Abstract
Purpose
The study aimed to investigate the efficacy and safety of SophorOx® (LN-OS-22) on oxidative stress and body composition in adults with excessive body weight and obesity.
Participants and Methods
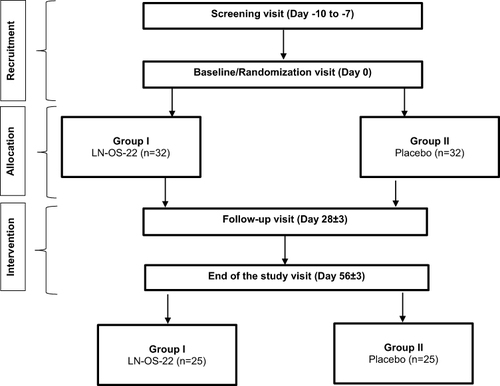
The 56-days randomized, double-blind, placebo-controlled, parallel-group, multi-centric clinical trial had individuals aged 30–60 years with body mass index (BMI) ≥25 to ≤34.9 kg/m2. 68 participants were randomly allocated to LN-OS-22 or placebo groups. The primary outcome was improvement in the oxidative stress. Secondary outcomes were changes in plasma lipopolysaccharide (LPS) and serum malondialdehyde (MDA) levels, weight and waist circumference, inflammatory markers, and quality of life.
Results
At day 56, a statistically significant change in the 8-Isoprostane levels between LN-OS-22 vs placebo was observed (p = 0.0222). As compared to placebo, at the end of study, statistically significant reductions were demonstrated in body weight, waist circumference and BMI in the LN-OS-22 group (p < 0.0001). Also, a statistically significant change when compared to placebo for the energy/stamina domain (p = 0.0300) of the Impact of Weight on Quality of Life-Lite-Clinical Trials Version (IWQOL-Lite-CT) questionnaire was depicted in LN-OS-22 group.
Conclusion
The study demonstrates that LN-OS-22 was effective in reducing the oxidative stress, anthropometrics and improving the quality of life in individuals with overweight and obesity.
Introduction
In a healthy individual, the balance between pro-oxidant generation such as Reactive Oxygen Species (ROS) and Reactive Nitrogen Species (RNS) and antioxidant defense is autonomic in nature. Exposure to toxic xenobiotics coupled with the sedentary lifestyle, results in an imbalance between the pro-oxidants and antioxidants, leading to oxidative stress. Pro-oxidant antioxidant balance in the cell is shifted towards the pro-oxidants if the production of oxygen species is increased greatly or when levels of antioxidants are diminished. This state is called oxidative stress.Citation1 This oxidative stress is known to be one of the primary mechanism through which these lifestyle changes adversely affect individual health leading to a disruption of redox signaling and control and/or molecular damage.Citation2–5 As demonstrated by several studies, overproduction of ROS can lead to damage of cellular molecules gradually resulting into white adipose tissue accumulation and release of several pro-inflammatory cytokines.Citation6,Citation7 Oxidative stress is known to trigger the deposition of white adipose tissue by increasing preadipocyte proliferation, adipocyte differentiation, and the size of mature adipocytes.Citation8 Other contributing factors of oxidative stress are low antioxidant defense,Citation9 chronic inflammation,Citation10 and postprandial ROS generation.Citation11 Therefore, obesity served as an ideal condition to investigate the antioxidant activity of LN-OS-22 extract.
Several dietary practices have been shown to alleviate oxidative stress thereby minimizing the health associated hazards.Citation12 Sophora japonica L (Styphnolobium japonicum) has a rich history of use in traditional Chinese medicine. A wide range of biological activities of Sophora japonica L in vitro and in vivo have been reported such as anti-inflammatory, antibacterial, antiviral, anti-osteoporotic, antioxidant, radical scavenging, anti-hyperglycemic, anti-obesity, antitumor, and hemostatic effects.Citation13,Citation14 Sophora Japonica L is one of the richest sources of rutin and quercetin flavonoids. Both of these flavonoids have been studied extensively for exploiting their use in improving human health.Citation15–20 Layn Natural Ingredients adopted Sophora japonica L under the “Adopt-an-Herb” initiative of the American Botanical Council and derived LN-OS-22. It was extracted from the flower bud of the plant, underwent ethanol/water extraction, and has been standardized to contain ≥90% flavonoids, primarily, quercetin, a rutin and other minor flavonoids. This extract has been tested in vitro in LPS-stimulated RAW 264.7 macrophages for its anti-inflammatory and antioxidant activity. The extract showed significant inhibition of pathways of inflammation and oxidative stress.Citation21 The biological activities were then confirmed by an in vivo study. Oral administration of this extract in exercised animals resulted in a significant decrease in oxidative stress and inflammatory biomarkers associated with the high-intensity exercise in a rodent model (Sprague-Dawley rat model).Citation22 The validation in humans of these in vitro and in vivo antioxidant activities was studied in the current randomized clinical trial.
A meta-analysisCitation23 of 50 clinical studies showed that individuals with overweight and obesity have a comparatively increased levels of 8-isoprostane, a reliable and stable biomarker of oxidative stress,Citation24 than healthy population. A reduction in the body weight is known to decrease oxidation markers in the body and thereby reduce obesity-related pathological risk factors.Citation8 Obesity is also known to be associated with chronic low-grade information.Citation25 A significant impact on the health-related quality of life is also seen in individuals with overweight and obesity.Citation26 Thus, the primary objective of the study was to assess the effect of the investigational product (IP), LN-OS-22 on oxidative stress. Body composition (BMI, body weight and waist circumference) were included as secondary objectives as clinical indicators of reduction in oxidative stress and inflammation. Furthermore, lipid peroxidation, metabolic endotoxemia as well as quality of life were also included as secondary objectives. The safety of 2-month administration of the Investigational Product (IP), LN-OS-22, was evaluated by monitoring the vitals as well as liver and kidney function biomarkers [serum glutamic oxaloacetic transaminase (SGOT), serum glutamic pyruvic transaminase (SGPT), alkaline phosphatase (ALP), and creatinine] throughout the study.
Materials and Methods
Study Approval and Registration
This study was conducted in compliance with the Declaration of Helsinki, International Conference on Harmonization (ICH) recommendation on Good Clinical Practice (GCP)-E6 (R2), 2016, and National Ethical Guidelines for Biomedical and Health Research involving Human Participants, 2017, India. The ethical approval for conduct of the study at three sites was provided by the independent ethics committee - Harmony Ethical Research Committee, Mumbai, India (Reg. No. ECR/1411/Inst/MH/2020). The eligible participants were enrolled in the study only after providing written consent for participation. The study was registered with the NIH ClinicalTrials.gov (Identifier: NCT05404217) and Clinical Trials Registry, India (Registration Number: CTRI/2022/06/043394). Vedic Lifesciences, Mumbai, India, monitored and audited the study to ensure the study protocol and ICH-GCP compliance. The study report conformed to the Consolidated Standard Reporting of Trials (CONSORT) guidelines.
Study Design
The current study is a randomized, double-blind, placebo-controlled, parallel-group multi-centric study intended for evaluating the effect of 56-days administration of LN-OS-22 on oxidative stress and body composition in individuals with overweight and obesity. The study was conducted at three clinical sites in Mumbai, Maharashtra, India, under the supervision of qualified physicians from June 2022 to March 2023.
Participants
The present study was conducted among adults aged ≥30 to ≤60 years with a BMI range of ≥25 to ≤34.9 kg/m2. Individuals with at least three of the following five metabolic risk factors were included in the study: 1) waist circumference: male: ≥102 cm; female ≥88 cm, 2) triglycerides >150 mg/dL, 3) systolic blood pressure ≥130 mm H and/or diastolic blood pressure ≥85 mm Hg, 4) fasting blood glucose ≥100 mg/dL, 5) high-density lipoproteins: male: <40 mg/dL; female: <50 mg/dL. Also, individuals who were physically inactive for 1/3rd of their wake time as per the Longitudinal Aging Study Amsterdam (LASA) sedentary behavior questionnaire were included. Individuals meeting the following criteria were excluded from the study: known cases of lactose intolerance, thyroid function test (TSH) level < 0.4 to > 4.2 mIU/L, known cases of type I diabetes mellitus and presence of uncontrolled type II diabetes mellitus [indicated by glycosylated hemoglobin (HbA1c) ≥ 6.5]. The other eligibility criteria are described in Table S1 (Supplementary Material).
The prospective participants fulfilling inclusion and exclusion criteria were randomized in the ratio of 1:1 for LN-OS-22 or placebo by the statistician who was not directly involved in the study. Based on the computer generated randomization chart, the participant identification numbers were generated which were printed on the product labels using a stratified block randomization method with a block of four. The randomization chart was generated by an independent statistician using StatsDirect software (Ver. 3.1.17). The participant, research staff, and Investigator were blinded to the study product allocation.
Intervention
The investigational product (IP), LN-OS-22, is a polyphenolic supplementation comprising of rutin, quercetin, and other minor flavonoids. Previous studies have shown that quercetin up to a dose of 2000 mg and Rutin in a dose of 500 mg when administered for 6–8 weeks is safe in humans.Citation27–30 Thus, in the present study, 500 mg capsule of LN-OS-22 was administered to participants in one of the arms. The detailed percentage composition of the study products is given in . The placebo capsule contained 500 mg of Maltodextrin. All the participants were instructed to take two capsules a day orally with a glass of milk after breakfast daily. At each follow-up visit, the compliance to the intervention was ensured by counting the remaining number of capsules in the bottles. Any concomitant medication taken was recorded in the source document and Electronic-Case Record Form (e-CRF). The participants having at least 90% compliance during the intervention period were considered “completed intervention”.
Table 1 Investigational Product Composition
To preserve the blinding, the study products and the placebo capsules were matched for size, shape, color, and texture and packed in identical packaging. Layn Natural Ingredients, USA supplied the products for the study. The products were manufactured in compliance with good manufacturing practices and applicable regulations.
Study Procedures
During the screening visit, the study procedures were thoroughly explained to the research participants in a language well understood by them, following which he/ she duly signed and dated informed consent. The participants were screened for the eligibility criteria, and those who qualified were instructed to report on day 0 for randomization and baseline assessments. The follow-up assessments were conducted on days 28 and 56, as depicted in (). Demographic and baseline characteristics such as anthropometric parameters (height, weight and BMI), vital signs (pulse rate and blood pressure), clinical examination and medical history with prior and concomitant medication were recorded. Participants were asked to self-administer the LASA questionnaire. Participants were required to have a minimum of 90% Investigational Product (IP) compliance during the study. The detailed method of assessments is explained in the Supplementary Material.
Study Outcomes
Primary Outcome
The primary objective of the study was to assess the effect of 56 days of IP intake on the oxidative stress as compared to placebo. This was assessed by the change in serum levels of 8-isoprostane (8-iso-PGF2α) which is considered a reliable and pivotal biomarker of generalized as well as cellular oxidative stress.Citation31 The 8-iso-PGF2α levels were analyzed using sandwich ELISA technique.
Secondary Outcomes
The secondary objective was to assess the effect of IP on body weight, waist circumference along with the inflammatory (CRP and IL-6) and lipid peroxidation biomarkers (MDA) along with the serum lipopolysaccharide (LPS) levels, a marker for the bacterial pollution. Lastly, effect of IP was assessed on the quality of life using Impact of Weight on Quality of Life-Lite-Clinical Trials Version (IWQOL-Lite-CT). The detailed study flow chart is shown in . The participants were instructed to maintain their routine diet and physical activity levels throughout the study.
Safety Outcomes
The safety of the IP was evaluated by measuring the liver and kidney function panel [serum glutamic-oxaloacetic transaminase (SGOT), serum glutamic-pyruvic transaminase (SGPT), serum alkaline phosphatase (ALP) and serum creatinine]. Blood pressure, pulse rate along with adverse events were monitored throughout the study.
Statistical Analysis
The sample size calculation was based on previous studies conducted to evaluate the efficacy of other products with similar objectives.Citation32–34 The null hypothesis was considered to be rejected in case LN-OS-22 was able to significantly reduce oxidative stress and body composition in a population with overweight and obesity as compared to the placebo. Data was checked for normality using Shapiro Wilk/Kolmogorov–Smirnov test. Mean change of serum levels of 8-isoprostane at 56 days from baseline was compared between the IP and placebo groups using ANCOVA. Mean change in the serum MDA, plasma LPS levels at 56 days from baseline were assessed on similar line of primary endpoints. Change in serum CRP, serum IL-6, weight and waist circumference, and IWQOL-Lite-CT from baseline to Day 28 and Day 56, in the IP and placebo groups was analyzed using multivariate ANCOVA. Data analyses were performed using R/R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/version4.0.5 and XLSTAT statistical and data analysis solution, New York, USA. https://www.xlstat.com./version2021.3.1.
Quality Assurance
Vedic Lifesciences monitored and audited the study to ensure compliance with the study protocol and ICH-GCP E6 (R2) guidelines.
Results
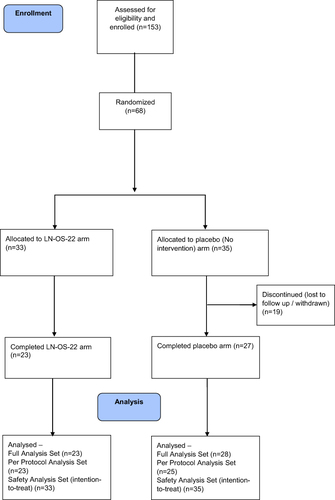
Out of 153 screened individuals, 68 met the predefined inclusion-exclusion criteria and were randomized into the study. Of these, 50 participants completed the study. The detailed participant disposition is presented in .
Demographic and Baseline Characteristics
Eligible individuals were randomized in a 1:1 ratio to receive either LN-OS-22 (n = 33) or placebo (n = 35). The enrolled participants had a mean age of 42 years and a mean BMI of 30.25 kg/m2. The demographic and baseline characteristics of all the randomized participants are summarized in .
Table 2 Demographics and Baseline Characteristics of Randomized Participants
Oxidative Stress
At the baseline, 8-Isoprostane levels matched between the groups (p = 0.1016). At day 28, the serum levels increased in both the LN-OS-22 and the placebo (p = 0.7634) groups. At day 56, the increase in the levels of the 8-isoprostane was significantly higher in the placebo group as compared to the IP (p = 0.0222) and this increase was significantly higher as compared to the baseline (p = 0.0710). Thus, at the end of the study, the LN-OS-22 helped restore the balance between anti- and pro-oxidative levels ().
Table 3 Summary of 8-Isoprostane Levels of the mITT Population
Body Composition
The LN-OS-22 study group demonstrated not only a statistically significant reduction in the body weight and BMI but also showed a clinically meaningful reduction in the waist circumference when compared with the placebo. Eight weeks’ administration of the IP reduced the body weight by 2.07 (1.62) kgs as opposed to the placebo group which showed only a marginal reduction by 0.14 (1.53) kgs (p < 0.0001). The IP group, thus, also showed a statistically significant reduction in the BMI as compared to placebo (p < 0.0001). Similarly, waist circumference reduced by 2.58 (1.69) cm in the LN-OS-22 group as compared to the placebo which showed only a 0.31 (1.71) cm of reduction over 56 days of administration (p < 0.0001). This decrease in waist circumference resulted in the significant reduction in the waist-to-height ratio (p = 0.0005) ().
Table 4 Summary of Anthropometric Measurements of the mITT Population
Inflammatory & Lipid Peroxidation Markers
At baseline, the groups seemed statistically similar for inflammatory and lipid peroxidation markers (p > 0.05). After 8 weeks of study period, the LN-OS-22 group showed lower levels of the CRP as compared to the placebo, however, the levels of CRP increased in both the groups from baseline. Similarly, the IL-6 levels were also lower in the intervention group as compared to the placebo group however the results were not statistically significant () By the end of the study, a reduction in the mean MDA levels was observed in both study groups. The LN-OS-22 showed a greater mean reduction of 5.52 (70.99) EU/mL of LPS levels in comparison to the placebo study group ().
Table 5 Summary of Inflammatory and Lipid Peroxidation Biomarkers of the mITT Population
Quality of Life
On days 28 and 56, the change in the quality of life in the IP group was statistically significant as indicated by p = 0.0029 and p = 0.0004, respectively, when compared to baseline. After 56 days of intervention, LN-OS-22 group demonstrated a statistically significant change when compared to placebo for the energy/stamina domain (p = 0.0300) of the IWQOL-Lite-CT questionnaire. Also, at day 56, when compared to baseline, statistically significant results were demonstrated in the IP group in the physical impact (p = 0.0047), emotions (p = 0.0003), self-confidence/self-esteem (p = 0.0236) domains ().
Table 6 Summary of IWQOL-Lite-CT of the mITT Population
Safety Outcomes
No significant change in safety parameters was observed during the study in the IP group compared to baseline and placebo. Only five adverse events were reported during the study in the IP group – 1) high SGPT and ALP levels, 2) dry cough, 3) high IL-6 levels, 4) high CRP levels, 5) high SGPT and SGOT levels. None of the adverse events were found related to the study products. No serious adverse event was reported during the study (Table S2) (Supplementary Material).
Discussion
Obesity has been purported as a global pandemic in recent times with a well-established relationship with oxidative imbalance. Studies have shown that individuals with higher BMI have increased productions of 8-isoprostane.Citation6,Citation35–37 The levels of 8-isoprostane are known to increase in response to oxidative stress.Citation38 Hence, 8-Isoprostane, which is considered as the most stable biomarker of oxidative stress was assessed in the serum of the participants enrolled in the study.Citation39 In the present study, participants with increased body weight and at a risk of metabolic syndrome showed a trend of increasing oxidative stress over next eight weeks. Dietary polyphenols (or flavonoids) serve as effective scavengers for free radicals and ROS. They possess the capacity to neutralize ROS or inhibit cellular oxidative stress, thereby preventing damage to biomolecules such as lipids, proteins, and DNA. This phenomenon is commonly referred to as the antioxidant effects of dietary polyphenols.Citation40 The IP consistent with this known antioxidant effect of polyphenols, helped to cease the increase of 8-isoprostane, whereas in the placebo group the oxidative stress continued to increase till the end of the study. Oxidative imbalance arises due to a deficiency in the antioxidant defense system (ADS) resulting from impaired production or distribution of antioxidants (AO) or surplus of ROS.Citation41 These results of the present study are similar to the effect of Vitamin E, wherein 6-month supplementation led to the reduction in the 8-Isoprostane levels thus ceasing oxidative stress.Citation42 In another study conducted by Askari et al, eight weeks of quercetin supplementation proved to be efficacious in reducing oxidative stress.Citation32
Obesity is known to disrupt the antioxidant tissue defenses and this disruption has been found to be related to a low intake of dietary phytochemicals and antioxidants that possess antioxidant capacity. An inverse correlation of oxidative stress with high BMI, waist circumference, waist-to-hip ratio and plasma oxidative stress was demonstrated in a study conducted by Vincent.Citation43 Polyphenols exhibit potential anti-obesity properties, with proposed mechanisms for weight management including the promotion of thermogenesis and increased energy expenditure,Citation44 inhibition of adipocyte differentiation and growth,Citation45 augmentation of lipolysis and induction of β-oxidation,Citation46 and reduction of appetite.Citation47 The anti-obesity effect of our present study is in line with the known effects of polyphenols like rutin and quercetin. After 56 days of intervention, a mean reduction in the BMI of 0.8 (0.61) kg/m2 was seen in the LN-OS-22 study group as opposed to placebo where the mean BMI showed an increase by 0.06 (0.6) kg/m2; and this result of the IP group was statistically significant when compared to placebo as well as baseline (p<0.0001). Also, at the end of the study, when compared with placebo, a statistically significant reduction was observed in the waist circumference (p < 0.0001), waist-to height ratio (p = 0.0005) as well as body weight (p < 0.0001) in the intervention group. Thus, the anthropometric endpoints of the present study proved to be positive in the LN-OS-22 study group.
Oxidative stress and the increased body weight can have a significant impact on the quality of life.Citation48 In the present study, a statistically significant change in the total scores of the IWQOL-Lite-CT was depicted after 56 days of intervention in the IP group (p = 0.0004). When compared to baseline, the IP group showed significant improvement in the physical impact, energy/stamina, emotions and self-confidence/self-esteem domains of the IWQOL-Lite-CT questionnaire. Thus, it can be concluded that the study group LN-OS-22 positively influenced the quality of life of individuals. No statistically significant results were demonstrated in the intervention group on inflammatory (CRP and IL-6), lipid peroxidation (MDA) as well as metabolic endotoxemia (LPS) biomarkers. This can be attributed to the small sample size of the study. Nevertheless, the investigational product LN-OS-22 proved to be efficacious in reducing the oxidative stress, body weight, BMI and waist circumference of individuals with overweight and obesity as well as improved the quality of life of individuals with overweight and obesity.
Conclusion
The present randomized clinical trial concludes that the LN-OS-22 has antioxidant potential thereby helping to maintain healthy body composition. It also has shown promise of reducing the risk of metabolic dysfunction by maintaining healthy waist to height ratio. The study product LN-OS-22 was found to be well tolerated.
Abbreviations
BMI, Body Mass Index; BP, Blood Pressure; CONSORT, Consolidated Standards Of Reporting Trials; CRP, C-Reactive Protein; EC, Ethics Committee; e-CRF, Electronic-Case Record Form; GMP, Good Manufacturing Practice; IP, Investigational Product; ICH-GCP, International Conference On Harmonization-Good Clinical Practice; IL, Interleukin; LASA, Longitudinal Aging Study Amsterdam (LASA); LPS, Lipopolysaccharide; MDA, Malondialdehyde; PR, Pulse rate; ROS, Reactive Oxygen Species; RNS, Reactive Nitrogen Species; ALP, Serum Alkaline Phosphatase; SGOT, Serum Glutamic-Oxaloacetic Transaminase; SGPT, Serum Glutamic-Pyruvic Transaminase; IWQOL-Lite-CT, Weight on Quality of Life-Lite-Clinical Trials Version.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
Mr James Roza is an employee for Layn, outside the submitted work; and Layn Corp. will use this research to market this ingredient (SophorOx®) as a dietary supplement worldwide. The authors report no other conflicts of interest in this work.
Acknowledgments
The authors are grateful to all the participants who volunteered for the study. The authors thank Layn Natural Ingredients, USA, for providing the study products and Vedic Lifesciences, India, for facilitating the study.
Data Sharing Statement
The data used in the study is available on reasonable request from the corresponding author with due permission from the sponsor.
Additional information
Funding
References
- Videla LA. Oxidative stress signaling underlying liver diseases and hepatoprotective mechanism. World J Hepatic. 2009;31(1):72–78. doi:10.4254/wjh.v1.i1.72
- Laufs U, Wassmann S, Czech T, et al. Physical inactivity increases oxidative stress, endothelial dysfunction, and atherosclerosis. Arterioscler Thromb Vasc Biol. 2005;25(4):809–814. doi:10.1161/01.ATV.0000158311.24443.af
- Aleksandrova K, Koelman L, Rodrigues CE. Dietary patterns and biomarkers of oxidative stress and inflammation: a systematic review of observational and intervention studies. Redox Biol. 2021;42,101869. doi:10.1016/j.redox.2021.101869
- Sies H, Jones D. Oxidative Stress. Encyclop Stress. 2007;45–48. doi:10.1016/B978-012373947-6.00285-3
- Sies H. Oxidative stress: a concept in redox biology and medicine. Redox. 2015;Biol(4):180–183. doi:10.1016/j.redox.2015.01.002
- Keaney JF, Larson MG, Vasan R, et al. Obesity and systemic oxidative stress: clinical correlates of oxidative stress in the Framingham Study. Arterioscler Thromb Vasc Biol. 2003;23:434–439. doi:10.1161/01.ATV.0000058402.34138.11
- Biswas SK. Does the Interdependence between oxidative stress and inflammation explain the antioxidant paradox? Oxid Med Cell Longev. 2016;2016:5698931. doi:10.1155/2016/5698931
- Manna P, Jain SK. Obesity, oxidative stress, adipose tissue dysfunction, and the associated health risks: causes and therapeutic strategies. Metabolic Synd Rel Disord. 2015;13(10):423–444. doi:10.1089/met.2015.0095
- Chrysohoou C, Panagiotakos DB, Pitsavos C, et al. The implication of obesity on total antioxidant capacity in apparently healthy men and women: the ATTICA study. Nutr Metab Cardiovasc Dis. 2007;17(8):590–597. doi:10.1016/j.numecd.2006.05
- Fernández-Sánchez A, Madrigal-Santillán E, Bautista M, et al. Inflammation, oxidative stress, and obesity. Int J Mol Sci. 2011;12(5):3117–3132. doi:10.3390/ijms12053117
- Patel C, Ghanim H, Ravishankar S, et al. Prolonged reactive oxygen species generation and nuclear factor-kappaB activation after a high-fat, high-carbohydrate meal in the obese. J Clin Endocrinol Metab. 2007;92(11):4476–4479. doi:10.1210/jc.2007-0778
- Jiang S, Liu H, Li C. Dietary regulation of oxidative stress in chronic metabolic diseases. Foods. 2021;10(8):1854. doi:10.3390/foods10081854
- He X, Bai Y, Zhao Z, et al. Local and traditional uses, phytochemistry, and pharmacology of Sophora japonica L.: a review. J Ethnopharmacol. 2016;187):160–182. doi:10.1016/J.JEP.2016.04.014
- Abd-Alla HI, Souguir D, Radwan MO. Genus Sophora: a comprehensive review on secondary chemical metabolites and their biological aspects from past achievements to future perspectives. Arch Pharm Res. 2021;44(11):903. doi:10.1007/S12272-021-01354-2
- Ha AT, Rahmawati L, You L, Hossain MA, Kim JH, Cho JY. Anti-inflammatory, antioxidant, moisturizing, and antimelanogenesis effects of quercetin 3-O-β-D-glucuronide in human keratinocytes and melanoma cells via activation of NF-κB and AP-1 pathways. Int J Mol Sci. 2021;23. doi:10.3390/IJMS23010433
- Alizadeh SR, Ebrahimzadeh MA. Quercetin derivatives: drug design, development, and biological activities, a review. Eur J Med Chem. 2021;229. doi:10.1016/J.EJMECH.2021.114068
- Bernini R, Velotti F. Natural polyphenols as immunomodulators to rescue immune response homeostasis: quercetin as a research model against severe COVID-19. Molecules. 2021;26(19):5803. doi:10.3390/MOLECULES26195803
- Di Petrillo A, Orrù G, Fais A, Fantini MC. Quercetin and its derivates as antiviral potentials: a comprehensive review. Phytother Res. 2022;36(1):266–278. doi:10.1002/PTR.7309
- Shen P, Lin W, Deng X, et al. Potential implications of quercetin in autoimmune diseases. Front Immunol. 2021;12. doi:10.3389/FIMMU.2021.689044
- Yi H, Peng H, Wu X, et al. The therapeutic effects and mechanisms of quercetin on metabolic diseases: pharmacological data and clinical evidence. Oxid Med Cell Longev. 2021;2021:1–16. doi:10.1155/2021/6678662
- Shanmugasundaram D, Roza JM. Assessment of anti-inflammatory and antioxidant activity of quercetin–rutin blend (SophorOx™) – an invitro cell based assay. J Complement Integr Med. 2022;19:637–644. doi:10.1515/jcim-2021-0568
- Shanmugasundaram D, Roza JM. Assessment of anti-inflammatory and antioxidant activities of a proprietary preparation of quercetin-rutin blend (SophorOx™) in exercised rats. ScientificWorldJournal. 2024;2024:9063936. doi:10.1155/2024/9063936 PMID: 38371227; PMCID: PMC10874291.
- Van’t Erve TJ, Kadiiska MB, London SJ, Mason RP. Classifying oxidative stress by F2-isoprostane levels across human diseases: a meta-analysis. Redox Biol. 2017;12(March):582–599. doi:10.1016/j.redox.2017.03.024
- Van’t Erve TJ, Lih FB, Jelsema C, et al. Reinterpreting the best biomarker of oxidative stress: the 8-iso-prostaglandin F2α/prostaglandin F2α ratio shows complex origins of lipid peroxidation biomarkers in animal models. Free Radic Biol Med. 2016;95:65–73. doi:10.1016/j.freeradbiomed.2016.03.001
- Castro AM, Macedo-de la Concha LE, Pantoja-Meléndez CA. Low-grade inflammation and its relation to obesity and chronic degenerative diseases. Revista Médica Del Hospital General de México. 2017;80(2):101–105. doi:10.1016/J.HGMX.2016.06.011
- Rozjabek H, Fastenau J, Laprade A, Sternbach N. Adult obesity and health-related quality of life, patient activation, work productivity, and weight loss behaviors in the United States. Diabet Metabo Synd obesit. 2020;13:2049–2055. doi:10.2147/DMSO.S245486
- Dehghani F, Sezavar Seyedi Jandaghi SH, Janani L, Sarebanhassanabadi M, Emamat H, Vafa M. Effects of quercetin supplementation on inflammatory factors and quality of life in post-myocardial infarction patients: a double blind, placebo-controlled, randomized clinical trial. Phytother Res PTR. 2021;35(4):2085–2098. doi:10.1002/ptr.6955
- Han MK, Barreto TA, Martinez FJ, Comstock AT, Sajjan US. Randomised clinical trial to determine the safety of quercetin supplementation in patients with chronic obstructive pulmonary disease. BMJ Open Respir Res. 2020;7(1):e000392. doi:10.1136/bmjresp-2018-000392
- Javadi F, Ahmadzadeh A, Eghtesadi S, et al. The effect of quercetin on inflammatory factors and clinical symptoms in women with rheumatoid arthritis: a double-blind, randomized controlled trial. J Am Coll Nutr. 2017;36(1):9–15. doi:10.1080/07315724.2016.1140093
- Boyle SP, Dobson VL, Duthie SJ, Hinselwood DC, Kyle J, Collins AR. Bioavailability and efficiency of rutin as an antioxidant: a human supplementation study. Eur J Clin Nutr. 2020;54:774–782. doi:10.1038/sj.ejcn.1601090
- Liu ZH, Cai Y, He J. High serum levels of 8-OHdG are an independent predictor of post-stroke depression in Chinese stroke survivors. Neuropsychiatr Dis Treat. 2018;14:587–596. doi:10.2147/NDT.S155144
- Askari G, Ghiasvand R, Feizi A, Ghanadian SM, Karimian J. The effect of quercetin supplementation on selected markers of inflammation and oxidative stress. J Res Med Sci. 2021;17(7):637–641.
- Wiswedel I, Hirsch D, Kropf S, et al. Flavanol-rich cocoa drink lowers plasma F(2)-isoprostane concentrations in humans. Free Radic Biol Med. 2004;37(3):411–421. doi:10.1016/j.freeradbiomed.2004.05.013
- O’Reilly JD, Mallet AI, McAnlis GT, et al. Consumption of flavonoids in onions and black tea: lack of effect on F2-isoprostanes and autoantibodies to oxidized LDL in healthy humans. Am J Clin Nutr. 2001;73(6):1040–1044. doi:10.1093/AJCN/73.6.1040
- Furukawa S, Fujita T, Shimabukuro M, et al. Increased oxidative stress in obesity and its impact on metabolic syndrome. J Clin Invest. 2004;114(12):1752–1761. doi:10.1172/JCI21625
- Morrow JD. Quantification of isoprostanes as indices of oxidant stress and the risk of atherosclerosis in humans. Arterioscler Thromb Vasc Biol. 2005;25(2):279–286. doi:10.1161/01.ATV.0000152605.64964.c0
- Urakawa H, Katsuki A, Sumida Y, et al. Oxidative stress is associated with adiposity and insulin resistance in men. J Clin Endocrinol Metab. 2003;88(10):4673–4676. doi:10.1210/jc.2003-030202
- Herasymchuk NM. 8-isoprostane as the main marker of oxidative stress. Zaporožskij Medicinskij Žurnal. 2018b;1(6). doi:10.14739/2310-1210.2018.6.146780
- Meera S, Sarangarajan R, Rajkumar K. 8-Isoprostane: a salivary oxidative stress biomarker for oral submucous fibrosis and oral squamous cell carcinoma. J Oral Maxillofac Pathol. 2020;24(2):279–284. doi:10.4103/jomfp.JOMFP_235_19 PMID: 33456237; PMCID: PMC7802855.
- Rudrapal M, Khairnar SJ, Khan J, et al. Dietary polyphenols and their role in oxidative stress-induced human diseases: insights into protective effects, antioxidant potentials and mechanism(s) of action. Front Pharmacol. 2022;13. doi:10.3389/fphar.2022.806470
- Montuschi P, Barnes PJ, Roberts LJ. Isoprostanes: markers and mediators of oxidative stress. THE FASEB Journal. 2004;18(15):1791–1800. doi:10.1096/fj.04-2330rev
- Sutherland WH, Manning PJ, Walker RJ, de Jong SA, Ryalls AR, Berry EA. Vitamin E supplementation and plasma 8-isoprostane and adiponectin in overweight subjects. Obesity. 2007;15(2):386–391. doi:10.1038/oby.2007.546
- Vincent HK, Bourguignon CM, Taylor AG. Relationship of the dietary phytochemical index to weight gain, oxidative stress and inflammation in overweight young adults. J Hum Nutr Diet. 2010;23(1):20–29. doi:10.1111/j.1365-277X.2009.00987.x
- Dulloo AG, Duret C, Rohrer D, et al. Efficacy of a green tea extract rich in catechin polyphenols and caffeine in increasing 24-h energy expenditure and fat oxidation in humans. The American Journal of Clinical Nutrition. 1999;70(6):1040–1045. doi:10.1093/ajcn/70.6.1040
- Sy M, Yang HS, Sg S, et al. Cocoa polyphenols suppress adipogenesis in vitro and obesity in vivo by targeting insulin receptor. Int J Obesity. 2012;37(4):584–592. doi:10.1038/ijo.2012.85
- Rupasinghe HV, Sekhon-Loodu S, Mantso T, Panayiotidis MΙ. Phytochemicals in regulating fatty acid β-oxidation: potential underlying mechanisms and their involvement in obesity and weight loss. Pharmacol Ther. 2016;165:153–163. doi:10.1016/j.pharmthera.2016.06.005
- Boix-Castejón M, Herranz-López M, Gago AP, et al. Correction: hibiscus and lemon verbena polyphenols modulate appetite-related biomarkers in overweight subjects: a randomized controlled trial. Food Funct. 2018;9(7):4037. doi:10.1039/c8fo90028k
- Kolotkin RL, Meter K, Williams GR. Quality of life and obesity. Obes Rev. 2001;2(4):219–229. doi:10.1046/j.1467-789x.2001.00040.x