alterations in the relative abundance of Bacteroidota and Bacillota in the PCOS group compared with the controls. In addition, the family Muribaculaceae and the species Bacteroides acidifaciens were enriched in the PCOS model mice. Both liraglutide and semaglutide could change this abnormal intestinal flora composition but in different ways. Liraglutide treatment recovered the imbalance of gut microbiota mainly by reversing the effects of DHEA-induced PCOS on microbiota composition at every taxonomic level, including increasing the ratio of Bacillota to Bacteroidota, reducing the specific bacteria enriched in the PCOS group and enriching the relative abundance of Lactobacillaceae and other probiotics. Nevertheless, semaglutide altered the gut microbiota composition mainly through enriching the relative abundance of the phylum Campylobacterota, the family Helicobacteraceae, the genus Helicobacter, and the species Alistipes muris.
Liraglutide and Semaglutide Restored the Disrupted Gut Microbial Functions in DHEA-Induced PCOS Mice
To explore disrupted microbiota functions in PCOS model mice and evaluate the effects of liraglutide and semaglutide treatment on functional bacteria genes, we conducted functional analyses using the KEGG database.
A total of 5363 genes were predicated in our functional analysis based on the Venn diagram (). The KEGG orthologous (KO) was clearly separated among the four groups based on the ANOSIM results (P=0.003, ). PCoA with Bray-Curtis distance based on KEGG modules indicated significant alterations in microbial functions among the different groups ().
Figure 4 Microbial gene functions annotated on KEGG were disrupted in DHEA-induced PCOS model mice. (A) The Venn diagram showing the numbers of genes among the four groups. (B) Comparison of the KEGG orthologous (KO) among the four groups. The black dot in the PCOS group represented an outlier. (C) PCoA based on the Bray-Curtis distance of KEGG among the four groups. (D) The average abundance of KEGG modules was differently enriched in the control group and the PCOS group according to level 1 (above) and level 2 (below). (E) LDA scores for microbial gene functions at the pathway level in the control group and the PCOS group. Positive and negative LDA scores indicate the dominant KEGG pathways in the control group and the PCOS group, respectively. Only the gene function with a LDA score > 2 is shown. *P <0.05, and **P < 0.01.
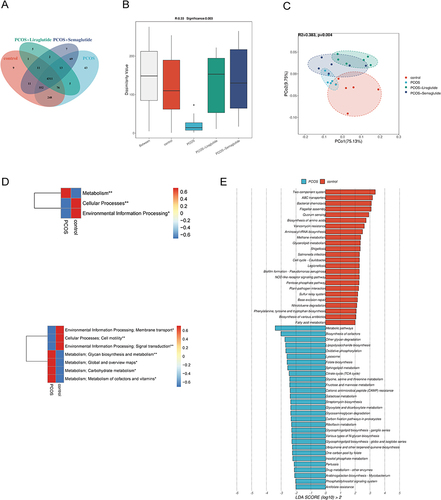
Compared with the control group based on level 1 and level 2 of KEGGE pathways, the function of gut microbiota was apparently disrupted in the PCOS group (). Pathways involved in metabolism, such as glycan biosynthesis and metabolism, global and overview maps, carbohydrate metabolism, and metabolism of cofactors and vitamins were all increased in the PCOS model mice. To be more specific, we identified 24 pathways in controls and 29 in the PCOS group at the pathway level using the LEfSe analysis (LDA > 2.0) (). Lipopolysaccharide biosynthesis, glyoxylate and dicarboxylate metabolism, sphingolipid metabolism, and citrate cycle (TCA cycle) were strongly enriched in the PCOS group.
Liraglutide treatment could restore the dysfunction of intestinal flora in mice with PCOS according to the LEfSe analysis (LDA > 2.0) at the pathway level (). Most of the pathways enriched in the PCOS+Liraglutide group were similar to the control group, including the two-component system and ABC transporters. The enriched pathways involved in glycan biosynthesis and metabolism as well as the metabolism of cofactors and vitamins in the PCOS group were significantly decreased after liraglutide intervention (). On the other hand, semaglutide could increase the pathways involved in starch and sucrose metabolism, porphyrin metabolism, and degradation of flavonoids while decreasing the metabolism of nucleotide, such as purine pyrimidine metabolism and pyrimidine metabolism in mice with PCOS ( and ).
Figure 5 Effects of liraglutide and semaglutide on the disrupted microbial gene functions annotated on KEGG in DHEA-induced PCOS model mice. (A)LDA scores for microbial gene functions at the pathway level in the PCOS group and the PCOS+Liraglutide group. Positive and negative LDA scores indicate the dominant KEGG pathways in the PCOS group and the PCOS+Liraglutide group, respectively. Only the gene function having a LDA score > 2 is shown. (B) The average abundance of KEGG modules was differently enriched in the PCOS group and the PCOS+Liraglutide group according to level 2. (C) The average abundance of KEGG modules was differently enriched in the PCOS group and the PCOS+Semaglutide group based on level 2. (D) LDA scores for KEGG pathways in the PCOS group and the PCOS+Semaglutide group. *P <0.05, and **P < 0.01.
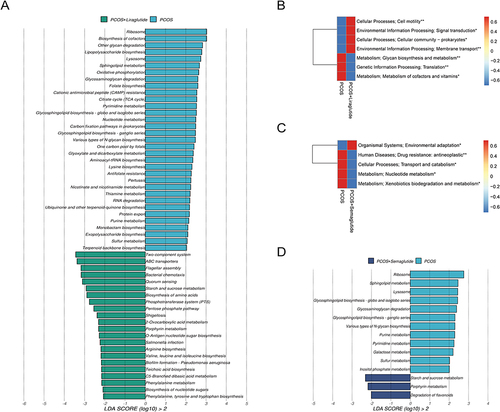
The results above support the notion that PCOS model mice showed gut microbial dysfunction in the pathways of the KEGG. The PCOS group displayed disruption of carbohydrate metabolism, glycan biosynthesis, and metabolism compared with the control group. Meanwhile, these dysfunctions could be dramatically altered by GLP-1 RAs. Liraglutide could reverse most of the KEGG pathways to normal, while semaglutide mainly increased the pathway of starch and sucrose metabolism and decreased nucleotide metabolism.
Correlation Analysis Between Gut Microbiota and Metabolic Parameters in DHEA-Induced PCOS Mice
Our previous study measured the hormone profile and biochemical indexes related to metabolism in the four groups.Citation13 Both liraglutide and semaglutide effectively improve metabolic disorders, such as hyperandrogenemia, insulin resistance, and inflammatory response. Interestingly, semaglutide showed a stronger weight-loss effect than liraglutide (semaglutide vs liraglutide: 14% vs 9% decrease). However, the reduction of food intake in the two groups showed no statistical difference. These results indicated that there may be more mechanisms underlying the effect of semaglutide on weight loss than by limiting the food intake alone. To identify the potential associations between these metabolic parameters and the gut microbiota in PCOS model mice, we performed correlation analysis both at the genus () and species level ().
Figure 6 Pearson correlation analysis between metabolic parameters and gut microbiota in each group at the genus level. *P < 0.05.
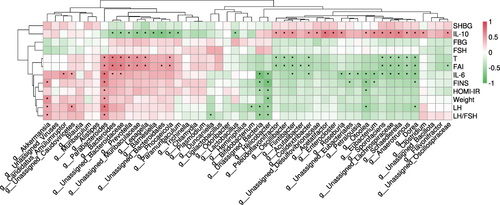
Figure 7 Pearson correlation analysis between metabolic parameters and gut microbiota in each group at the species level. *P < 0.05.
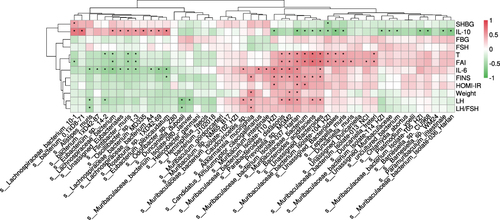
Most of the genera increased in the PCOS group positively correlated with testosterone, fasting insulin (FINS), and interleukin-6 (IL-6), including Bacteroides and Parabacteroides (both belong to the phylum Bacteroidota). The dominant species Lepagella muris and Bacteroides acidifaciens also had a positive relationship with the testosterone level, which implied that they may contribute to hyperandrogenemia in the development of PCOS. Bacteroides acidifaciens was also positively related to many other metabolic parameters, such as FINS and IL-6. In addition, the species Muribaculaceae bacterium Isolate-037 Harlan and Parabacteroides distasonis had a significantly positive relationship with so many clinical variables including body weight.
On the contrary, the genera (such as Bifidobacterium) belonging to the phylum Bacillota were negatively associated with these metabolic parameters, and they were all increased in the PCOS+Liraglutide group. The genus Helicobacter, which was one of the dominant bacterial genera in the PCOS +Semaglutide group, was the only genus that was found to be negatively associated with body weight. This result suggested that the enriched genus Helicobacter may play a role in the significant weight-sparing effect of Semaglutide. This genus also negatively correlated with other metabolic parameters such as FINS, IL-6, and LH/FSH. The species Alistipes muris which was also increased after semaglutide treatment showed a negative relationship with IL-6 and LH/FSH.
The result indicated that the enrichment of specific bacteria belonging to the phylum Bacteroidota might make a great contribution to insulin resistance, obesity, and other dysmetabolism in the pathophysiology of PCOS. The genus Helicobacter and the species Alistipes muris may have beneficial effects on improving hormone levels and chronic inflammation in PCOS.
Discussion
Based on metagenomic sequencing, our study found that the diversity and composition of gut microbiota were significantly altered in the PCOS group, resulting in disrupted microbiota functions. Meanwhile, GLP-1 receptor agonists liraglutide and semaglutide showed strong effects on restoring the imbalance of gut microbiota induced by PCOS, and these changes were closely associated with improvements in metabolic parameters. To the best of our knowledge, the current study first evaluated the potential effects of liraglutide and semaglutide on gut microbiota in mice with PCOS and provided strong evidence that particular phenotypes of gut bacteria could affect the pathophysiology of PCOS, which contributes to the clinical use of gut microbiota for PCOS in the future.
The hypothesis that dysbiosis of the gut microbiota played a role in the development of PCOS was first brought up by Tremellen and Pearce in 2012.Citation32 Since then, many studies have shown that the imbalance of the gut microbiota is associated with PCOS, especially the two core dysmetabolic changes, hyperandrogenism and insulin resistance.Citation33–35 In our study, the beta diversity and abundance of the gut microbiota were decreased in the PCOS group by enriching the phylum Bacteroidota, which contributed to a reduced ratio of Bacillota to Bacteroidota. As reported, the Bacillota-to-Bacteroidota ratio was negatively correlated with the weight-gain effect.Citation36,Citation37 Consistent with the aforementioned results, most bacteria that showed a positive relationship with body weight in the current correlation analysis belong to the phylum Bacteroidota. The abundance of the family Muribaculaceae, the genus Lepagella and Bacteroides were also observed to increase in the PCOS group. According to previous studies, these enriched bacteria were associated with intestinal inflammation and metabolic disorders.Citation17 The genus Bacteroides has showed diagnostic value in PCOS and has already been considered as a key microbial biomarker, the enrichment of which was found both in PCOS patients and PCOS model mice.Citation33 At the species level, the dominant bacteria Lepagella muris and Bacteroides acidifaciens were observed to have positive relationships with many metabolic disorders, especially hyperandrogenemia. Besides that, Muribaculaceae bacterium Isolate-037 Harlan was also found to positively correlate to metabolic parameters. To date, no studies have shown the harmful effect of this species on PCOS or other metabolic diseases. These mentioned differential species which were identified in mice with PCOS may have potential as biomarkers in the clinical diagnosis of PCOS, although further explorations such as monocolonization were needed to be more conclusive.
Besides changing the structure of gut microbiota, the KEGG annotations revealed that PCOS has also disrupted bacteria functions. The pathway of Lipopolysaccharide (LPS) biosynthesis was enriched in the PCOS group, which was in accordance with the findings of gut microbiota composition in our study. The phylum Bacteroidota enriched in mice with PCOS was the main group of Gram-negative bacteria, the outer cell walls of which were full of LPS.Citation38 The high level of LPS was reported to activate the immune system and may be involved in the pathogenesis of PCOS by promoting androgen and insulin secretion.Citation17 Another increased pathway in the PCOS group was the glyoxylate and dicarboxylate metabolism. The glyoxylate and dicarboxylate metabolism has already been found to be associated with many metabolic diseases (type 2 diabetes, obesity, and atherosclerosis cardiovascular disease).Citation39 Metabolites in the glyoxylate and dicarboxylate metabolic pathways, such as tartrate, could be fermented to short-chain fatty acids (SCFAs), especially producing acetate in the colon.Citation40 The high production of acetate was reported to promote glucose-stimulated insulin secretion (GSIS), pancreatic β-cell activity, and obesity by activating the parasympathetic nervous system.Citation41 To our knowledge, it’s the first study to report that disturbed glyoxylate and dicarboxylate metabolism was linked to PCOS. Moreover, sphingolipid metabolism which was found to be enriched in the PCOS group in our study was reported to be involved with obesity-related diseases.Citation42 In fact, sphingolipids were found to induce insulin resistance by suppressing mitochondrial function.Citation43 Interestingly, dysfunction of sphingolipid metabolism was recently found to be closely associated with oocyte development in patients with PCOS accepting assisted reproductive techniques (ART).Citation44 Taken together, these disrupted metabolic pathways may contribute to the underlying mechanism in the etiology of PCOS.
We then evaluate the effects of liraglutide and semaglutide on alleviating metabolic disorders in PCOS via modulating the changed gut microbiota. Although both liraglutide and semaglutide could alter the diversity of gut microbiota, they had different points of focus on the alterations of the intestinal flora community as well as disrupted microbial functions.
Liraglutide, as a promoting agent for obesity and/or diabetes treatment due to its weight-sparing effect, mainly reversed the gut microbiota community of PCOS model mice to normal at different taxonomic levels. Basically, liraglutide treatment decreased the species richness with a significant enriching effect on the phylum Bacillota, resulting in an increased ratio of Bacillota to Bacteroidota which was lower in the PCOS group. The dominant family Muribaculaceae in PCOS model mice was markedly decreased after treating them with liraglutide, and most of the abundant Bacillota were from the family Lachnospiraceae and Oscillospiraceae. Importantly, Lachnospiraceae and Oscillospiraceae are recognized as butyrate-producing members of gut microbiota.Citation45 As previously reported, gut microbiota-derived butyrate has beneficial effects on intestinal homeostasis, insulin sensitivity enhancement, and immune response regulation, the level of which was found to be decreased in obesity and metabolic diseases.Citation46–49 This information further suggested that the potential mechanism underlying the effect of liraglutide on improving metabolic disorders of PCOS may be related to these increased butyrate-producing bacteria. Further metabolic analysis is surely needed to determine their correlations. At the genus level, under the modulation of liraglutide, the abundance of well-known probiotics Bifidobacterium and Ileibacterium was elevated. Bifidobacterium was widely reported to display beneficial effects on human health by alleviating obesity and controlling blood glucose levels,Citation50 while Ileibacterium could prevent mice from gaining weight.Citation51 The application of Bifidobacterium in food and therapeutic use has already been extensively studied due to its health advantages.Citation52 Importantly, its potential for long-term gut colonization has been recently evaluated,Citation53 which may contribute to novel treatments of PCOS. Besides recovering the imbalance of gut microbiota composition in PCOS, liraglutide also restored the disrupted bacterial function. Based on the KEGG annotations, the enriched pathways after liraglutide intervention were similar to those in the controls. The two-component system, as an essential signal transduction mechanism, plays an important role in the survival and adaptation of bacteria via perceiving environmental changes and regulating bacterial virulence.Citation54,Citation55 Adenosine triphosphate (ATP)-binding cassette (ABC) transporters which are located downstream of the two-component system help to export antimicrobial peptides (AMPs) during biosynthesis and provide resistance.Citation56,Citation57 The enrichment of these pathways in the PCOS+Liraglutide group surely showed a positive influence on the stability and integrity of microbial cells, thus contributing to the whole balance of gut microbiota disrupted by PCOS.
Semaglutide is a novel long-acting GLP-1 receptor agonist, with a longer half-life of 168–184 hours compared to liraglutide. Instead of making significant alterations to the abundance of the two main phyla Bacteroidota and Bacillota, semaglutide made differential changes to the composition of gut microbiota in PCOS by increasing the abundance of the phylum Campylobacterota. The main phylotype responsible for the increased phylum in semaglutide-treated mice was in the genus Helicobacter within the family Helicobacteraceae. The phylum Campylobacterota, used to known as Epsilonproteobacteria,Citation31 is a major bacterial cause of diarrhea in the world.Citation58 According to the systematic review and meta-analysis of clinical trials, semaglutide treatment did have frequent gastrointestinal events like diarrhea.Citation59 The current study may show the correlation between this side effect caused by semaglutide and the increased abundance of the phylum Campylobacterota. Iqbal et al investigated the weight-loss effect of GLP-1 RAs in clinical patients with obesity but without diabetes.Citation60 Consistent with their findings, our study demonstrated that semaglutide had greater efficacy in weight loss. The result may have positive relationships with the increased genus Helicobacter based on our correlation analysis. The species that showed beneficial effects on metabolic disorders in the PCOS+Semaglutide group was Alistipes muris. To date, there have been no studies that found the benign effect of the species Alistipes muris on PCOS. However, the genus Alistipes has been reported to have protective effects on diseases like liver fibrosis, colitis, and cardiovascular disease.Citation61 In addition, it was one of the most reported lean-associated genera in patients with obesity and metabolic disordersCitation62 and was found to decrease in patients with diabetes.Citation63 Further studies including fecal transplantation of this specific bacteria may validate the potential effect on PCOS. Semaglutide also could alter the disrupted bacterial function in PCOS. The starch and sucrose metabolism was increased after semaglutide intervention. These major dietary fibers could be efficiently used to produce energy for hosts.Citation64 Porphyrin mainly exists in hemin mobilized in animals and is crucial for a variety of physiological processes.Citation65 Hemin was found to have the ability to enhance the expression of ovarian total antioxidants and improve insulin resistance as well as dyslipidemia in letrozole-induced PCOS model rats.Citation66 Moreover, the higher serum level of glycine (a precursor of porphyrin) was reported to be associated with the improvement of insulin resistance in obese people and the decreased risk of incident type 2 diabetes.Citation67 Therefore, the enrichment of porphyrin metabolism pathways after semaglutide treatment may contribute to the improving effect of this long-acting GLP-1 RA on metabolic disorders. Another enriched pathway in the PCOS+Semaglutide group was flavonoid degradation. Flavonoids are an important group of secondary metabolites frequently found in plants and have a variety of medical and nutraceutical applications due to their anti-inflammatory,Citation68 anti-oxidative,Citation69 and hypoglycemicCitation70 capacities. Recently, a review summarized the potential of flavonoids in alleviating and treating PCOS.Citation71 For example, naringin showed a beneficial effect on alleviating hormone levels and insulin resistance via modulating the composition of gut microbiota and activating the SIRT1/PGC-1ɑ signaling pathway.Citation72 Soy isoflavones could improve ovarian function in rats with letrozole-induced PCOS by returning the gut microbiota to normal at the genus level.Citation73 These studies suggested pathways involved in flavonoids may be related to the regulation of gut microbiota and the treatment effect of semaglutide on PCOS. However, much more further experiments are needed to validate this conjecture.
The limitation of our study is that the current results can only represent the alterations of fecal microbiota in the PCOS model mice induced by DHEA. Following studies on clinical patients with a larger sample scale are surely required to testify to our present results. The study shows the differential changes in the gut microbiota made by liraglutide (daily given) and semaglutide (weekly given), which to some extent suggested the correlation between the treatment effect of GLP-1 RAs on PCOS with the modulation of gut microbiota. Nevertheless, the detailed mechanism of single or multiple bacteria identified in the intervention groups is necessary to be further clarified.
Conclusion
The analysis provided convincing evidence that GLP-1 RAs, both liraglutide and semaglutide, could ameliorate metabolic disorders in PCOS by modulating the disrupted gut microbiota. Liraglutide increased the ratio of Bacillota to Bacteroidota via enriching the abundance of butyrate-producing bacteria in the phylum Bacillota. The novel GLP-1 RA semaglutide may have advantages over liraglutide in the treatment of PCOS in the weight-sparing respect due to the up-regulated abundance of the genus Helicobacter. These increased bacteria species may provide practical insights into the potential mechanisms underlying the anti-dysmetabolic effect of GLP-1 RAs on PCOS and future clinical applications.
Disclosure
The authors report no conflicts of interest in this work.
Acknowledgments
We thank all the participants in this study.
Data Sharing Statement
Data and materials supporting the results of this study can be obtained from the corresponding authors.
Additional information
Funding
References
- Joham AE, Norman RJ, Stener-Victorin E, et al. Polycystic ovary syndrome. Lancet Diabetes Endocrinol. 2022;10(9):668–680. doi:10.1016/S2213-8587(22)00163-2
- Zeng X, Xie YJ, Liu YT, Long SL, Mo ZC. Polycystic ovarian syndrome: correlation between hyperandrogenism, insulin resistance and obesity. Clin Chim Acta. 2020;502:214–221. doi:10.1016/j.cca.2019.11.003
- Oliver-Williams C, Vassard D, Pinborg A, Schmidt L. Risk of cardiovascular disease for women with polycystic ovary syndrome: results from a national Danish registry cohort study. Eur J Prev Cardiol. 2021;28(12):e39–e41. doi:10.1177/2047487320939674
- Jensterle M, Rizzo M, Haluzík M, Janež A. Efficacy of GLP-1 RA Approved for Weight Management in Patients With or Without Diabetes: a Narrative Review. Adv Ther. 2022;39(6):2452–2467. doi:10.1007/s12325-022-02153-x
- Gilbert MP, Pratley RE. GLP-1 Analogs and DPP-4 Inhibitors in Type 2 Diabetes Therapy: review of Head-to-Head Clinical Trials. Front. Endocrinol. 2020;11:178. doi:10.3389/fendo.2020.00178
- Xing C, Zhang J, Zhao H, He B. Effect of Sex Hormone-Binding Globulin on Polycystic Ovary Syndrome: mechanisms, Manifestations, Genetics, and Treatment. Int J Womens Health. 2022;14:91–105. doi:10.2147/IJWH.S344542
- Abdalla MA, Deshmukh H, Atkin S, Sathyapalan T. The potential role of incretin-based therapies for polycystic ovary syndrome: a narrative review of the current evidence. Ther Adv Endocrinol Metab. 2021;12:2042018821989238. doi:10.1177/2042018821989238
- Siamashvili M, Davis SN. Update on the effects of GLP-1 receptor agonists for the treatment of polycystic ovary syndrome. Expert Rev Clin Pharmacol. 2021;14(9):1081–1089. doi:10.1080/17512433.2021.1933433
- Cena H, Chiovato L, Nappi RE. Obesity, Polycystic Ovary Syndrome, and Infertility: a New Avenue for GLP-1 Receptor Agonists. J Clin Endocrinol Metab. 2020;105(8):e2695–2709. doi:10.1210/clinem/dgaa285
- Ma X, Liu Z, Ilyas I, et al. GLP-1 receptor agonists (GLP-1RAs): cardiovascular actions and therapeutic potential. Int J Biol Sci. 2021;17(8):2050–2068. doi:10.7150/ijbs.59965
- Lin CH, Shao L, Zhang YM, et al. An evaluation of liraglutide including its efficacy and safety for the treatment of obesity. Expert Opin Pharmacother. 2020;21(3):275–285. doi:10.1080/14656566.2019.1695779
- Chao AM, Tronieri JS, Amaro A, Wadden TA. Semaglutide for the treatment of obesity. Trends Cardiovasc Med. 2023;33(3):159–166. doi:10.1016/j.tcm.2021.12.008
- Zhang Y, Lin Y, Li G, et al. Glucagon-like peptide-1 receptor agonists decrease hyperinsulinemia and hyperandrogenemia in dehydroepiandrosterone-induced polycystic ovary syndrome mice and are associated with mitigating inflammation and inducing browning of white adipose tissue†. Biol Reprod. 2023;108(6):945–959. doi:10.1093/biolre/ioad032
- Qi X, Yun C, Pang Y, Qiao J. The impact of the gut microbiota on the reproductive and metabolic endocrine system. Gut Microbes. 2021;13(1):1–21. doi:10.1080/19490976.2021.1894070
- Wang L, Zhou J, Gober H-J, et al. Alterations in the intestinal microbiome associated with PCOS affect the clinical phenotype. Biomed Pharmacother. 2021;133:110958. doi:10.1016/j.biopha.2020.110958
- Qi X, Yun C, Sun L, et al. Gut microbiota-bile acid-interleukin-22 axis orchestrates polycystic ovary syndrome. Nat Med. 2019;25(8):1225–1233. doi:10.1038/s41591-019-0509-0
- Chu W, Han Q, Xu J, et al. Metagenomic analysis identified microbiome alterations and pathological association between intestinal microbiota and polycystic ovary syndrome. Fertil Steril. 2020;113(6):1286–1298. doi:10.1016/j.fertnstert.2020.01.027
- Liang Z, Di N, Li L, Yang D. Gut microbiota alterations reveal potential gut-brain axis changes in polycystic ovary syndrome. J Endocrinol Invest. 2021;44(8):1727–1737. doi:10.1007/s40618-020-01481-5
- Dong S, Jiao J, Jia S, et al. 16S rDNA full-length assembly sequencing technology analysis of intestinal microbiome in polycystic ovary syndrome. Front Cell Infect Microbiol. 2021;11:634981. doi:10.3389/fcimb.2021.634981
- Su YN, Wang MJ, Yang JP, et al.Effects of Yulin Tong Bu formula on modulating gut microbiota and fecal metabolite interactions in mice with polycystic ovary syndrome. Front Endocrinol. 2023;(14):1122709. doi:10.3389/fendo.2023.1122709
- Zhao L, Chen Y, Xia F, et al. A Glucagon-Like Peptide-1 Receptor Agonist Lowers Weight by Modulating the Structure of Gut Microbiota. Front. Endocrinol. 2018;9:233. doi:10.3389/fendo.2018.00233
- Charpentier J, Briand F, Lelouvier B, et al. Liraglutide targets the gut microbiota and the intestinal immune system to regulate insulin secretion. Acta Diabetol. 2021;58(7):881–897. doi:10.1007/s00592-020-01657-8
- Han Q, Wang J, Li W, Chen ZJ, Du Y. Androgen-induced gut dysbiosis disrupts glucolipid metabolism and endocrinal functions in polycystic ovary syndrome. Microbiome. 2021;9(1):101. doi:10.1186/s40168-021-01046-5
- Chen S, Zhou Y, Chen Y, Gu J. fastp: an ultra-fast all-in-one FASTQ preprocessor. Bioinformatics. 2018;34(17):i884–i890. doi:10.1093/bioinformatics/bty560
- Li D, Liu CM, Luo R, Sadakane K, Lam TW. MEGAHIT: an ultra-fast single-node solution for large and complex metagenomics assembly via succinct de Bruijn graph. Bioinformatics. 2015;31(10):1674–1676. doi:10.1093/bioinformatics/btv033
- Hyatt D, Chen GL, Locascio PF, Land ML, Larimer FW, Hauser LJ. Prodigal: prokaryotic gene recognition and translation initiation site identification. BMC Bioinf. 2010;11(1):119. doi:10.1186/1471-2105-11-119
- Steinegger M, Söding J. MMseqs2 enables sensitive protein sequence searching for the analysis of massive data sets. Nat Biotechnol. 2017;35(11):1026–1028. doi:10.1038/nbt.3988
- Buchfink B, Xie C, Huson DH. Fast and sensitive protein alignment using DIAMOND. Nat Methods. 2015;12(1):59–60. doi:10.1038/nmeth.3176
- Aramaki T, Blanc-Mathieu R, Endo H, et al. KofamKOALA: KEGG Ortholog assignment based on profile HMM and adaptive score threshold. Bioinformatics. 2020;36(7):2251–2252. doi:10.1093/bioinformatics/btz859
- Segata N, Izard J, Waldron L, et al. Metagenomic biomarker discovery and explanation. Genome Biol. 2011;12(6):R60. doi:10.1186/gb-2011-12-6-r60
- Oren A, Garrity GM. Valid publication of the names of forty-two phyla of prokaryotes. Int J Syst Evol Microbiol. 2021;71(10):005056. doi:10.1099/ijsem.0.005056
- Tremellen K, Pearce K. Dysbiosis of Gut Microbiota (DOGMA)--a novel theory for the development of Polycystic Ovarian Syndrome. Med Hypotheses. 2012;79(1):104–112. doi:10.1016/j.mehy.2012.04.016
- Yang YL, Zhou WW, Wu S, et al. Intestinal Flora is a Key Factor in Insulin Resistance and Contributes to the Development of Polycystic Ovary Syndrome. Endocrinology. 2021;162(10):bqab118. doi:10.1210/endocr/bqab118
- Insenser M, Murri M, Del Campo R, Martínez-García MÁ, Fernández-Durán E, Escobar-Morreale HF. Gut Microbiota and the Polycystic Ovary Syndrome: influence of Sex, Sex Hormones, and Obesity. J Clin Endocrinol Metab. 2018;103(7):2552–2562. doi:10.1210/jc.2017-02799
- Torres PJ, Siakowska M, Banaszewska B, et al. Gut Microbial Diversity in Women With Polycystic Ovary Syndrome Correlates With Hyperandrogenism. J Clin Endocrinol Metab. 2018;103(4):1502–1511. doi:10.1210/jc.2017-02153
- Wang L, Li P, Tang Z, Yan X, Feng B. Structural modulation of the gut microbiota and the relationship with body weight: compared evaluation of liraglutide and saxagliptin treatment. Sci Rep. 2016;6(1):33251. doi:10.1038/srep33251
- Aragón-Vela J, Solis-Urra P, Ruiz-Ojeda FJ, Álvarez-Mercado AI, Olivares-Arancibia J, Plaza-Diaz J. Impact of Exercise on Gut Microbiota in Obesity. Nutri. 2021;13(11):3999. doi:10.3390/nu13113999
- Magne F, Gotteland M, Gauthier L, et al. The Firmicutes/Bacteroidetes Ratio: a Relevant Marker of Gut Dysbiosis in Obese Patients? Nutri. 2020;12(5):1474. doi:10.3390/nu12051474
- Proffitt C, Bidkhori G, Lee S, et al. Genome-scale metabolic modelling of the human gut microbiome reveals changes in the glyoxylate and dicarboxylate metabolism in metabolic disorders. iScience. 2022;25(7):104513. doi:10.1016/j.isci.2022.104513
- Younes M, Aquilina G, Castle L, et al. Re-evaluation of l(+)-tartaric acid (E 334), sodium tartrates (E 335), potassium tartrates (E 336), potassium sodium tartrate (E 337) and calcium tartrate (E 354) as food additives. Efsa j. 2020;18(3):e06030. doi:10.2903/j.efsa.2020.6030
- Bose S, Ramesh V, Locasale JW. Acetate Metabolism in Physiology, Cancer, and Beyond. Trends Cell Biol. 2019;29(9):695–703. doi:10.1016/j.tcb.2019.05.005
- Juchnicka I, Kuźmicki M, Szamatowicz J. Ceramides and Sphingosino-1-Phosphate in Obesity. Front. Endocrinol. 2021;12:635995. doi:10.3389/fendo.2021.635995
- Roszczyc-Owsiejczuk K, Zabielski P. Sphingolipids as a Culprit of Mitochondrial Dysfunction in Insulin Resistance and Type 2 Diabetes. Front. Endocrinol. 2021;12:635175. doi:10.3389/fendo.2021.635175
- Liu L, Yin TL, Chen Y, et al. Follicular dynamics of glycerophospholipid and sphingolipid metabolisms in polycystic ovary syndrome patients. J Steroid Biochem Mol Biol. 2019;185:142–149. doi:10.1016/j.jsbmb.2018.08.008
- Leth ML, Pichler MJ, Abou Hachem M. Butyrate-producing colonic clostridia: picky glycan utilization specialists. Essays Biochem. 2023;67:415–428. doi:10.1042/EBC20220125
- Coppola S, Avagliano C, Calignano A, Berni Canani R. The Protective Role of Butyrate against Obesity and Obesity-Related Diseases. Molecules. 2021;26(3):682. doi:10.3390/molecules26030682
- Bridgeman SC, Northrop W, Melton PE, Ellison GC, Newsholme P, Mamotte CDS. Butyrate generated by gut microbiota and its therapeutic role in metabolic syndrome. Pharmacol Res. 2020;160:105174. doi:10.1016/j.phrs.2020.105174
- Zhang L, Liu C, Jiang Q, Yin Y. Butyrate in Energy Metabolism: there Is Still More to Learn. Tren Endocrinol Metab. 2021;32(3):159–169. doi:10.1016/j.tem.2020.12.003
- van Deuren T, Blaak EE, Canfora EE. Butyrate to combat obesity and obesity-associated metabolic disorders: current status and future implications for therapeutic use. Obes Rev. 2022;23(10):e13498. doi:10.1111/obr.13498
- Ohue-Kitano R, Taira S, Watanabe K, et al. 3-(4-Hydroxy-3-methoxyphenyl)propionic Acid Produced from 4-Hydroxy-3-methoxycinnamic Acid by Gut Microbiota Improves Host Metabolic Condition in Diet-Induced Obese Mice. Nutri. 2019;11(5):1036. doi:10.3390/nu11051036
- Xie B, Zu X, Wang Z, Xu X, Liu G, Liu R. Ginsenoside Rc ameliorated atherosclerosis via regulating gut microbiota and fecal metabolites. Front Pharmacol. 2022;13:990476. doi:10.3389/fphar.2022.990476
- Kalam Saleena L A, Chang SK, Simarani K, et al. A comprehensive review of Bifidobacterium spp: as a probiotic, application in the food and therapeutic, and forthcoming trends. Crit Rev Microbiol;2023:1–17. doi:10.1080/1040841X.2023.2243617
- Xiao Y, Zhao J, Zhang H, Zhai Q, Chen W. Mining Lactobacillus and Bifidobacterium for organisms with long-term gut colonization potential. Clin Nutr. 2020;39(5):1315–1323. doi:10.1016/j.clnu.2019.05.014
- Casado J, Á L, González A. Two-component regulatory systems in Helicobacter pylori and Campylobacter jejuni: attractive targets for novel antibacterial drugs. Front Cell Infect Microbiol. 2022;12:977944. doi:10.3389/fcimb.2022.977944
- Xie Y, Li J, Ding Y, et al. An atlas of bacterial two-component systems reveals function and plasticity in signal transduction. Cell Rep. 2022;41(3):111502. doi:10.1016/j.celrep.2022.111502
- Ahmad A, Majaz S, Nouroz F. Two-component systems regulate ABC transporters in antimicrobial peptide production, immunity and resistance. Microbiology (Reading). 2020;166(1):4–20. doi:10.1099/mic.0.000823
- Moore JM, Bell EL, Hughes RO, Garfield AS. ABC transporters: human disease and pharmacotherapeutic potential. Trends Mol Med. 2023;29(2):152–172. doi:10.1016/j.molmed.2022.11.001
- Dai L, Sahin O, Grover M, Zhang Q. New and alternative strategies for the prevention, control, and treatment of antibiotic-resistant Campylobacter. Transl Res. 2020;223:76–88. doi:10.1016/j.trsl.2020.04.009
- Li A, Su X, Hu S, Wang Y. Efficacy and safety of oral semaglutide in type 2 diabetes mellitus: a systematic review and meta-analysis. Diabet Res Clin Pract. 2023;198:110605. doi:10.1016/j.diabres.2023.110605
- Iqbal J, Wu HX, Hu N, et al. Effect of glucagon-like peptide-1 receptor agonists on body weight in adults with obesity without diabetes mellitus-a systematic review and meta-analysis of randomized control trials. Obes Rev. 2022;23(6):e13435. doi:10.1111/obr.13435
- Parker BJ, Wearsch PA, Veloo ACM, Rodriguez-Palacios A. The Genus Alistipes: gut Bacteria With Emerging Implications to Inflammation, Cancer, and Mental Health. Front Immunol. 2020;11:906. doi:10.3389/fimmu.2020.00906
- Xu Z, Jiang W, Huang W, Lin Y, Chan FKL, Ng SC. Gut microbiota in patients with obesity and metabolic disorders - a systematic review. Genes Nutr. 2022;17(1):2. doi:10.1186/s12263-021-00703-6
- Letchumanan G, Abdullah N, Marlini M, et al. Gut Microbiota Composition in Prediabetes and Newly Diagnosed Type 2 Diabetes: a Systematic Review of Observational Studies. Front Cell Infect Microbiol. 2022;12:943427. doi:10.3389/fcimb.2022.943427
- Tappy L. Metabolism of sugars: a window to the regulation of glucose and lipid homeostasis by splanchnic organs. Clin Nutr. 2021;40(4):1691–1698. doi:10.1016/j.clnu.2020.12.022
- Reddi AR, Hamza I. Heme Mobilization in Animals: a Metallolipid’s Journey. Acc Chem Res. 2016;49(6):1104–1110. doi:10.1021/acs.accounts.5b00553
- Ragy MM, Abdel-Hamid HA, Toni NDM. Pathophysiological changes in experimental polycystic ovary syndrome in female albino rats: using either hemin or L arginine. J Cell Physiol. 2019;234(6):8426–8435. doi:10.1002/jcp.27757
- Alves A, Morio B. Alterations in glycine metabolism in obesity and chronic metabolic diseases - an update on new advances. Curr Opin Clin Nutr Metab Care. 2023;26(1):50–54. doi:10.1097/MCO.0000000000000883
- Maleki SJ, Crespo JF, Cabanillas B. Anti-inflammatory effects of flavonoids. Food Chem. 2019;299:125124. doi:10.1016/j.foodchem.2019.125124
- Shen N, Wang T, Gan Q, Liu S, Wang L, Jin B. Plant flavonoids: classification, distribution, biosynthesis, and antioxidant activity. Food Chem. 2022;383:132531. doi:10.1016/j.foodchem.2022.132531
- Shamsudin NF, Ahmed QU, Mahmood S, et al. Flavonoids as Antidiabetic and Anti-Inflammatory Agents: a Review on Structural Activity Relationship-Based Studies and Meta-Analysis. Int J Mol Sci. 2022;23(20):12605. doi:10.3390/ijms232012605
- Luo ED, Jiang HM, Chen W, et al. Advancements in lead therapeutic phytochemicals polycystic ovary syndrome: a review. Front Pharmacol. 2022;13:1065243. doi:10.3389/fphar.2022.1065243
- Wu YX, Yang XY, Han BS, et al. Naringenin regulates gut microbiota and SIRT1/ PGC-1ɑ signaling pathway in rats with letrozole-induced polycystic ovary syndrome. Biomed Pharmacother. 2022;153:113286. doi:10.1016/j.biopha.2022.113286
- Liyanage GSG, Inoue R, Fujitani M, et al. Effects of Soy Isoflavones, Resistant Starch and Antibiotics on Polycystic Ovary Syndrome (PCOS)-Like Features in Letrozole-Treated Rats. Nutri. 2021;13(11):3759. doi:10.3390/nu13113759
