Abstract
Purpose
Arterial stiffness is often increased in overweight or obese individuals before the development of hypertension (HT). This study aimed to determine the connection between pancreatic fat and atherosclerosis in overweight and obese people without HT.
Patients and methods
We included 128 patients who were non-hypertensive and overweight or obese in a study between December 2019 and November 2022. Medical history was collected, and all participants underwent a physical examination and blood tests. Pancreatic fat content was measured by magnetic resonance imaging (MRI) and was grouped into quartiles based on pancreatic fat fraction (PFF). The upper three quartiles (PFF≥10.33%) were defined as non-alcoholic fatty pancreas disease (NAFPD) and the first quartile (PFF<10.33%) as non-NAFPD. High baPWV (H-baPWV) and low baPWV (L-baPWV) were classified according to the median baPWV (1159 cm/s). The effect of NAFPD on baPWV was examined using binary logistic regression. The study population consisted of 96 NAFPD and 32 non-NAFPD cases.
Results
Participants with NAFPD had significantly higher levels of baPWV than people without. The rates of NAFPD and the PFF values varied significantly in the L-baPWV and H-baPWV groups. Logistic regression analysis suggested that the presence of NAFPD was independently correlated with increased baPWV after adjusting for age, smoking, body mass index, blood pressure, lipid profiles, and glycemic index.
Conclusion
NAFPD is an independent risk factor for increased baPWV in individuals with overweight and obesity but no HT, suggesting that the presence of NAFPD may be a warning signal of early atherosclerosis.
Introduction
Obesity is an independent risk factor for cardiovascular disease (CVD) and influences the process of other cardiovascular risk factors such as dyslipidemia, hypertension (HT), and type 2 diabetes mellitus (T2DM).Citation1 An expanding corpus of studies indicates that compared with subcutaneous fat, visceral fat is a more reliable indicator of cardiovascular risk.Citation2,Citation3 Non-alcoholic fatty pancreas disease (NAFPD), a type of visceral obesity, is also correlated with CVD risk factors.Citation4 However, whether NAFPD directly contributes to an increased risk of CVD is unknown.
Few studies have shown that NAFPD is related to subclinical atherosclerosis. Kim et al found that computed tomography (CT)-assessed fatty pancreas was directly correlated with higher carotid intima-media thickness (IMT) and brachial-ankle pulse wave velocity (baPWV) in individuals with no obesity and T2DM but not in patients with obesity and T2DM.Citation5 Ozturk et al and Sahin et al reported that pancreatic fat accumulation measured using transabdominal ultrasonography was associated with higher carotid IMT among people with non-alcoholic fatty liver disease (NAFLD).Citation6,Citation7 Ultrasonography-determined NAFPD and elevated aortic IMT were also reported to be independently associated in a Turkish study.Citation8 Moreover, a Chinese study suggested that CT-estimated pancreatic fat content correlated with carotid plaque in patients with T2DM.Citation9 Yet another study found a direct link between carotid artery calcification and pancreatic fat.Citation10 However, the direct connection between NAFPD and atherosclerosis became weaker after adjusting for confounding factors. Additionally, this association is further complicated by differences in the methods for quantifying pancreatic fat and the characteristics of the individuals. Hence, further research in this field is required.
Aim
In the present research, we sought to analyze the connection between baPWV and pancreatic fat quantified using magnetic resonance imaging (MRI) in individuals with non-hypertension and overweight or obesity. MRI, which is non-invasive, nonradiative, and highly accurate, is regarded as the best imaging tool for quantifying intrapancreatic fat deposition in clinical practice.Citation11,Citation12 Arterial stiffness, one of the early markers of increased CVD risk, frequently increases in patients who are overweight or obese prior to the onset of hypertension.Citation13 Therefore, our study population included individuals without hypertension to exclude the interference of hypertension in atherosclerosis.Citation14,Citation15
Methods
Study Population
This research adopted baseline data of patients with overweight and obesity attending the weight-loss clinic of the affiliated Wuxi People’s Hospital of Nanjing Medical University for individualized multidisciplinary weight management (ChiCTR1900022948). This research was authorized by the hospital’s ethics committee and carried out in line with the Declaration of Helsinki (KS2019020). Each participant provided written informed consent.
One hundred twenty-eight eligible participants were enrolled in this study between December 2019 and November 2022. The requirements for inclusion were: age between 18–65 years, body mass index (BMI) ≥ 28.0 kg/m2 or ≥ 24.0 kg/m2 with one or more comorbidities (dyslipidemia, sleep apnea, or abnormal glucose metabolism), and steady weight over the past 3 months. The Working Group on Obesity in China recommends a BMI of 24.0 kg/m2 as the cutoff point for overweight and a BMI of 28.0 kg/m2 as the cutoff point for obesity.Citation16 Individuals with the following conditions were excluded: (1) medical history of HT or a baseline systolic blood pressure (SBP) ≥140 mmHg or diastolic blood pressure (DBP) ≥90 mmHg; (2) secondary obesity (such as Cushing’s syndrome and hypothyroidism) or drug usage to change fat metabolism; (3) consumption of alcohol per day: intake >10g in females and >20g in males (Supplementary Table); (4) pancreatitis, pancreatic cancer, or any other types of pancreatic illnesses; (5) medical history of malignancy, cardiovascular disease, or severe liver or kidney dysfunction; and (6) inappropriate for MRI examination (metallic substance implanted in the body, a history of claustrophobia, or pregnancy).
Medical history was gathered from each participant, as well as physical examination, blood tests, arterial stiffness testing, and pancreatic MRI. These tests were completed within 7 days of the first visit.
Anthropometric and Laboratory Measurements
All clinical evaluations were conducted by qualified personnel following recognized procedures. Body weight and height were recorded using a calibrated scale with participants dressed in light attire and barefoot. BMI was computed as follows: weight (kg)/height squared (m2). Waist circumference (WC) was determined by measuring the midpoint of the lowest rib and the superior border of the iliac crest with a regular tape measure. Waist-to-height ratio (WHtR) was computed as follows: WC (cm)/height (cm). After a 15-min rest period, SBP and DBP were assessed twice using a mercury gravity manometer with the individual in the seated position.
After fasting for at least 10 h overnight, venous blood samples were gathered to measure total cholesterol (TC), triglyceride (TG), high-density lipoprotein cholesterol (HDL-C), alanine aminotransferase(ALT); aspartate aminotransferase(AST), low-density lipoprotein cholesterol (LDL-C), and fasting blood glucose (FBG) levels using photometric assays (Chemistry Immunoanalyzer AU5800, Beckman Coulter, USA). Glycosylated hemoglobin (HbA1c) levels were measured using high-pressure liquid chromatography (VARIANT II Hemoglobin Testing System, BIORAD, USA).
Assessment of baPWV
baPWV, which can be measured by a straightforward and nonintrusive technique, reflects arterial stiffness and was determined using a BP-203RPE III detection device (Omron Health Medical Co., Ltd., China). The detection procedure was performed by trained nurses following the manufacturer’s recommendations. Cigarettes, alcohol, tea, and coffee were prohibited for 30 min before testing. After more than 5 min of rest, the participants underwent baPWV measurements in the supine position. Both arms and ankles were fitted with blood pressure cuffs that were linked to plethysmographic and oscillometric pressure sensors. A heart sound detector was positioned at the left margin of the sternal border. This procedure was performed twice for each participant, and the results from the second examination were valuable. The mean baPWV values of the left and right sides were used in the analysis. Participants were separated into high baPWV (H-baPWV) and low baPWV (L-baPWV) groups based on the median value of baPWV (1159 cm/s).
Assessment of NAFPD
The six-point Dixon technique was used to determine pancreatic fat content using a 3T MRI system (SIEMENS 3.0T MAGNETOM Prisma) with an eighteen-channel body array coil. The imaging parameters were as follows: TE1, 2.38 ms; TE2, 4.76 ms; TE3, 7.15 ms; TE4, 9.53 ms; TE5, 11.91 ms; TE6, 14.29 ms; TR, 15.60 ms; flip angle, 4°; FOV, 420 mm × 420 mm, and a 3.5-mm slice thickness. The Philips IntelliSpace Portal software was used with MRI workstations for post-processing of the mDixon sequence images. A trained radiologist manually drew the outline of the entire pancreas at each level of the proton density fat fraction (PDFF) maps to calculate the average pancreatic fat fraction (PFF). Based on the PFF, pancreatic fat was divided into quartiles. The 25th percentile corresponds to a PFF value of 10.33%. The first quartile of PFF (PFF<10.33%) was considered non-NAFPD and the upper three quartiles (PFF≥10.33%) were defined as NAFPD.
Statistical Analysis
In this cross-sectional study, the descriptive data are displayed as means ± standard deviations or medians (first quartile, third quartile). The chi-square statistical test was used for categorical variables, whereas Student’s t-test or the Mann–Whitney U-test was used for continuous variables. Spearman correlation analysis was used to evaluate the relationship between baPWV and many variables. To assess the effect of NAFPD on baPWV, odds ratios (OR) and 95% confidence intervals (CI) were calculated by binary logistic regression analysis. Statistical analyses were conducted using SPSS software version 27.0 (Armonk, NY: IBM Corp). A P-value of less than 0.05 was considered significant.
Results
Demographic and Clinical Characteristics of Study Participants with the Presence or Absence of NAFPD
As shown in , individuals with NAFPD tended to be male, smokers, and older. In the NAFPD group, the levels of ALT and AST were 1.7 and 1.3 times greater, respectively, than those in the non-NAFPD group. This group also had higher BMI, WC, WHtR, and HbA1c. Moreover, compared with individuals without NAFPD, those with NAFPD had higher baPWV values (1159.68 [1087.00, 1249.62] vs 1109.50 [1032.37, 1167.79] cm/s, P=0.032). However, lipid levels (TG, TC, LDL-C, HDL-C, TG/HDL-C), FBG levels, HOMA-IR, prevalence of diabetes, and blood pressure (SBP, DBP) did not differ significantly between the two groups.
Table 1 Characteristics of Study Participants with and without NAFPD
Demographic and Clinical Characteristics of Study Participants According to baPWV Value
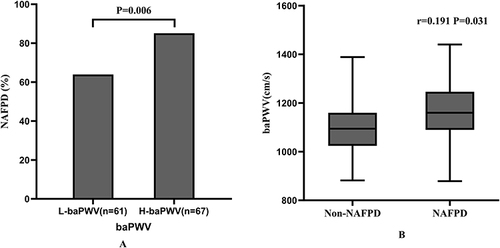
As shown in , the proportion of patients with diabetes in the H-baPWV group was nearly four times higher than that in the L-baPWV group; further, the proportion of smokers in the H-baPWV group was twice that in the L-baPWV group. Participants in the H-baPWV group were 1.2 times older than those in the L-baPWV group, and TG and AST levels were both 1.3 times higher than those in the L-baPWV group. The H-baPWV group also had higher SBP, DBP, TG/HDL-C, HbA1c, and FBG levels. However, the difference in sex distribution, BMI, WC, WHtR, TC, LDL-C, HDL-C, ALT, or HOMA-IR between the two groups was not statistically significant.
Table 2 Characteristics of Study Participants According to baPWV
The univariate analysis showed a positive association between baPWV and age (r=0.317, P<0.001), SBP (r=0.239, P=0.007), DBP (r=0.303, P<0.001), HbA1c (r=0.194, P=0.029), and FBG (r=0.225, P<0.011). However, there was no association between baPWV and BMI, ALT, AST, HOMA-IR, and lipid profiles. ()
Table 3 Univariate Correlation Coefficients of baPWV
Relationship Between NAFPD and baPWV
Comparisons between the H-baPWV and L-baPWV groups revealed that individuals with H-baPWV had higher PFFs (14.76 [11.85,17.52] vs.12.43 [8.64,15.74] %, P=0.003) as well as a greater prevalence of NAFPD (85.1 vs 63.9%, P=0.006) (). Furthermore, the presence of NAFPD was strongly related with baPWV (r=0.191, P=0.031) ().
Impact of NAFPD on baPWV After Adjusting for Confounding Factors
The impact of pancreatic fat on atherosclerosis was evaluated using logistic regression analysis. Individuals with NAFPD, compared with those without, had an OR of 3.22 (95% CI: 1.38–7.53, P=0.007) for H-baPWV. After adjusting for age, smoking, BMI, BP, lipid profiles, hepatic enzymes, FBG, HOMA-IR, and HbA1c, the ORs of NAFPD on baPWV were slightly attenuated, but remained significant ().
Table 4 Unadjusted and Adjusted Odds Ratios (ORs) of baPWV by NAFPD
Discussion
This study demonstrated that people with overweight and obesity but no HT, with MRI-proven NAFPD, had increased baPWV compared with those without NAFPD. In contrast, individuals with H-baPWV had a higher PFF than those with L-baPWV. Furthermore, logistic regression analysis demonstrated that the presence of NAFPD was independently correlated with increased baPWV. These results indicate that the likelihood of developing CVD can be increased by the presence of NAFPD, suggesting that individuals with NAFPD require a more proactive approach to assess their cardiovascular risk.
There are several risk factors for atherosclerosis, including age, blood pressure, and smoking.Citation17 In addition, a stronger correlation between physical activity and arterial stiffness than other risk factors has been observed in older people.Citation18 Therefore, the effect of old age should be taken into account. Studies have shown that age also affects the amount of fat in the pancreas.Citation19,Citation20 A cross-sectional study showed that the volume of pancreatic fat plateaus between the ages of 20 and 60 years.Citation19 The age range of our actual enrollment population was 18 to 53 years old, and the average age was 32 years old. Therefore, age grouping was not performed. Furthermore, smoking and alcohol consumption are also important confounding factors. Smoking and alcohol consumption are strongly associated with subclinical atherosclerosisCitation21,Citation22 and studies have shown that smoking is a more influential factor in NAFPD than alcohol consumption.Citation23 In this study, we excluded people who consumed excessive amounts of alcohol. Consequently, to clarify the confounding effect of these factors, we performed multiple logistic analysis adjusted for age, smoking, and other confounding factors.
Some studies have suggested that NAFPD is correlated with different non-invasive indicators of atherosclerosis, such as IMT, carotid plaque, carotid-femoral PWV (cfPWV), and carotid artery calcification.Citation5–10 Most of these studies reported that pancreatic steatosis is independently correlated with atherosclerosis markers after adjusting for confounders,Citation7–9 which is consistent with our observations. However, in a study by Ozturk et al, the association of fatty pancreas with cfPWV and carotid IMT ceased to be significant after adjusting for confounding factors.Citation6 Kim et al indicated that a significant correlation between fatty pancreas and carotid atherosclerosis remained in non-obese individuals with T2DM, but not in the obese group, after adjusting for age, sex, and BMI.Citation5 A large community-based cohort study that evaluated the association between pancreatic fat and systemic calcified atherosclerosis demonstrated that NAFPD was only independently linked to carotid calcification.Citation10 Thus, it is clear that conventional risk factors have some effect on the relationship between fatty pancreas and atherosclerosis. In addition, the study populations in previous studies mainly involved patients with NAFLDCitation6,Citation7 and T2DM,Citation5,Citation9 whereas the participants selected for this study were patients with overweight and obesity.
To the best of our knowledge, this is the first study to assess the relationship between MRI-determined NAFPD and atherosclerosis; previous studies used ultrasound or CT to measure NAFPD. Comparing the echogenicity of the pancreas with that of the liver or kidney is somewhat subjective; therefore, pancreatic fat cannot be quantitatively measured using ultrasound.Citation24 While pancreatic fat can be visualized with CT, it is challenging to accurately measure the tissue fat content of the pancreas with CT.Citation25 In addition, the radioactivity of CT restricts it from being widely used in clinical studies. Therefore, MRI is the ideal imaging modality for precisely quantifying pancreatic fat because of its nonradiative nature and high accuracy.Citation12,Citation26
In this study, we used baPWV to evaluate arterial stiffness. As stated in earlier reports, cfPWV is considered the highest standard for evaluating arterial stiffness;Citation27 however, cfPWV is not suitable for routine clinical use because of its high cost and inconvenience associated with measuring devices. Based on the current evidence,Citation28 baPWV and cfPWV exhibit similar associations with atherosclerotic CVD. Because of its simplicity and convenience, baPWV is an ideal method for assessing early atherosclerosis in clinical practice.Citation29 Carotid IMT and plaques are also reliable indicators of atherosclerosis.Citation30,Citation31 Compared with carotid IMT or arterial stiffness, carotid plaques signify the advanced stages of atherosclerosis.Citation32 Carotid IMT quantitatively assesses arterial morphology composed of intimal lesions,Citation33 whereas baPWV reflects central arterial stiffness.Citation29 However, carotid IMT measurements are influenced by the operating physician.
Our study has some limitations. First, owing to the strict inclusion criteria, the sample size was small. Second, the cross-sectional design made it impossible to identify causal connections. Third, we did not examine the pancreatic histology; pancreatic biopsy is highly invasive and cannot be performed ethically in healthy individuals. Therefore, we used MRI which is the best non-invasive test for pancreatic fat. Finally, all of the participants were Chinese, aged between 18 and 65, and were overweight or obese. Studies have shown racial differences in the prevalence of NAFPD and propensity for high PFF. The prevalence of NAFPD was 16.1% in Hong Kong,Citation34 30.7% in mainland China,Citation35 35% in Indonesia,Citation36 and 27.8% in America.Citation37 The differences are partly influenced by the method of examination and ethnicity. Several studies have looked into the likelihood of having high PFF in different racial and/or ethnic groups and found that PFF occurrence was highest among individuals of East/South-East/South Asian descent and lowest among Black African people.Citation38–41 All published investigations, however, were restricted to comparing no more than two or three racial or ethnic groups. Combined with previous studies, Sun et al’s study in Asia supported the role of NAFPD as an independent predictor of atherosclerosis,Citation9 similar to our conclusion. Therefore, more research across more ethnic groups is required to draw more definitive conclusions.
Conclusion
In summary, this research demonstrated that the presence of NAFPD is associated with an increased risk of atherosclerosis in individuals with no HT but with overweight and obesity. The findings we obtained support the potential role of pancreatic fat in the development of atherosclerosis. Therefore, it is necessary to assess cardiovascular risk in patients with NAFPD for the early prevention of CVD.
Author Contributions
Wenjun Wu used to work at The Affiliated Wuxi People’s Hospital of Nanjing Medical University and now works at Jinshan Branch of Shanghai Sixth People’s Hospital.
Disclosure
The authors report no conflicts of interest in this work.
Acknowledgments
Chenxi Li and Xiaolei Chen are co-first authors for this study. We are grateful to Editage for their assistance with English language editing.
Data Sharing Statement
All Data has been provided in the manuscript and no further Data will be shared.
Additional information
Funding
References
- Powell-Wiley TM, Poirier P, Burke LE, et al. Obesity and cardiovascular disease: a scientific statement from the American heart association. Circulation. 2021;143(21):e984–e1010. doi:10.1161/cir.0000000000000973
- Shah RV, Murthy VL, Abbasi SA, et al. Visceral adiposity and the risk of metabolic syndrome across body mass index: the Mesa Study. JACC Cardiovasc Imaging. 2014;7(12):1221–1235. doi:10.1016/j.jcmg.2014.07.017
- Kaess BM, Pedley A, Massaro JM, Murabito J, Hoffmann U, Fox CS. The ratio of visceral to subcutaneous fat, a metric of body fat distribution, is a unique correlate of cardiometabolic risk. Diabetologia. 2012;55(10):2622–2630. doi:10.1007/s00125-012-2639-5
- Singh RG, Yoon HD, Wu LM, Lu J, Plank LD, Petrov MS. Ectopic fat accumulation in the pancreas and its clinical relevance: a systematic review, meta-analysis, and meta-regression. Metabolism. 2017;69:1–13. doi:10.1016/j.metabol.2016.12.012
- Kim MK, Chun HJ, Park JH, et al. The association between ectopic fat in the pancreas and subclinical atherosclerosis in type 2 diabetes. Diabetes Res Clin Pract. 2014;106(3):590–596. doi:10.1016/j.diabres.2014.09.005
- Ozturk K, Dogan T, Celikkanat S, et al. The association of fatty pancreas with subclinical atherosclerosis in nonalcoholic fatty liver disease. Eur J Gastroenterol Hepatol. 2018;30(4):411–417. doi:10.1097/meg.0000000000001059
- Sahin S, Karadeniz A. Pancreatic fat accumulation is associated with subclinical atherosclerosis. Angiology. 2022;73(6):508–513. doi:10.1177/00033197211038334
- Kul S, Karadeniz A, Dursun İ, et al. Non-alcoholic fatty pancreas disease is associated with increased epicardial adipose tissue and aortic intima-media thickness. Acta Cardiologica Sinica. 2019;35(2):118–125. doi:10.6515/acs.201903_35(2).20181009a
- Sun P, Fan C, Wang R, et al. Computed tomography-estimated pancreatic steatosis is associated with carotid plaque in type 2 diabetes mellitus patients: a cross-sectional study from China. Diabete Metab Synd Obes. 2021;14:1329–1337. doi:10.2147/dmso.S299060
- Koo BK, Denenberg JO, Wright CM, Criqui MH, Allison MA. The association between pancreatic fat and systemic calcified atherosclerosis. Pancreas. 2020;49(1):e16–e18. doi:10.1097/mpa.0000000000001447
- Idilman IS, Tuzun A, Savas B, et al. Quantification of liver, pancreas, kidney, and vertebral body MRI-PDFF in non-alcoholic fatty liver disease. Abdominal Imaging. 2015;40(6):1512–1519. doi:10.1007/s00261-015-0385-0
- Kameda F, Tanabe M, Onoda H, et al. Quantification of pancreas fat on dual-energy computed tomography: comparison with six-point Dixon magnetic resonance imaging. Abdom Radiol. 2020;45(9):2779–2785. doi:10.1007/s00261-020-02583-7
- Femia R, Kozakova M, Nannipieri M, et al. Carotid intima-media thickness in confirmed prehypertensive subjects: predictors and progression. Arteriosclerosis Thrombosis Vasc Biol. 2007;27(10):2244–2249. doi:10.1161/atvbaha.107.149641
- Wang Y, Yuan Y, Gao WH, et al. Predictors for progressions of brachial-ankle pulse wave velocity and carotid intima-media thickness over a 12-year follow-up: hanzhong adolescent hypertension study. J Hypertens. 2019;37(6):1167–1175. doi:10.1097/hjh.0000000000002020
- Sheng S. Mean arterial pressure and arterial stiffness in Japanese population: a secondary analysis based on a cross-sectional study. Blood Pressu Monit. 2020;25(6):310–317. doi:10.1097/mbp.0000000000000471
- Zhou BF. Predictive values of body mass index and waist circumference for risk factors of certain related diseases in Chinese adults--study on optimal cut-off points of body mass index and waist circumference in Chinese adults. Biomed Environ Sci. 2002;15(1):83–96.
- Lopes-Vicente WRP, Rodrigues S, Cepeda FX, et al. Arterial stiffness and its association with clustering of metabolic syndrome risk factors. Diabetol Metab Syndr. 2017;9:87. doi:10.1186/s13098-017-0286-1
- Hill H, Elliot CA, Lizamore CA, Hamlin MJ. Physical activity has a stronger correlation with arterial stiffness than strength, balance, or BMI in an older population. Front Aging. 2023;4:1279479. doi:10.3389/fragi.2023.1279479
- Saisho Y, Butler AE, Meier JJ, et al. Pancreas volumes in humans from birth to age one hundred taking into account sex, obesity, and presence of type-2 diabetes. Clin Anat. 2007;20(8):933–942. doi:10.1002/ca.20543
- Murakami R, Saisho Y, Watanabe Y, et al. Pancreas fat and β cell mass in humans with and without diabetes: an analysis in the Japanese population. J Clin Endocrinol Metab. 2017;102(9):3251–3260. doi:10.1210/jc.2017-00828
- Hisamatsu T, Miura K, Arima H, et al. Smoking, smoking cessation, and measures of subclinical atherosclerosis in multiple vascular beds in Japanese men. J Am Heart Assoc. 2016;5:9.
- Laguzzi F, Baldassarre D, Veglia F, et al. Alcohol consumption in relation to carotid subclinical atherosclerosis and its progression: results from a European longitudinal multicentre study. Eur J Nutr. 2021;60(1):123–134. doi:10.1007/s00394-020-02220-5
- Stuart CE, Ko J, Modesto AE, et al. Implications of tobacco smoking and alcohol consumption on ectopic fat deposition in individuals after pancreatitis. Pancreas. 2020;49(7):924–934. doi:10.1097/mpa.0000000000001600
- Hung CS, Tseng PH, Tu CH, et al. Increased pancreatic echogenicity with US: relationship to glycemic progression and incident diabetes. Radiology. 2018;287(3):853–863. doi:10.1148/radiol.2018170331
- Koç U, Taydaş O. Evaluation of pancreatic steatosis prevalence and anthropometric measurements using non-contrast computed tomography. Turk J Gastroenterol. 2020;31(9):640–648. doi:10.5152/tjg.2020.19434
- Chen Y, Long L, Jiang Z, Zhang L, Zhong D, Huang X. Quantification of pancreatic proton density fat fraction in diabetic pigs using MR imaging and IDEAL-IQ sequence. BMC Med Imaging. 2019;19(1):38. doi:10.1186/s12880-019-0336-2
- Van Bortel LM, Laurent S, Boutouyrie P, et al. Expert consensus document on the measurement of aortic stiffness in daily practice using carotid-femoral pulse wave velocity. J Hypertens. 2012;30(3):445–448. doi:10.1097/HJH.0b013e32834fa8b0
- Tanaka H, Munakata M, Kawano Y, et al. Comparison between carotid-femoral and brachial-ankle pulse wave velocity as measures of arterial stiffness. J Hypertens. 2009;27(10):2022–2027. doi:10.1097/HJH.0b013e32832e94e7
- Sánchez Bacaicoa C, Rico-Martín S, Morales E, et al. Brachial-ankle pulse wave velocity with a custom device. Rev Clin Esp. 2021;221(3):145–150. doi:10.1016/j.rceng.2019.12.008
- Fernández-Alvarez V, Linares Sánchez M, López Alvarez F, et al. Evaluation of Intima-media thickness and arterial stiffness as early ultrasound biomarkers of carotid artery atherosclerosis. Cardiol Ther. 2022;11(2):231–247. doi:10.1007/s40119-022-00261-x
- Johri AM, Nambi V, Naqvi TZ, et al. Recommendations for the assessment of carotid arterial plaque by ultrasound for the characterization of atherosclerosis and evaluation of cardiovascular risk: from the American society of echocardiography. J Am Soc Echocardiography. 2020;33(8):917–933. doi:10.1016/j.echo.2020.04.021
- Kurkowska-Jastrzebska I, Karlinski MA, Błazejewska-Hyzorek B, Sarzynska-Dlugosz I, Filipiak KJ, Czlonkowska A. Carotid intima media thickness and blood biomarkers of atherosclerosis in patients after stroke or myocardial infarction. Croatian Med J. 2016;57(6):548–557. doi:10.3325/cmj.2016.57.548
- Simon A, Gariepy J, Chironi G, Megnien JL, Levenson J. Intima-media thickness: a new tool for diagnosis and treatment of cardiovascular risk. J Hypertens. 2002;20(2):159–169. doi:10.1097/00004872-200202000-00001
- Wong VW, Wong GL, Yeung DK, et al. Fatty pancreas, insulin resistance, and β-cell function: a population study using fat-water magnetic resonance imaging. Am J Gastroenterol. 2014;109(4):589–597. doi:10.1038/ajg.2014.1
- Zhou J, Li ML, Zhang DD, et al. The correlation between pancreatic steatosis and metabolic syndrome in a Chinese population. Pancreatology. 2016;16(4):578–583. doi:10.1016/j.pan.2016.03.008
- Lesmana CR, Pakasi LS, Inggriani S, Aidawati ML, Lesmana LA. Prevalence of Non-Alcoholic Fatty Pancreas Disease (NAFPD) and its risk factors among adult medical check-up patients in a private hospital: a large cross sectional study. BMC Gastroenterol. 2015;15:174. doi:10.1186/s12876-015-0404-1
- Sepe PS, Ohri A, Sanaka S, et al. A prospective evaluation of fatty pancreas by using EUS. Gastrointest Endosc. 2011;73(5):987–993. doi:10.1016/j.gie.2011.01.015
- Lê KA, Ventura EE, Fisher JQ, et al. Ethnic differences in pancreatic fat accumulation and its relationship with other fat depots and inflammatory markers. Diabetes Care. 2011;34(2):485–490. doi:10.2337/dc10-0760
- Szczepaniak LS, Victor RG, Mathur R, et al. Pancreatic steatosis and its relationship to β-cell dysfunction in humans: racial and ethnic variations. Diabetes Care. 2012;35(11):2377–2383. doi:10.2337/dc12-0701
- Roh E, Kim KM, Park KS, et al. Comparison of pancreatic volume and fat amount linked with glucose homeostasis between healthy Caucasians and Koreans. Diabetes Obesity Metab. 2018;20(11):2642–2652. doi:10.1111/dom.13447
- Hakim O, Bonadonna RC, Mohandas C, et al. Associations between pancreatic lipids and β-cell function in black African and White European men with type 2 diabetes. J Clin Endocrinol Metab. 2019;104(4):1201–1210. doi:10.1210/jc.2018-01809