Abstract
Background
The monocyte-to-high-density lipoprotein cholesterol (MHR) ratio has been linked to metabolic disorders. However, there is limited research on the predisposition to MHR and prediabetes. Hence, we conducted a study to investigate the relationship between MHR and the prevalence of prediabetes.
Methods
In total, 85,293 participants were included in our cross-sectional observational study. Multivariable regression analysis, subgroup analyses, and interaction testing were used to determine the relationship between MHR and prediabetes. To explore the non-linear association of MHR with prediabetes risk, generalized additive model (GAM) and smoothing splines were applied. The threshold effect analysis of MHR on the risk of prediabetes was further employed to identify the turning point.
Results
After controlling for covariates, the results indicated that a positive correlation persisted between MHR and prediabetes (odds ratio (OR) =1.64, 95% confidence interval (CI), 1.48–1.82), and subgroup analyses found a more robust correlation between MHR and prediabetes in individuals with lower age, SBP, DBP, TG, TC and higher values of BMI and LDL-C than in their counterparts. Additionally, the correlation between MHR and the risk of prediabetes was found to be non-linear, with a turning point of −0.4 (Log-Likelihood Ratio, P< 0.001). The impact of variables on the two sides of the turning point were 1.94 (1.72, 2.19) and 0.88 (0.69, 1.14).
Conclusion
The positive correlation between MHR and the risk of prediabetes in Chinese participants was observed to be non-linear, and MHR ≤ −0.4 was strongly positively correlated with prediabetes risk.
Introduction
Prediabetes refers to a stage where an individual’s blood glucose levels are higher than the normal reference range, but have not yet reached the diagnostic criteria for diabetes.Citation1 According to the 2015–2017 epidemiological survey on diabetes in adults in China, the prevalence of prediabetes among Chinese adults was approximately 35.2% (95% CI, 33.5% to 37.0%).Citation2 As an important risk factor of developing diabetes, the annual conversion rate from prediabetes to diabetes was estimated to be around 5–10%,Citation3 although some patients with prediabetes remained stable or converted back to normal.Citation4 Significantly, individuals with prediabetes are at a heightened risk of developing numerous complications typically associated with diabetes in the future, including cardiovascular and diabetic kidney disease, diabetic retinopathy, and neuropathy.Citation5–8 The swiftly escalating global prevalence of prediabetes, coupled with its associated complications, positions blood glucose disorders as a significant public health issue. Thus, it becomes crucial to pinpoint early interventions that could prevent the progression of prediabetes to diabetes and its subsequent complications.
Emerging evidence has suggested that inflammatory responses have a vital part in the pathogenesis of glucose metabolism disorders.Citation9 Prediabetes is a condition that characterized by high inflammatory burden.Citation10 Several inflammatory factors have been found to be connected with the risks of prediabetes, such as neutrophil-to-lymphocyte ratio(NLR),Citation11 white blood cell (WBC),Citation12 C-reactive protein (CRP),Citation13 NLRP3 inflammasome,Citation14 various interleukins (IL-1β, IL-6, TNF-α, and IL-18),Citation15–17 and an increase in number and activation of monocytes.Citation15,Citation18 Similarly, high density lipoprotein (HDL) based inflammation markers are associated with inflammatory diseases such as hypertension,Citation19 hepatosteatosis,Citation20 diabetes mellitus type 2 (T2DM),Citation21 thyroiditis,Citation22 metabolic syndrome,Citation23 diabetic kidney disease,Citation24 and prediabetes.Citation25,Citation26 Furthermore, MHR is also linked with inflammatory conditions.Citation27 Given these results, it would be reasonable to study MHR in prediabetes. However, the evidence regarding the role of MHR in the progression of prediabetes is unclear. To address this knowledge gap, we conducted a multi-centered, cross-sectional study to explore the association between MHR and prediabetes.
Materials and Methods
Study Population
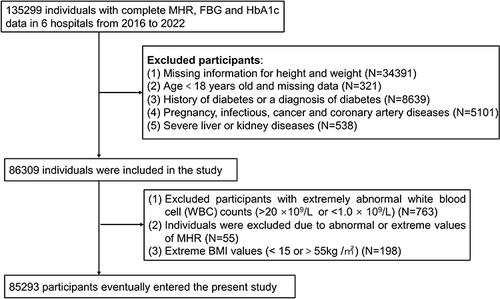
The investigation was a national population-based, multi-centered and cross-sectional observational study, including the routine health checkup data of 3,652,054 participants, involving 49 hospitals from 10 provinces, covering widely seven geographic regions (North, North-East, Central, South, East, North-West, and South-West regions) in China. The data are available from 6 hospitals. A total of 135,299 study participants with measurements of MHR, Hemoglobin A1c (HbA1c) and fasting plasma glucose (FPG) were initially included in the survey. The following exclusion criteria were applied: 1) missing information for height and weight; 2) age < 18 years old or missing data; 3) history of diabetes or a diagnosis of diabetes; 4) pregnancy, infectious disease, cancer, or coronary artery disease; 5) severe liver or kidney diseases; 6) extremely abnormal WBC (>20×10^9/L or <1.0×10^9/L); 7) abnormal or extreme values of MHR; 8) extreme body mass index (BMI) values (<15 or >55 kg/m2). Finally, 85,293 subjects were included in the analysis. ().
The Ethics Committee of Shandong Provincial Hospital granted ethical approval for this study, and all procedures were carried out according to the guidelines specified in the Declaration of Helsinki. In this research, participants were not required to provide written informed consent. Regarding the application for the waiver of informed consent, we have received approval from the Ethics Committee for Biomedical Research Involving Humans at Shandong Provincial Hospital (SWYX: NO. 2022–028). As the study was a retrospective analysis based on previously collected data, the need for formal patient consent was avoided. Throughout the study, it was particularly important that no areas related to patient privacy were violated or interfered with. The practice of this study strictly adheres to the ethical guidelines and management regulations related to the retrospective study. We have taken great care to ensure that the data we use is maintained to the maximum extent possible in terms of patient privacy and confidentiality.
Variables
The MHR can be assessed by dividing the monocyte count by the level of HDL. Based on the diagnostic criteria for prediabetes established by the Expert Consensus on Intervention for Prediabetes in Chinese Adults (2023 edition) according to the World Health Organization (WHO) in 1999 and the American Diabetes Association (ADA) 2022 guidelines,Citation3,Citation28 the diagnostic criteria for prediabetes in Chinese adults are as follows: a fasting blood glucose level between 6.1mmol/L and <7.0mmol/L, or a glycated hemoglobin (HbA1c) level between 5.7% and 6.4%. If an individual meets either of these criteria, they can be diagnosed with prediabetes. However, if the fasting blood glucose level is <6.1mmol/L and the HbA1c level is <5.7%, it indicates a normal blood glucose (NG) level. The covariates included age, gender, BMI, systolic blood pressure (SBP), diastolic blood pressure (DBP), total cholesterol (TC), triglycerides (TG), low-density lipid cholesterol (LDL-C), serum creatinine (Scr), uric acid (UA), FPG and WBC. The data were collected through a standardized process.
Statistical Analysis
Based on whether the data follows a normal distribution, continuous variables are shown as mean ± SD or median (interquartile range). Categorical variables are presented as frequencies and percentages. The MHR was a skewed distribution, when the MHR was log10-transformed, a normality analysis was conducted in the study. For comparisons between groups, Student’s t-test or Mann–Whitney U-test for continuous variables, the chi-square tests for categorical variables. The relationship between MHR and prediabetes was assessed via multivariate logistic regression to calculate OR and 95% CI. Model 1 was unadjusted data. In Model 2, the data were adjusted for age, gender, BMI. In Model 3, the results were adjusted for age, gender, BMI, SBP, DBP, Scr, UA, TG, TC, LDL-C, FPG, WBC.
We performed subgroup analyses using stratified factors, including age (<45, 45–60, >60 years), gender, BMI (18.5–23.9, 24–27.9, >28kg/m²), SBP (<140, ≥140 mmHg), DBP (<90, ≥90 mmHg), TG (<1.7, ≥ 1.7 mmol/L), TC (<5.2, ≥5.2mmol/L), LDL-C (<3.4, ≥3.4 mmol/L). The interaction test in the logistic regression model was employed to compare the OR among the various examined subgroups. A generalized additive model (GAM) with penalized smoothing splines was leveraged to investigate the nonlinear association between MHR and prediabetes. To dissect the threshold effect, we used the piecewise regression model and the logarithmic likelihood ratio test with the goal of identifying the inflection points on the curve and analyzing the effect in a piecewise manner. Subgroup analyses also employed a GAM.
Statistical analyses were performed using R software (version 4.2.3, http://www.R-project.org/) and SPSS (v.25.0; IBM Corporation, Armonk, New York, USA). Two-tailed P value below 0.05 was considered statistically significant.
Results
Sample Characteristics
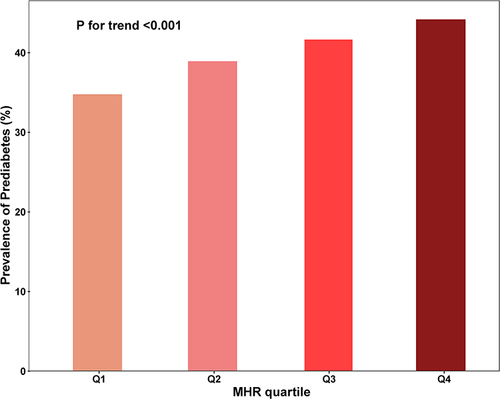
The clinical attributes of 85,293 participants were classified based on the presence or absence of prediabetes. The mean age was 50.29 ± 13.24 years and 63.88% were male. 33,992 participants (39.85%) were diagnosed with prediabetes. Compared with NG, patients with prediabetes tended to be older, female, higher levels of BMI, SBP, DBP, HbA1c, FPG, Scr, ALT, TG, UA, WBC and MHR. (). Subjects were also divided into four groups based on MHR quartiles. Compared with the low MHR group, the high MHR group were older and had higher levels of BMI, SBP, DBP, HbA1c, FPG, Scr, ALT, TG, UA, WBC, and monocytes, but had lower levels of TC, LDL-C, HDL-C (Supplementary Table 1). Furthermore, the prevalence of prediabetes was higher with increasing MHR quartile ().
Table 1 Baseline Characteristics of Study Participants
Relationship Between MHR and Prediabetes
showed the results of the multivariable regression analysis between MHR and prediabetes. This relationship was expressed to be significant in both the non-adjusted model (OR= 2.19; 95% CI), 2.04–2.31; P < 0.001) and the minimally-adjusted model (OR = 1.72; 95% CI, 1.59–1.87; P < 0.001). A positive correlation between MHR and prediabetes was still evident in the fully controlled model (OR = 1.64; 95% CI, 1.48–1.82, P < 0.001). This means that a one-unit increase in MHR corresponded to a 64% increase in prediabetes prevalence. Moreover, the trend remained statistically significant when MHR was converted into a categorical variable (quartiles). Compared with Q1 in the unadjusted model, the MHR in Q4 was connected with an increased risk of prediabetes (crude OR, 1.49; 95% CI, 1.43–1.55, P < 0.001). After accounting for confounders, the association has been impaired but still remains significant (adjusted OR=1.29; 95% CI, 1.23–1.35, P < 0.001) (adjusted OR=1.22; 95% CI, 1.15–1.29, P < 0.001).
Table 2 The Association Between MHR and Prediabetes
Subgroup Analyses
The subgroup analysis identified variations in the association between MHR and the risk of prediabetes across diverse stratified factors. A slightly stronger association was observed in individuals with lower age, SBP, DBP, TG, TC and higher levels of BMI and LDL-C than in their counterparts. On the other hand, a weaker correlation was observed in participants with higher levels of age, SBP, DBP, TG, TC and lower levels of BMI and LDL-C. Interaction tests were used to evaluate how age, BMI, SBP, DBP, TG, TC, and LDL influenced the positive relationship between MHR and prediabetes. The results showed that these variables produced statistically significant interactions (All P for interaction < 0.05) ().
Table 3 Subgroup Analysis for the Association Between MHR and Prediabetes
The Non-Linear Association Between MHR and Prediabetes
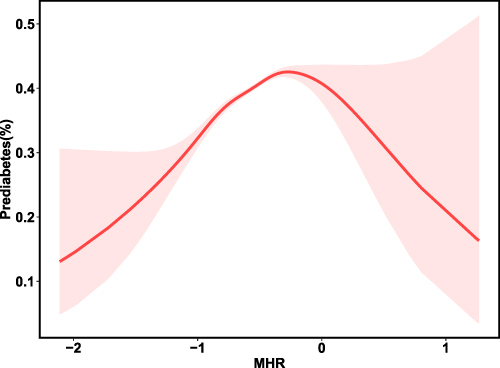
We employed GAM with smoothing splines to further analyze the correlation between MHR and prediabetes risk. After adjusting for several confounding factors (age, gender, BMI, SBP, DBP, Scr, UA, TG, TC, LDL-C, FPG, WBC), we discovered a significant nonlinear connection between MHR and prediabetes (), with an inverted U-shaped curve. Simultaneously, we identified the inflection point of MHR was −0.4 by two-piecewise linear regression model (P < 0.001). When the turning point was ≤ −0.4, MHR had a positive connection with prediabetes risk (OR=1.94; 95% CI, 1.72–2.19, P < 0.001). However, it is important to note that when the turning point was above −0.4, the correlation between MHR and prediabetes did not demonstrate statistical significance (OR = 0.88; 95% CI 0.69–1.14; P = 0.348), as evidenced by the data presented in . Besides, in the subgroup analysis stratified by age, gender, BMI, SBP, DBP, TG, TC, and LDL, the non-linear relationships between MHR and the risk of prediabetes still existed (Supplementary Figure 1).
Table 4 Threshold Effect Analysis of MHR on Prediabetes Using a Two-Piecewise Linear Regression Model
Figure 3 Association between MHR and the prevalence of prediabetes.
Discussion
For the first time, this cross-sectional study demonstrated that a higher value of MHR was connected with the escalated risk of prediabetes in a Chinese sample. Besides, the results of the subgroup analyses and interaction testing indicated that lower levels of age, SBP, DBP, TG, TC and higher levels of BMI and LDL-C strengthened MHR-associated prediabetic risks. An inverted U-shape relationship between MHR and prediabetes was also discovered, with an inflection point of −0.4. When MHR ≤ −0.40, MHR is an independent risk factor for prediabetes.
The pathological characteristics of prediabetes, particularly insulin resistance (IR) and β-cell dysfunction, make it a crucial period for early intervention and prevention.Citation29,Citation30 Previous evidence suggested that inflammation contributed to the development of IR and impaired pancreatic β-cell function.Citation31–33 Proteomics indicated that the changes in pancreatic function during prediabetes was largely associated with the activation of immune cells from congenital and acquired immunity.Citation34 Long-term chronic inflammation associated with glucose and lipid metabolism can recruit monocytes in the circulation to migrate to tissues and organs which regulate insulin sensitivity, and then differentiate into tissue-resident macrophages to exacerbate inflammatory responses and tissue function damage.Citation35 Consequently, circulating monocytes may be important indicators for reflecting tissue-related inflammation activation.
As the MHR rose, there was a corresponding increase in risks for metabolic syndrome, diabetes mellitus, coronary artery disease, and nonalcoholic fatty liver disease.Citation36–40 Recent observational studies suggested that the MHR as an independent factor could predict long-term prognosis in Takayasu arteritis.Citation41 In addition, MHR has been indicated to have greater diagnostic significance in conditions such as tuberculosis and osteoarthritis.Citation42,Citation43 In the prediabetic stage, an increase in monocytes count and pro-inflammatory subtypes has been observed.Citation18 In a 5-year cohort study, it was found that the dysregulation of circulating immune cells and inflammatory factors was associated with dynamic disruptions in glucose metabolism. In particular, innate immune cells, such as granulocytes and monocytes, exhibited a significant and progressively increasing risk for prediabetes and diabetes.Citation15 It has been observed that there was an increase in pro-inflammatory CD14 and CD18 monocytes, as well as recruiting factors and inflammatory cytokines such as CRP and TNF-α in prediabetes and diabetes.Citation44 When HDL-C levels was low, there was an accumulation of cholesterol within monocytes, leading to enhanced activation of signaling pathways and resulting in a more severe inflammatory response produced by monocytes.Citation45–47 A large prospective cohort study in Tangshan, North China, has shown that metabolic inflammation evaluated by the Cumulative MHR, was a valuable complement to hsCRP for a more thorough evaluation of inflammatory risk in the development of T2DM.Citation38 Simultaneously, interventions targeting inflammation have demonstrated efficacy in improving IR and potentially reversing prediabetes. Several studies suggested that interventions like exercise, weight loss, dietary modifications, and medication interventions can effectively reduce inflammation and immune responses in individuals with prediabetes.Citation48–51 Therefore, MHR might contribute to the progression of prediabetes and its conversion to T2DM.
In the present study, we found that each unit in the MHR increased the risk of prediabetes by 64% (OR: 1.64 95% CI 1.48–1.82, P<0.001), after adjusting for age, gender, BMI, SBP, DBP, Scr, UA, TG, TC, LDL-C, FPG, WBC. It was the first study to find a correlation between MHR and prediabetes, which may provide valuable insight into the primary prevention of prediabetes.
Additionally, in subgroup analyses, a more robust correlation was noticed in individuals with lower age, SBP, DBP, TG and TC, and higher values of BMI and LDL-C than in their counterparts. These variables may change the association between MHR and prediabetes. Clinically, by intervening with the levels of SBP, DBP, BMI, TG, TC, and LDL-C, it’s feasible to lower the risk of prediabetes by changing the degree of the correlation between MHR and prediabetes.
Moreover, we observed a nonlinear relationship between MHR and the risk of prediabetes. The turning point was −0.4 after controlling for confounding variables (age, gender, BMI, SBP, DBP, Scr, UA, TG, TC, LDL-C, FPG, WBC). When the MHR was ≤ −0.4, a 1-unit increase in MHR levels was correlated to a 94% increase in adjusted OR of prediabetes risk (OR: 1.94, 95% CI: 1.72–2.19, P < 0.001). However, when MHR was > −0.4, there was no correlation with prediabetes risk (OR = 0.88, 95% CI 0.69–1.14; P = 0.348). The reason may be that variables other than MHR also affected prediabetes. In comparison with the MHR ≤ −0.4 group, participants in the MHR > −0.4 group displayed higher average levels of age, BMI, SBP, DBP, Scr, TG, LDL-C, UA, WBC and a greater percentage of males. Nevertheless, the noted indicators were strongly associated with prediabetes. When MHR was > −0.4, owing to the existence of these prediabetes risk factors, MHR had a mild impact on prediabetes risk. Conversely, when MHR was below −0.4, the lower levels of related risk factors such as age, BMI, SBP, DBP, Scr, TG, LDL-C, UA, and WBC were found in prediabetes, and the influence on prediabetes was lessened, during which the relative impact of MHR was seen to be strengthened (Supplementary Table 2).
In our study, we discerned a non-linear relationship and found a critical juncture between MHR and the risk of prediabetes. Notably, in individuals with a lesser metabolic risk profile, we observed a substantial relationship between MHR and prediabetes under mildly inflammatory conditions. Despite the high prevalence of prediabetes, it often remains undiagnosed in clinical practice. The practical application of MHR coupled with identifying the pivotal point, could prove to be invaluable tools for both prevention and early detection of prediabetes. Furthermore, our study provides compelling evidence for interventions aimed at addressing inflammation.
Prediabetes is a condition characterized by marked by heightened systemic inflammation.Citation10 This condition’s link to the MHR, a key marker of inflammation, is therefore of notable interest. Monocytes can migrate to sites of inflammation or infection, which amplifies the inflammatory response, leading to tissue damage and exacerbated insulin resistance, a cardinal trait of prediabetes.Citation15,Citation18,Citation32 On the other hand, HDL-C serves a crucial anti-inflammatory role.Citation52 A decline in HDL-C concentrations heightens the inflammatory status within the body. In essence, the association between prediabetes and MHR likely stems from inflammation, which reinforces the potential value of MHR in prediabetes, particularly in assessing the risks associated with inflammation.
Our research possesses several strengths. Firstly, we used a relatively large sample size and included individuals from different centers. Secondly, through the application of GAM and smoothing splines, our study primarily unveiled a significant non-linear relationship between MHR and the risk of prediabetes. Thirdly, we executed meticulous statistical adjustments to reduce the impact of potential confounders. Moreover, subgroup analyses were carried out to identify potential confounding variables which could change the relationship between MHR and prediabetes.
Our study has several limitations. Firstly, the absence of oral glucose tolerance tests (OGTTs) may lead to an underestimation of prediabetic cases. Secondly, given the cross-sectional design of the study, it was not feasible to determine a causal relationship between the variables studied. In the future, prospective research will be necessary to validate the potential causality. Thirdly, despite controlling for confounding factors such as SBP, DBP, BMI, TC, LDL-C, there could still be confounding factors that are not controlled or measured, including dietary habits, exercise, smoking and alcohol intake. These factors may influence the observed associations and should be considered in future research.
Conclusions
There was a threshold effect between MHR and prediabetes. When MHR was < −0.4, there was a substantial and positive correlation observed in relation to the risk of prediabetes. This study for the first time provided epidemiological insights into the association between MHR and prediabetes. Particularly in participants at low risk of prediabetes, targeted assessment and management of MHR can significantly decrease the risk of prediabetes when MHR is below the inflection point.
Ethics Statement
The Ethics Committee of Shandong Provincial Hospital granted ethical approval for this study, and all procedures were carried out according to the guidelines specified in the Declaration of Helsinki. In this research, participants were not required to provide written informed consent.
Disclosure
The authors declare no competing interests in this work.
Acknowledgments
The authors desired to thank all the participants and investigators who were involved in this study.
Additional information
Funding
References
- Echouffo-Tcheugui JB, Perreault L, Ji L, Dagogo-Jack S. Diagnosis and Management of Prediabetes: a Review. JAMA. 2023;329(14):1206–1216. doi:10.1001/jama.2023.4063
- Li Y, Teng D, Shi X, et al. Prevalence of diabetes recorded in mainland China using 2018 diagnostic criteria from the American Diabetes Association: national cross sectional study. BMJ. 2020;369:m997. doi:10.1136/bmj.m997
- Tabák AG, Herder C, Rathmann W, Brunner EJ, Kivimäki M. Prediabetes: a high-risk state for diabetes development. Lancet. 2012;379(9833):2279–2290. doi:10.1016/S0140-6736(12)60283-9
- Perreault L, Kahn SE, Christophi CA, Knowler WC, Hamman RF. Regression from pre-diabetes to normal glucose regulation in the diabetes prevention program. Diabetes Care. 2009;32(9):1583–1588. doi:10.2337/dc09-0523
- Chen C, Liu G, Yu X, Yu Y, Liu G. Association between prediabetes and renal dysfunction from a community-based prospective study. Int J Med Sci. 2020;17(11):1515–1521. doi:10.7150/ijms.46477
- Cai X, Zhang Y, Li M, et al. Association between prediabetes and risk of all cause mortality and cardiovascular disease: updated meta-analysis. BMJ. 2020;370:m2297.
- Zheng Y, Ley SH, Hu FB. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat Rev Endocrinol. 2018;14(2):88–98. doi:10.1038/nrendo.2017.151
- Melsom T, Schei J, Stefansson VT, et al. Prediabetes and risk of glomerular hyperfiltration and albuminuria in the general nondiabetic population: a prospective cohort study. Am J Kidney Dis. 2016;67(6):841–850. doi:10.1053/j.ajkd.2015.10.025
- Barzilay JI, Abraham L, Heckbert SR, et al. The relation of markers of inflammation to the development of glucose disorders in the elderly: the Cardiovascular Health Study. Diabetes. 2001;50(10):2384–2389. doi:10.2337/diabetes.50.10.2384
- Cheng ZJ, Wang YF, Jiang XY, et al. High sensitivity C-reactive protein and prediabetes progression and regression in middle-aged and older adults: a prospective cohort study. J Diabetes Invest. 2024;15(1):78–86. doi:10.1111/jdi.14090
- Mertoglu C, Gunay M. Neutrophil-Lymphocyte ratio and Platelet-Lymphocyte ratio as useful predictive markers of prediabetes and diabetes mellitus. Diabetes Metab Syndr. 2017;11(Suppl 1):S127–S131.
- Zang X, Meng X, Wang Y, et al. Six-year follow-up study on the association between white blood cell count and fasting blood glucose level in Chinese adults: a community-based health examination survey. Diabetes/Metab Res Rev. 2019;35(4):e3125. doi:10.1002/dmrr.3125
- Brahimaj A, Ligthart S, Ghanbari M, et al. Novel inflammatory markers for incident pre-diabetes and type 2 diabetes: the Rotterdam Study. Eur J Epidemiol. 2017;32(3):217–226. doi:10.1007/s10654-017-0236-0
- Alfadul H, Sabico S, Ansari MGA, et al. Differences and associations of NLRP3 inflammasome levels with interleukins 1α, 1β, 33 and 37 in adults with prediabetes and type 2 diabetes mellitus. Biomedicines. 2023;11(5):1315. doi:10.3390/biomedicines11051315
- Grossmann V, Schmitt VH, Zeller T, et al. Profile of the immune and inflammatory response in individuals with prediabetes and type 2 diabetes. Diabetes Care. 2015;38(7):1356–1364. doi:10.2337/dc14-3008
- AlAfaleq NO, Hussein TM, Al-Shouli ST, et al. Proinflammatory cytokine profiles in prediabetic Saudi patients. Saudi J Biol Sci. 2023;30(8):103714. doi:10.1016/j.sjbs.2023.103714
- Huang K, Liang Y, Ma Y, Wu J, Luo H, Yi B. The variation and correlation of serum adiponectin, nesfatin-1, IL-6, and TNF-α levels in prediabetes. Front Endocrinol. 2022;13:774272. doi:10.3389/fendo.2022.774272
- Fadini GP, Cappellari R, Mazzucato M, Agostini C, Vigili de Kreutzenberg S, Avogaro A. Monocyte-macrophage polarization balance in pre-diabetic individuals. Acta diabetologica. 2013;50(6):977–982. doi:10.1007/s00592-013-0517-3
- Aktas G, Khalid A, Kurtkulagi O, et al. Poorly controlled hypertension is associated with elevated serum uric acid to HDL-cholesterol ratio: a cross-sectional cohort study. Postgraduate Med. 2022;134(3):297–302. doi:10.1080/00325481.2022.2039007
- Kosekli MA, Kurtkulagii O, Kahveci G, et al. The association between serum uric acid to high density lipoprotein-cholesterol ratio and non-alcoholic fatty liver disease: the abund study. Revista da Associacao Medica Brasileira. 2021;67(4):549–554. doi:10.1590/1806-9282.20201005
- Aktas G, Kocak MZ, Bilgin S, Atak BM, Duman TT, Kurtkulagi O. Uric acid to HDL cholesterol ratio is a strong predictor of diabetic control in men with type 2 diabetes mellitus. Aging Male. 2020;23(5):1098–1102. doi:10.1080/13685538.2019.1678126
- Kurtkulagi O, Tel BMA, Kahveci G, et al. Hashimoto’s thyroiditis is associated with elevated serum uric acid to high density lipoprotein-cholesterol ratio. Roman J Internal Med. 2021;59(4):403–408. doi:10.2478/rjim-2021-0023
- Kocak MZ, Aktas G, Erkus E, Sincer I, Atak B, Duman T. Serum uric acid to HDL-cholesterol ratio is a strong predictor of metabolic syndrome in type 2 diabetes mellitus. Revista da Associacao Medica Brasileira. 2019;65(1):9–15. doi:10.1590/1806-9282.65.1.9
- Aktas G, Yilmaz S, Kantarci DB, et al. Is serum uric acid-to-HDL cholesterol ratio elevation associated with diabetic kidney injury? Postgraduate Med. 2023;135(5):519–523. doi:10.1080/00325481.2023.2214058
- Li M, Zhang W, Zhang M, et al. Nonlinear relationship between untraditional lipid parameters and the risk of prediabetes: a large retrospective study based on Chinese adults. Cardiovasc Diabetol. 2024;23(1):12. doi:10.1186/s12933-023-02103-z
- Mo Z, Hu H, Han Y, Cao C, Zheng X. Association between high-density lipoprotein cholesterol and reversion to normoglycemia from prediabetes: an analysis based on data from a retrospective cohort study. Sci Rep. 2024;14(1):35. doi:10.1038/s41598-023-50539-w
- Romo-Cordero A, González-Sierra M, Quevedo-Abeledo JC, et al. The ratio of monocytes to HDL-cholesterol is associated with cardiovascular risk and insulin resistance in patients with rheumatoid arthritis. Life. 2023;13(10). doi:10.3390/life13101995
- Diabetes Care. 2. Classification and diagnosis of diabetes: standards of medical care in diabetes-2022. Diabetes Care. 2022;45(Suppl 1):S17–S38. doi:10.2337/dc22-S002
- Abdul-Ghani MA, Tripathy D, DeFronzo RA. Contributions of beta-cell dysfunction and insulin resistance to the pathogenesis of impaired glucose tolerance and impaired fasting glucose. Diabetes Care. 2006;29(5):1130–1139. doi:10.2337/dc05-2179
- Magkos F, Lee MH, Lim M, et al. Dynamic assessment of insulin secretion and insulin resistance in Asians with prediabetes. Metabolism. 2022;128:154957. doi:10.1016/j.metabol.2021.154957
- Festa A, Hanley AJ, Tracy RP, D’Agostino R, Haffner SM. Inflammation in the prediabetic state is related to increased insulin resistance rather than decreased insulin secretion. Circulation. 2003;108(15):1822–1830. doi:10.1161/01.CIR.0000091339.70120.53
- Weaver JR, Odanga JJ, Breathwaite EK, et al. An increase in inflammation and islet dysfunction is a feature of prediabetes. Diabetes/Metab Res Rev. 2021;37(6):e3405. doi:10.1002/dmrr.3405
- Weber KS, Nowotny B, Strassburger K, et al. The role of markers of low-grade inflammation for the early time course of glycemic control, glucose disappearance rate, and β-cell function in recently diagnosed type 1 and type 2 diabetes. Diabetes Care. 2015;38(9):1758–1767. doi:10.2337/dc15-0169
- Diamanti K, Cavalli M, Pereira MJ, et al. Organ-specific metabolic pathways distinguish prediabetes, type 2 diabetes, and normal tissues. Cell Rep Med. 2022;3(10):100763. doi:10.1016/j.xcrm.2022.100763
- Park MD, Silvin A, Ginhoux F, Merad M. Macrophages in health and disease. Cell. 2022;185(23):4259–4279. doi:10.1016/j.cell.2022.10.007
- Wang P, Guo X, Zhou Y, et al. Monocyte-to-high-density lipoprotein ratio and systemic inflammation response index are associated with the risk of metabolic disorders and cardiovascular diseases in general rural population. Front Endocrinol. 2022;13:944991. doi:10.3389/fendo.2022.944991
- Wang W, Chen ZY, Guo XL, Tu M. Monocyte to high-density lipoprotein and apolipoprotein A1 ratios: novel indicators for metabolic syndrome in Chinese newly diagnosed type 2 diabetes. Front Endocrinol. 2022;13:935776. doi:10.3389/fendo.2022.935776
- Wu D, Lan Y, Xu Y, et al. Association of cumulative monocyte to high-density lipoprotein ratio with the risk of type 2 diabetes: a prospective cohort study. Cardiovasc Diabetol. 2022;21(1):268. doi:10.1186/s12933-022-01701-7
- Zhao Y, Xia J, He H, Liang S, Zhang H, Gan W. Diagnostic performance of novel inflammatory biomarkers based on ratios of laboratory indicators for nonalcoholic fatty liver disease. Front Endocrinol. 2022;13:981196. doi:10.3389/fendo.2022.981196
- Chen J, Wu K, Cao W, Shao J, Huang M. Association between monocyte to high-density lipoprotein cholesterol ratio and multi-vessel coronary artery disease: a cross-sectional study. Lipids Health Dis. 2023;22(1):121. doi:10.1186/s12944-023-01897-x
- Ci W, Wan J, Han J, et al. Monocyte-to-high-density lipoprotein ratio as a predictor for patients with Takayasu arteritis and coronary involvement: a double-center, observational study. Front Immunol. 2023;14:1120245. doi:10.3389/fimmu.2023.1120245
- Yu Q, Yan J, Tian S, et al. A scoring system developed from a nomogram to differentiate active pulmonary tuberculosis from inactive pulmonary tuberculosis. Front Cell Infect Microbiol. 2022;12:947954. doi:10.3389/fcimb.2022.947954
- Cao J, Hua L, Dong L, Wu Z, Xue G. The value of the monocyte to high-density lipoprotein cholesterol ratio in assessing the severity of knee osteoarthritis: a retrospective single center cohort study. J Inflamm Res. 2023;16:595–604. doi:10.2147/JIR.S395229
- Fogelstrand L, Hulthe J, Hultén LM, Wiklund O, Fagerberg B. Monocytic expression of CD14 and CD18, circulating adhesion molecules and inflammatory markers in women with diabetes mellitus and impaired glucose tolerance. Diabetologia. 2004;47(11):1948–1952. doi:10.1007/s00125-004-1553-x
- Yvan-Charvet L, Pagler T, Gautier EL, et al. ATP-binding cassette transporters and HDL suppress hematopoietic stem cell proliferation. Science. 2010;328(5986):1689–1693. doi:10.1126/science.1189731
- Natali A, Baldi S, Bonnet F, et al. Plasma HDL-cholesterol and triglycerides, but not LDL-cholesterol, are associated with insulin secretion in non-diabetic subjects. Metabolism. 2017;69:33–42. doi:10.1016/j.metabol.2017.01.001
- Harsløf M, Pedersen KM, Nordestgaard BG, Afzal S. Low high-density lipoprotein cholesterol and high white blood cell counts: a Mendelian randomization study. Arterioscler Thromb Vasc Biol. 2021;41(2):976–987. doi:10.1161/ATVBAHA.120.314983
- Zhang T, Tian J, Fan J, Liu X, Wang R. Exercise training-attenuated insulin resistance and liver injury in elderly pre-diabetic patients correlates with NLRP3 inflammasome. Front Immunol. 2023;14:1082050. doi:10.3389/fimmu.2023.1082050
- Viardot A, Lord RV, Samaras K. The effects of weight loss and gastric banding on the innate and adaptive immune system in type 2 diabetes and prediabetes. J Clin Endocrinol Metab. 2010;95(6):2845–2850. doi:10.1210/jc.2009-2371
- Luotola K. IL-1 receptor antagonist (IL-1Ra) levels and management of metabolic disorders. Nutrients. 2022;14(16):3422. doi:10.3390/nu14163422
- Ren X, Sun Y, Guo Q, et al. Ameliorating effect of the total flavonoids of Morus nigra L. on prediabetic mice based on regulation of inflammation and insulin sensitization. J Agric Food Chem. 2022;70(39):12484–12501. doi:10.1021/acs.jafc.2c04970
- Denimal D. Antioxidant and anti-inflammatory functions of high-density lipoprotein in type 1 and type 2 diabetes. Antioxidants. 2023;13(1):57. doi:10.3390/antiox13010057