Abstract
Background
Generally, people with type 2 diabetes mellitus in various countries experience a significant rate of sensorineural hearing impairment. Nonetheless, there is scant evidence of sensorineural hearing impairment among type 2 diabetes mellitus patients in Northwest Ethiopian. Therefore, the objective of this study was to evaluate the occurrence rate and contributing factors of sensorineural hearing impairment in type 2 diabetics at comprehensive and specialized referral hospitals in Northwest Ethiopia.
Methods
A facility-based cross-sectional study design was carried out from May 3, 2022, to June 14, 2022, on 846 study participants in Ethiopia, with a response rate of 99.65%. The research subjects were chosen by simple random sampling techniques. Data was gathered by using audiometric measurements and structured interview-administered questionnaires and then entered into EPI data version 4.6. Finally, it was exported to STATA 14 for analysis. Binary logistic regression, chi-square test, and odds ratio were done to verify the assumptions and degree of association. Ultimately, factors exhibiting a p-value < 0.05 with a 95% CI were regarded as significant predictors of hearing impairment.
Results
The magnitude of sensorineural hearing impairment in this investigation was 50.49% (95% CI: 45.67%, 55.26%). Factors significantly associated with sensorineural hearing impairment were age (AOR=1.10, 95% CI: 1.07, 1.14), hyperlipidemia (AOR=2.86, 95% CI: 1.05, 7.82), duration of diabetes (AOR=2.26, 95% CI: 1.26, 4.06), hypertension (AOR=1.94, 95% CI: 1.02, 3.69) and regular physical exercise (AOR=0.25, 95% CI: 0.09, 0.68).
Conclusions and Recommendations
In this study, relatively high rates of sensorineural hearing impairment were observed. Stakeholders should establish routine hearing screening, and participants will advise to incorporate regular physical exercise into their routines.
Introduction
Sensorineural hearing impairment (SNHI) is a disability in which an individual is unable to hear sounds in one or both ears within 25 dB.Citation1,Citation2 In recent times, SNHI resulting from prolonged exposure to diabetes mellitus has emerged as a significant global public health issue.Citation2 Type 2 diabetes mellitus (T2DM) leads to a gradual, bilateral, and permanent SNHI.Citation3 Previous research has verified that prolonged exposure to elevated glucose levels can result in SNHI.Citation4 This was due to the excess production of free oxygen radicals in the body. Because of their high reactivity and ability to attach to a variety of molecules, free oxygen radicals have the potential to harm the inner ear’s mitochondrial deoxyribonucleic acid.Citation5 If the mitochondria were impaired, oxidative phosphorylation and adenosine triphosphate production would be diminished, causing anoxia. Anoxia in the inner ear causes a decline in endocochlear potential.Citation4 This results in SNHI by interfering with impulse conduction to the endocochlear nerveCitation4,Citation5. The World Health Organization (WHO) estimates that 20.3% of people worldwide have SNHI.Citation6 Furthermore, the European Center for Disease Control and Prevention (CDC) reports that the SNHI was 30% higher in type 2 diabetics than non-diabetic individuals.Citation7,Citation8 In addition to this, the American Diabetes Association revealed that the magnitude of SNHI is twice as high in T2DM patients than without T2DM.Citation9 Moreover, a meta-analysis conducted in Canada reported that the prevalence of SNHI among T2DM patients ranged between 44–69.7%.Citation10 The prevalence of T2DM-related SNHI varied globally;Citation11 as well as in African countries. Sensorineural hearing impairment imposes a substantial social and economic burden on numerous nations.Citation12 As a prevalent public health concern, hearing impairment adversely affects an individual’s capacity to learn, integrate into society, perform effectively at work, sustain relationships with others, and contribute productively. Furthermore, it exerts a negative impact on one’s overall quality of life and well-being.Citation12–14 In Sub-Saharan Africa, particularly in Ethiopia, T2DM has become a significant public health concern.Citation2 Despite the rising prevalence of T2DM in Ethiopia, the extent of SNHI attributable by T2DM remains unclear. Therefore, the aim of this study was to assess the magnitude and factors associated with sensorineural hearing impairment among type 2 diabetes mellitus individuals in northwest Ethiopia.
Methodology
Study Design, Area, and Period
A multicenter, hospital-based cross-sectional study was conducted in comprehensive and specialized referral hospitals (CSRHs) within the Amhara region, Northwest Ethiopia, from May 3 to June 14, 2022. The Amhara region is one of the largest regions in Ethiopia, with its capital city being Bahir Dar, situated 565 km from Addis Ababa (the capital city of Ethiopia). Among all people, 16,375 people in the Amhara region had T2DM. In the Amhara region, there are eight CSRH, including Felege Hiwot, Woldia, Debre Tabor, Debre Berhan, the University of Gondar, Tibebe Gihon, Dessie, and Debre Markos. By using simple random sampling techniques, Tibebe Gihon, Debre Markos, and Debre Tabor CSRHs were selected. Debre Markos Comprehensive Specialized Referral Hospital was established in Debre Markos City, located 297 km from Addis Ababa. The hospital is providing comprehensive diabetic care services for 2104 T2DM individuals. Debre Tabor CSRH is situated in Debre Tabor City, which is 668 kilometers distant from Addis Ababa. It offers diabetic support services for 1052 type 2 diabetics. Tibebe Gihon CSRH is located in Bahir Dar, 565 kilometers away from the capital city of Ethiopia. It also offers diabetic services for 1644 individuals with T2DM.
Population
The source population for this study comprised all individuals diagnosed with T2DM in the Amhara region. The study population included all T2DM patients in the selected Amhara region in comprehensive and specialized referral hospitals.
Eligibility Criteria
The study included individuals with T2DM who were ≥ 18 years old, not severely ill, and non-congenitally impaired. Those who did not meet these criteria were excluded from the study.
Sampling Techniques and Sample Size Estimation
Since, no previous similar study has been conducted in Ethiopia, the sample size of the study was established using a single population proportion formula by taking the following parameters: proportion of sensorineural hearing impairment (P) = 50%, CI: 95%, error of margin (d) = 5%, and design effect (De) = 2.
Where, ni = initial sample size
With a 10% contingency rate, the final sample size was 846.
The study hospitals were chosen through a simple random sampling technique. Subsequently, for the selection of study participants, a systematic random sampling technique was employed after proportional allocation was made for each of the selected hospitals.
Study Variables
Dependent variable: Hearing impairment
Independent variables: Sociodemographic characteristics like age, sex, occupation, educational level, income, family history, and institutional noise level were assessed. Behavioral and medical elements such as cigarette smoking, alcohol drinking, regular physical exercise, earphone utilization, hypertension, ear infection, hyperlipidemia, cardiac disease, body mass index, family history, glycemic control, and ototoxic drug utilization (Aminoglycoside, Aspirin, Chemotherapy, Loop diuretics, and NSAID) were also assessed.
Operational Definitions
Sensorineural hearing impairment: Was an average hearing threshold above 25 decibels at 0.5, 1, 2, 4 and 8 kHz.Citation1,Citation2
Severity of hearing impairment: Categorized as mild (25–40 dB), moderate (40–55 dB), moderately severe (55–70 dB), severe (70–90 dB), and profound (91+ dB).Citation6
Body mass index: according to WHO BMI is classified as underweight, normal weight, overweight, and obese when weight over height is <18.5 kg/m2, 18.5–24.9 kg/m2, 25–29.9 kg/m2, and ≥30 kg/m2Citation15 respectively.
Blood glucose regulation: Good glycemic control was defined as having a fasting blood glucose level within the range of 70–126 mg/dl, including the boundary points. Conversely, poor glycemic control was determined when the fasting blood glucose level was below 70 or above 126 mg/dl.Citation16
Data Collection Procedures and Instruments
Structured interviewer-administered questionnaires were utilized to collect data from participants (Supplementary File 1). The questionnaire, originally crafted in English, underwent translation into the participant’s local language (Amharic). To ensure accuracy, the translated version was then back translated into English by experts proficient in both languages. Three individuals with Bachelor of Science degrees in Nursing conducted the interviews under the supervision of a principal investigator. In addition to this, audiometric and institutional noise levels were used to measure the hearing threshold of participants and environmental sound levels, respectively.
Audiometric Measurement
The study participants’ hearing status was assessed by audiologists using a pure-tone audiometer
Daily calibration of the pure-tone audiometer was carried out using individuals with presumed normal hearing prior to any audiometric evaluations. Audiometric tests were consistently conducted in a noise-controlled environment with a background noise level maintained between 36 and 40 dB. This practice was implemented prior to workers entering their workstations to mitigate the potential impact of transitory threshold shifts caused by ongoing exposure to noise in the workplace. This precautionary measure aims to ensure the accuracy and reliability of the audiometric evaluations by minimizing any extraneous noise interference during the testing process. Participants were informed in advance about the scheduled audiometric test, allowing them the opportunity to observe an “acoustic rest” or “quiet time” ideally lasting 16 hours before the actual test.Citation17 Respondents received comprehensive instructions about the test, including guidance to sit still and refrain from talking. The audiometer was carefully plugged and placed on the participants’ ears. Before determining the threshold, respondents were introduced to the signal by presenting it at an intensity sufficient to elicit a clear response. Subsequently, the audiometer machine was adjusted to consecutively test the right and left ears of participants. Participants were then instructed to press the audiometer pointer when they heard the sound. Three successive audiometric measurements were conducted, and the average of these three records was analyzed. Ultimately, a diagnosis of hearing impairment was established if an individual exhibited a hearing threshold of ≥25 dB.Citation18,Citation19
Institution Noise Level
A sound level meter (Model SL-5868I) was employed to measure the noise level in workstations, providing a reliable indicator of the ambient noise in the working area. The meter has a measurement range spanning from 25 to 130 dB. The initial step preceding the measurement of institutional sound involved identifying the location of the major source of noise. After identifying the location of the noise source, the subsequent step involved selecting a suitable position for the instrument. This placement was strategically chosen to be away from any obstruction or vibration. Subsequently, the instrument was elevated to a height of 1.2–1.5 meters above the ground and positioned 3.5 meters away from the noise source. Following this, the sound level meter was activated, and measurements were taken. The recorded data included the sound pressure level obtained from the display screen. Measurements were conducted during specific periods: from 7 a.m. to 6 p.m. during the daytime, from 6 p.m. to 10 p.m. in the evening, and from 10 p.m. to 7 a.m. at night on three days. A sound level of greater than 85 dB for more than eight hours caused SNHI.Citation20,Citation21
Data Quality Control
To guarantee the quality of data, data collectors underwent three days of training. To determine the validity of the questionnaire, Cronbach’s alpha was examined, and the result was 76.54%. Additionally, 5% of the total sample size of T2DM patients at Bahir Dar General Hospital participated in the questionnaire pretesting. The main investigator closely monitored the data collectors each day. Before entry, the data underwent a thorough check for both consistency and completeness.
Data Processing and Analysis
Once the data was collected, it was exported into STATA version 14.0 for analysis. Descriptive statistics, including frequency, median, mean, interquartile range, and percentage, were utilized to provide a comprehensive summary of the participants. The normality of the data was examined using the Shapiro–Wilk test, revealing a p-value of 0.001. Furthermore, the goodness-of-fit for the model was assessed through the Hosmer-Lemeshow test, with a calculated value of 0.2654. Variables with a p-value of ≤ 0.2 in bivariable regression were included in multivariable logistics regression. To assess the strength of the association between the independent variables and outcome variables, odds ratios (OR) at a 95% confidence interval (CI) were calculated. In the multivariable regression, variables with a p-value of ≤ 0.05 were regarded as significantly associated.
Results
Demographic and Social Attributes of Participants
A total of 846 participants were recruited with a 99.65% response rate. Among these, 58.8% were men. The participants’ age span ranged from 24 to 70 years, with a mean age of 51.6 ± 10.10. In terms of religious affiliation, 74% identified as Christians. Regarding educational attainment, 36.19% reported not having formal education. Additionally, 59% of participants indicated residing in urban areas, while 28.57% identified as civil servants. The median monthly income among respondents was 3000 ETB. Institutional sound levels of DMCRH, TGCRH, and DTCRH were 61.5, 65.3, and 51.2 dB, respectively ().
Table 1 Socio-demographic characteristics of T2DM patients and Chi-square test in Amhara region, CSRH, Northwest Ethiopia, 2022
Behavioral Characteristics of Study Participants
In this study, 17.14% of participants did regular physical exercise, 6.43% had a history of cigarette smoking, 19.5% were heavy alcohol drinkers, and 89.52% were earphone utilizers ().
Table 2 Behavioral characteristics of T2DM patients and Chi-square test in Amhara region, CSRH, Northwest Ethiopia, 2022 (n=843)
Clinical Characteristics of Participants
The average duration of T2DM was 5 years, with an interquartile range of 8.25 years. Out of all the study participants, 91.19% started antidiabetic medication. From those, 41.90% took oral antidiabetics. A history of hypertension, hyperlipidemia, thyroid, and cardiovascular disease was present in 28.33%, 10.24%, 5.95%, and 6.19% of the individuals, respectively. Among participants, 61.67% had poor glycemic control, 8.33% had a history of ear infection, and 41.43% used ototoxic medications. The average BMI of the participants was 24 ± 4 kg/m2 ().
Table 3 Clinical characteristics of T2DM patients and Chi-square test in Amhara region, CSRH, Northwest Ethiopia, 2022 (n=843)
Prevalence of Sensorineural Hearing Impairment
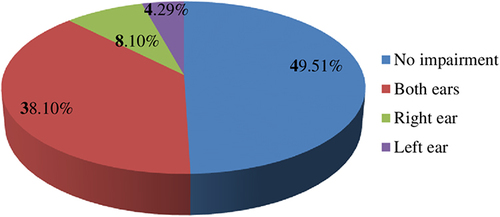
The study found that 50.5% of participants had SNHI (95% CI: 45.69%, 55.26%). Of those, a majority of the participants (75.46%) had a bilateral SNHI ().
Severity of Sensorineural Hearing Impairment
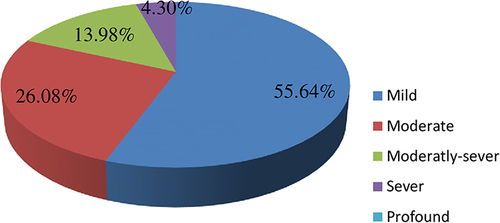
Among all patients with SNHI, 55.64, 26.08, 13.98, and 4.30% experienced mild, moderate, moderately-severe, and severe SNHI respectively. Notably, none of the patients exhibited profound HI ().
Associated Factors of Hearing Impairment
In the bivariable logistic regression analysis, age, regular physical exercise, smoking history, hypertension, ear infections or trauma, duration of T2DM, antidiabetic medication, BMI, hyperlipidemia, use of ototoxic drugs, and glycemic control were identified as factors significantly associated with the SNHI. However, in the multivariable logistic regression analysis, age, duration of T2DM, hypertension, hyperlipidemia, and regular physical exercise exhibited significant associations with the SNHI.
As age increased by one-unit, the likelihood of developing SNHI increased by 1.1 times, as indicated by an adjusted odds ratio (AOR) of 1.10, with a 95% confidence interval ranging from 1.07 to 1.14. Also, the odds of developing SNHI among participants with a diabetic duration of ≥ 10 years were 2.26 times higher than those with a diabetic duration of < 10 years (AOR = 2.26, 95% CI: 1.26, 4.06). Moreover, the odds of developing SNHI in hypertensive patients were 1.94 times higher than in non-hypertensive individuals, as evidenced by an AOR of 1.94 with a 95% CI ranging from 1.02 to 3.69. In addition, the odds of developing SNHI in hyperlipidemic respondents were 2.86 times higher than in respondents with normal lipid levels (AOR =2.86, 95% CI: 1.05, 7.82). However, Individuals engaging in regular physical exercise for two or more hours were 75% less likely to develop SNHI compared to those who did not engage in regular physical exercise ().
Table 4 Significantly associated variables in bivariable and multivariable logistic regression analysis among T2DM patients in Amhara region, CSRH, Northwest Ethiopia, 2022
Discussion
The objective of the current study was to evaluate the prevalence of sensorineural hearing impairment and factors associated with it among patients with type 2 diabetes mellitus in Ethiopia.
In the current study, the overall magnitude of SNHI among the participants was 50.49% (95% CI; 45.69%, 55.26%). It was consistent with studies conducted in Australia 50%,Citation22 Pakistan 46.1%,Citation23 Switzerland 46.9%,Citation11 Taiwan 47%,Citation24 Saudi Arabia 49%,Citation25 South Africa 55%,Citation26 and Egypt 51.3%.Citation27 The consistency in study design and participant characteristics may account for the observed trend. However, the findings from this study revealed lower values compared to similar research conducted in Japan (60.2%),Citation28 Iran (85%),Citation29 Nepal (72.5%),Citation1 India (58%),Citation30 North India (81%),Citation31 and Nigeria (71.8%).Citation32 This possible discrepancy might be due to natural disasters such as volcanic eruptions, flooding, and tsunamis that regularly affect Asian countries.Citation33 Another possible reason might be due to environmental factors; Southeast Asia is region where barotraumas frequently occur that causes HI.Citation34 In contrast, this result was higher than the results reported in the USA (43.6%),Citation3 Mexico (21.7%),Citation35 UK (7.5%),Citation36 China (45.1%),Citation37 Canada (44%-69.7%),Citation10 and Kenya (39.7%).Citation38 This might be due to differences in economic and public health institutions coverage. In Ethiopia, there is limited access to health institutions, early screening, sufficient immunization, and a high prevalence of inner ear infections.Citation39,Citation40 This factor could potentially contribute to the high prevalence of SNHI in this research. This study showed that the magnitude of bilateral SNHI (38.10%) was higher than unilateral SNHI (12.39%). This observation finds support in studies conducted in Saudi Arabia,Citation25 China 45.1%,Citation37 and USA.Citation3 This might be due to the nature of hyperglycemia, which distributes equally in both ears and leads to progressive, bilateral, and irreversible SNHI.Citation3 Factors significantly associated with predictor variables in multivariable logistic regression were age, duration of T2DM, hypertension, hyperlipidemia, and regular physical exercise. Age, duration of diabetes mellitus, hypertension, and hyperlipidemia were positively associated with SNHI. However, regular physical exercise was negatively associated with the occurrence of SNHI. The current study revealed that as age increased, the opportunity of developing SNHI also increased. This was substantiated by research conducted in Pakistan,Citation23 the USA,Citation41 Korea,Citation42 Iran,Citation43 Japan,Citation44 China,Citation45 Jammu,Citation46 India,Citation30 and North India.Citation31 This may be the result of vascular insufficiency, decreased mitochondrial activity, and increased ROS generation with aging.Citation4,Citation19 ROS disrupts mitochondrial DNA, which results in decreased oxygen and energy supply in the inner ear.Citation47 The results of the current study suggested that individuals with a long duration of T2DM had a higher chance of developing SNHI than those with a short-term duration. This was in line with studies done in Mexico,Citation35 Indonesia,Citation48 Turkey,Citation49 Nepal,Citation1 Iran,Citation29 China,Citation37 Jammu,Citation46 India,Citation50 Saudi Arabia,Citation25 and South Africa.Citation26 The possible reason might be that chronic hyperglycemia affects the inner ear nerve tracts and blood vessels by increased reactive oxygen species.Citation51 Excess production of oxygen-free radicals in the body caused a potentially toxic effect on the inner ear, which eventually caused neuronal degeneration in the auditory system and led to SNHI.Citation52 In addition, persistent hyperglycemia causes diabetic microangiopathy in the ear. In hyperglycemia, there was a notable thickening of the vascular wall of the basilar membrane and stria vascularis, accompanied by the loss of outer hair cells. This caused SNHI.Citation53 According to the current study, hypertension was significantly associated with SNHI in T2DM patients. This was supported by studies in Mexico,Citation35 Turkey,Citation49 Qatar,Citation54 Saudi Arabia,Citation25 and Nigeria.Citation55 This might be due to reduced blood flow and oxygen transport in the stria vascularis secondary to hypertension.Citation56 In addition to this, hypertension caused hypertension-induced vascular injury in the blood-labyrinth barrier (BLB) of the cochlea. This has the potential to disrupt the ionic balance within the endolymph, potentially resulting in SNHI.Citation56 Furthermore, participants who had hyperlipidemia were more likely to develop SNHI than their counterparts. This was supported by studies conducted in KoreaCitation57 and Mexico.Citation35 The possible explanation might be the deterioration of inner ear perfusion due to atherosclerosis and increased blood viscosity secondary to hyperlipidemia.Citation58–60 Finally, the current study demonstrated that engaging in regular physical exercise had a protective effect against SNHI, with an AOR of 0.25 and a 95% CI ranging from 0.09 to 0.68. This finding is consistent with research conducted in the United States.Citation41 The possible reason might be that regular physical exercise reduces glucose ototoxicity by elevating glucose uptake by hepatic and skeletal muscle tissues.Citation61 Also, regular physical exercise increases oxygen-rich blood flow to the inner ear by increasing cardiac output.Citation61 Generally, regular physical exercise protects the inner ear from glucose toxicity.
Strengths and Limitations of the Study
This research was carried out in multiple centers and encompassed a diverse population, thereby enhancing its generalizability. In addition to this, primary data was employed to enhance the credibility of the evidence. However, due to its cross-sectional study design, the research did not demonstrate a cause-and-effect relationship. In addition, the dose of ototoxic drugs was not accurately determined because of recall bias and the utilization of unrecorded drugs by the patients. Overall, the study serves as a foundation for future investigators to conduct more robust study designs, such as experimental and cohort in this setting to yield enhanced conclusions.
Conclusion and Recommendations
The magnitude of sensorineural hearing impairment in type 2 diabetes mellitus participants in the Amhara region was notably high. This study revealed that factors such as age, duration of diabetes mellitus, hypertension, and hyperlipidemia are associated with increased odds of experiencing sensorineural hearing impairment in type 2 diabetes mellitus participants. Therefore, health policymakers should develop a policy for routine hearing screening for all type 2 diabetic patients. To mitigate the impact of sensorineural hearing impairment, it is recommended that medical staff and stakeholders in each referral hospital prioritize early management of diabetes mellitus, hypertension, and cholesterol. Additionally, individuals with type 2 diabetes mellitus should actively participate in regular physical activity to minimize the potential effects.
Abbreviations
CSRH, Comprehensive and Specialized Referral Hospitals; dB, Decibel; KHz, Kilo Hertz; SNHI, Sensorineural Hearing Impairment, T2DM, Type Two Diabetes Mellitus.
Data Sharing Statement
The dataset are available upon request from the corresponding author.
Ethical Consideration
The research received scrutiny and approval from the University of Gondar, School of Medicine, College of Medicine and Health Science Institutional Review Board (IRB No. SOM/1482/2022). An official letter of permission was acquired from the University of Gondar. The study adhered to the ethical principles outlined in the Helsinki Declaration for Medical Research involving Human Subjects.Citation62 Prior to the commencement of actual data collection, formal authorization was obtained from the medical directorates of each hospital. Additionally, written informed consent was obtained from every study participant after a thorough explanation of the study’s significance.
Author Contributions
Each author played a substantial role in the undertaken work, contributing to various aspects such as conception, study design, execution, data acquisition, analysis, and interpretation. They actively participated in drafting, revising, or critically reviewing the article and provided final approval for the version intended for publication. All authors reached a consensus on the journal to which the article was submitted and willingly commit to being accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
Acknowledgments
The authors express their gratitude to the University of Gondar for providing ethical clearance. Additionally, appreciation is extended to the Comprehensive Specialized Hospital of Northwest Ethiopia. The authors would also like to acknowledge the study participants and express thanks to the data collectors for their valuable contributions to the success of this study.
Additional information
Funding
References
- Khakurel G, Mahato NB. Evaluation of hearing loss by pure tone audiometry in type 2 Diabetes Mellitus. Nepalese Med J. 2020;3(1):286–289. doi:10.3126/nmj.v3i1.28221
- Melese M, Adugna DG, Mulat B, Adera A. Hearing loss and its associated factors among metal workshop workers at Gondar city, Northwest Ethiopia. Front Public Health. 2022;10:919239. doi:10.3389/fpubh.2022.919239
- Pemmaiah KD, Srinivas DR. Hearing loss in Diabetes Mellitus. Int J Collab Res Inter Med Public Health. 2011;3(10):725–731.
- Aladag I, Eyibilen A, Güven M, Atış Ö, Erkokmaz Ü. Role of oxidative stress in hearing impairment in patients with type two diabetes mellitus. J Laryngol Otol. 2009;123(9):957–963. doi:10.1017/S0022215109004502
- Wang L, Gao P, Zhang M, et al. Prevalence and ethnic pattern of diabetes and prediabetes in China in 2013. JAMA. 2017;317(24):2515–2523. doi:10.1001/jama.2017.7596
- Haile LM, Kamenov K, Briant PS, et al. Hearing loss prevalence and years lived with disability, 1990–2019: findings from the Global Burden of Disease Study 2019. Lancet. 2021;397(10278):996–1009. doi:10.1016/S0140-6736(21)00516-X
- Centers for Disease Control and Prevention. Diabetes and Hearing Loss. US Department of Health & Human Services; 2021.
- Glantz G. Diabetes & hearing loss: action items for audiologists. Hearing J. 2021;74(11):16–18. doi:10.1097/01.HJ.0000800712.25761.0a
- Schade DS, Lorenzi GM, Braffett BH, et al. Hearing impairment and type 1 diabetes in the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) cohort. Diabetes Care. 2018;41(12):2495–2501. doi:10.2337/dc18-0625
- Akinpelu OV, Mujica-Mota M, Daniel SJ. Is type 2 diabetes mellitus associated with alterations in hearing? A systematic review and meta-analysis. Laryngoscope. 2014;124(3):767–776. doi:10.1002/lary.24354
- Ashkezari SJ, Namiranian N, Rahmanian M, Atighechi S, Mohajeri-Tehrani M-R, Gholami S. Is hearing impairment in diabetic patients correlated to other complications? J Diabetes Metab Disord. 2018;17(2):173–179. doi:10.1007/s40200-018-0357-3
- World Health Organization. Primary Ear and Hearing Care Training Resource. Geneva 27 Switzerland: World Health Organization; 2006.
- Krug E, Cieza A, Chadha S, et al. Childhood Hearing Loss: Strategies for Prevention and Care. World Health Organization; 2016:31–39.
- World Health Organization. Addressing the Rising Prevalence of Hearing Loss. Geneva: World Health Organization; 2018:3–28.
- Lim JU, Lee JH, Kim JS, et al. Comparison of World Health Organization and Asia-Pacific body mass index classifications in COPD patients. Int J Chronic Obstr. 2017;12:2465. doi:10.2147/COPD.S141295
- Yosef T, Nureye D, Tekalign E. Poor glycemic control and its contributing factors among type 2 diabetes patients at Adama Hospital Medical College in East Ethiopia. Diabetes Metabol Syndr Obes. 2021;14:3273. doi:10.2147/DMSO.S321756
- Hailu A. Assessment of Noise Induced Hearing Loss and Associated Factors Among Workers in Akaki Basic Metal Industry, Addis Ababa, Ethiopia. Addis Ababa University; 2015.
- Feder K, Michaud D, McNamee J, Fitzpatrick E, Davies H, Leroux T. Prevalence of hazardous occupational noise exposure, hearing loss, and hearing protection usage among a representative sample of working Canadians. J Occup Environ Med. 2017;59(1):92. doi:10.1097/JOM.0000000000000920
- Louw C, Swanepoel DW, Eikelboom RH, Hugo J. Prevalence of hearing loss at primary health care clinics in South Africa. Afr Health Sci. 2018;18(2):313–320. doi:10.4314/ahs.v18i2.16
- Masterson EA, Tak S, Themann CL, et al. Prevalence of hearing loss in the United States by industry. Am J Ind Med. 2013;56(6):670–681. doi:10.1002/ajim.22082
- Kim M-B, Zhang Y, Chang Y, et al. Diabetes mellitus and the incidence of hearing loss: a cohort study. Int J Epidemiol. 2017;46(2):717–726. doi:10.1093/ije/dyw243
- Mitchell P, Gopinath B, McMahon CM, et al. Relationship of Type 2 diabetes to the prevalence, incidence and progression of age‐related hearing loss. Diabetic Med. 2009;26(5):483–488. doi:10.1111/j.1464-5491.2009.02710.x
- Majeed S, Mumtaz N, Saqulain G. Prevalence of sensorineural hearing loss among patients of diabetes mellitus in Southern Punjab, Pakistan. J Shifa Tameer-e-Millat Univ. 2018;1(1):32–36. doi:10.32593/jstmu/Vol1.Iss1.36
- Shen FC, Hsieh CJ. Severity of hearing impairment is positively associated with urine albumin excretion rate in patients with type 2 diabetes. J Diabetes Invest. 2014;5(6):743–747. doi:10.1111/jdi.12196
- Al-Rubeaan K, AlMomani M, AlGethami AK, et al. Hearing loss among patients with type 2 diabetes mellitus: a cross-sectional study. Ann Saudi Med. 2021;41(3):171–178. doi:10.5144/0256-4947.2021.171
- Hlayisi V-G, Petersen L, Ramma L. High prevalence of disabling hearing loss in young to middle-aged adults with diabetes. Int J Diabetes Dev Countries. 2019;39(1):148–153. doi:10.1007/s13410-018-0655-9
- Rajamani S, Senniappan S, Radhakrishnan S. Prevalence and factors influencing sensorineural hearing loss among type II diabetes mellitus patients. ESC Heart Fail. 2018;5(3):732–737. doi:10.1002/ehf2.12289
- Sakuta H, Suzuki T, Yasuda H, Ito T. Type 2 diabetes and hearing loss in personnel of the Self-Defense Forces. Diabetes Res Clin Pract. 2007;75(2):229–234. doi:10.1016/j.diabres.2006.06.029
- Hosseini MS, Saeedi M, Khalkhal SA. Prevalence of hearing disorders among type 2 diabetes mellitus patients with and without vitamin D deficiency. Mædica. 2020;15(1):32. doi:10.26574/maedica.2020.15.1.32
- Meena R, Sonkhya D, Sonkhya N. Evaluation of hearing loss in patients with type 2 diabetes mellitus. Int J Res Med Sci. 2016;4(6):2281–2287. doi:10.18203/2320-6012.ijrms20161800
- Abraham AM, Varghese A, Jacob JJ. Prevalence of hearing loss in type 2 diabetes mellitus and its association with severity of diabetic neuropathy; 2022.
- Stephen YS, Roberts IK, Ahmad SA, Hassan SJ, Kingsley O. Hearing thresholds among type 2 diabetics in Sokoto, Nigeria: a Comparative Study. Int J Innovat Res Dev. 2017;6(3):184–190.
- Islam MR, Khan NA. Threats, vulnerability, resilience and displacement among the climate change and natural disaster-affected people in South-East Asia: an overview. J Asia Pac Econ. 2018;23(2):297–323. doi:10.1080/13547860.2018.1442153
- Mulwafu W, Kuper H, Ensink R. Prevalence and causes of hearing impairment in Africa. Trop Med Int Health. 2016;21(2):158–165. doi:10.1111/tmi.12640
- Lerman-Garber I, Cuevas-Ramos D, Valdés S, et al. Sensorineural hearing loss-a common finding in early-onset type 2 diabetes me llitus. Endocr Pract. 2012;18(4):549–557. doi:10.4158/EP11389.OR
- Morrison C, Morar P, Morrison G, Purewal T, Weston P. Hearing loss and type 2 diabetes: is there a link? Pract Diabetes. 2014;31(9):366–369. doi:10.1002/pdi.1904
- Li J, Zhang Y, Fu X, et al. Alteration of auditory function in type 2 diabetic and pre-diabetic patients. Acta Oto Laryngologica. 2018;138(6):542–547. doi:10.1080/00016489.2017.1422084
- Okwiri N. Prevalence and Pattern of Sensorineural Hearing Impairment Among Patients with Type 2 Diabetes Mellitus at the Kenyatta National Hospital. University of Nairobi; 2016.
- Musani MA, Rauf A, Ahsan M, Khan FA. Frequency and causes of hearing impairment in tertiary care center. JPMA. 2011;61(2):141.
- Yazew KG, Walle TA, Azagew AW. Prevalence of anti-diabetic medication adherence and determinant factors in Ethiopia: a systemic review and meta-analysis, 2019. Int J Africa Nurs Sci. 2019;11:100167. doi:10.1016/j.ijans.2019.100167
- Daniel E. Noise and hearing loss: a review. J Sch Health. 2007;77(5):225–231. doi:10.1111/j.1746-1561.2007.00197.x
- Jang T-W, Kim B-G, Kwon Y-J, H-J I. The association between impaired fasting glucose and noise-induced hearing loss. J Occup Health. 2011;53(4):1106070189.
- Nemati S, Hassanzadeh R, Mehrdad M, Kia SS. Hearing status in patients with type 2 diabetes mellitus according to blood-sugar control: a comparative study. Iranian J Otorhinolaryngol. 2018;30(99):209.
- Fukui M, Kitagawa Y, Nakamura N, et al. Idiopathic sudden hearing loss in patients with type 2 diabetes. Diabetes Res Clin Pract. 2004;63(3):205–211. doi:10.1016/j.diabres.2003.09.013
- Ren H, Wang Z, Mao Z, et al. Hearing loss in type 2 diabetes in association with diabetic neuropathy. Archiv Med Res. 2017;48(7):631–637. doi:10.1016/j.arcmed.2018.02.001
- Sachdeva K, Azim S. Sensorineural hearing loss and type II diabetes mellitus. Int J Otorhinolaryngol Head Neck Surg. 2018;4(2):499–507. doi:10.18203/issn.2454-5929.ijohns20180714
- Wei PZ, Szeto CC. Mitochondrial dysfunction in diabetic kidney disease. Clin Chim Acta. 2019;496:108–116. doi:10.1016/j.cca.2019.07.005
- Virgin Joena M. Eighth Nerve Function in Type 2 Diabetes Mellitus. Madurai: Madurai Medical College; 2012.
- Bener A, Hanoglu L, Cincik H, Oztürk M. Is neuropathy and hypertensionassociated with increased risk of hearing loss among type 2 diabetic patients? Int J Behav Sci. 2017;1(2):41–46.
- Srinivas C, Shyamala V, Shiva Kumar B. Clinical study to evaluate the association between sensorineural hearing loss and diabetes mellitus in poorly controlled patients whose HbA1c> 8. Indian J Otolaryngol Head Neck Surg. 2016;68(2):191–195. doi:10.1007/s12070-016-0973-5
- Bear MF, Connors BW, Paradiso MA. Neuroscience Exploring the Brain. 3rd ed. New York, London: Lippincott Williams & Wilkins; 2007.
- Samocha-Bonet D, Wu B, Ryugo DK. Diabetes mellitus and hearing loss: a review. Ageing Res Rev. 2021;71:101423. doi:10.1016/j.arr.2021.101423
- Nukada H. Ischemia and diabetic neuropathy. Handbook Clin Neurol. 2014;126:469–487.
- Bener A, Salahaldin AH, Darwish SM, Al-Hamaq A, Gansan L. Association between hearing loss and type 2 diabetes mellitus in elderly people in a newly developed society. Biomed Res. 2008;19(3):187–195.
- Adebola SO, Olamoyegun MA, Sogebi OA, Iwuala SO, Babarinde JA, Oyelakin AO. Otologic and audiologic characteristics of type 2 diabetics in a tertiary health institution in Nigeria. Brazilian J Otorhinolaryngol. 2016;82(5):567–573. doi:10.1016/j.bjorl.2015.10.016
- Toyama K, Mogi M. Hypertension and the development of hearing loss. Hypertens Res. 2022;45(1):172–174. doi:10.1038/s41440-021-00789-w
- Lee JS, Choi HG, Jang JH, et al. Analysis of predisposing factors for hearing loss in adults. J Korean Med Sci. 2015;30(8):1175–1182. doi:10.3346/jkms.2015.30.8.1175
- Csige I, Ujvárosy D, Szabó Z, et al. The Impact of Obesity on the Cardiovascular System. J Diabetes Res. 2018;2018:3407306. doi:10.1155/2018/3407306
- Rodrigues JCL, McIntyre B, Dastidar AG, et al. The effect of obesity on electrocardiographic detection of hypertensive left ventricular hypertrophy: recalibration against cardiac magnetic resonance. J Human Hypertens. 2016;30(3):197–203. doi:10.1038/jhh.2015.58
- Nasir JM, Rubal BJ, Jones SO, Shah AD. The effects of body mass index on surface electrocardiograms in young adults. J Electrocardiol. 2012;45(6):646–651. doi:10.1016/j.jelectrocard.2012.07.022
- Hulett NA, Scalzo RL, Reusch JE. Glucose uptake by skeletal muscle within the contexts of type 2 diabetes and exercise: an integrated approach. Nutrients. 2022;14(3):647. doi:10.3390/nu14030647
- World Medical Association. World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191–2194. doi:10.1001/jama.2013.281053