Abstract
Objective
This study assessed possible associations among physical activity (PA), sitting time (ST), metabolic syndrome (MetS), and the individual components thereof. We analyzed the entire study sample and subpopulations stratified by visceral fat area (VFA). We hypothesized that individuals with elevated VFA might respond differently to modifiers of metabolic health, including PA and ST.
Methods
This cross-sectional study, conducted between March and May 2010, enrolled 957 adults with abdominal magnetic resonance imaging (MRI) aged 40–65 years living in the urban communities in Hangzhou, China. PA and ST were recorded using the standard International Physical Activity Questionnaire (IPAQ) and categorized into three levels. The ethnicity-specific cutoff for central obesity was VFA ≥ 80 cm2 on MRI according to Chinese population-based research. Multiple logistic regression models were used to analyze the associations between PA, ST, MetS and its components.
Results
In the total subject population, participants reporting high level of PA were at a lower risk of MetS (OR = 0.46, 95% CI: 0.25, 0.86) than those declaring low PA. In the subgroup population with VFA ≥ 80 cm2 (ie, with central obesity), moderate-to-high PA levels were associated with a lower risk of MetS (p for trend < 0.05) and a lower risk of decreased high-density lipoprotein cholesterol (HDL-C) concentrations (p for trend < 0.05). In addition, ST > 3 h/day was a risk factor for both MetS (p for trend < 0.05) and hypertriglyceridemia (p for trend < 0.05) in the total subject population. While in the central obesity subgroup, ST > 3 h/day was found a stronger risk factor.
Conclusion
Our study suggests that moderate-to-high levels of PA may have a role in prevention of MetS, and ST > 3 h/day was associated with a higher risk of MetS, particularly in individuals with central obesity.
Introduction
Non-communicable diseases (NCDs) are rapidly increasing in prevalence in both developed and underdeveloped countries, imposing a major burden on health systems worldwide. Notably, metabolic syndrome has become the major NCD. MetS is defined as a cluster of risk factors for both cardiovascular disease and type 2 diabetes mellitus. Although various institutions have presented slightly different definitions of MetS over the past decade, MetS can be broadly summarized as a combination of central obesity, abnormal glycemia, dyslipidemia, and hypertension.Citation1 Recent research indicates that, together with obesity, MetS affects more than 30% of all adults and approximately 5% of all adolescents worldwide.Citation2,Citation3
Both observational and interventional studies suggest that PA significantly reduces the risk of cardiometabolic issues and insulin-resistance.Citation4–6 Regular PA aids weight loss, lowers blood pressure, and improves dyslipidemia by raising the level of HDL-C and reducing that of triglycerides (TGs).Citation7–9 Emerging evidence shows that less sedentary time may also reduce metabolic risk.Citation10–12 Recently, the American Diabetes Association and equivalent bodies in other countries have added sedentary behavior guidelines to their PA recommendations.Citation13–15
Many researchers have shown that overweight and obesity are associated with a greater risk of MetS than is normal weight. Inappropriate body fat distribution constitutes a strong metabolic and cardiovascular risk factor. Larger VFA is associated with the development of obesity-related comorbidities and increased all-cause mortality; the level of abdominal subcutaneous adipose tissue (SAT) is a much weaker indicator of cardiovascular risk.Citation16,Citation17 Although the underlying mechanisms are not fully understood, three plausible suggestions have been made. First, the metabolic properties of visceral adipose tissue (VAT) may differ from those of SAT; second, excess VAT may induce inflammation; and third, VAT may be a marker of increased ectopic fat.Citation18,Citation19 Hence, identifying individuals with central obesity is important, and it is essential to initiate effective behavioral interventions at an early stage.
To date, no study has explored whether the two separate health consciousness behavior of PA and ST independently affect MetS risk by VFA status. Our study aims to evaluate associations among different PA, ST levels, and the risk of MetS and components thereof in Chinese Han individuals stratified by central obesity status.
Methods
Study Subjects
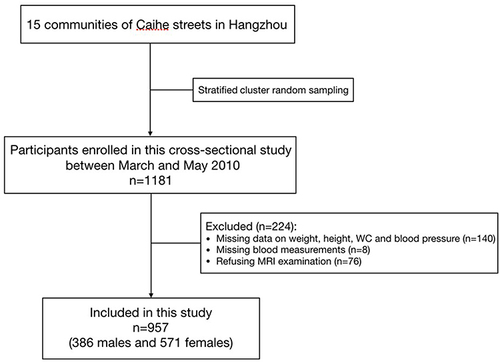
This population-based cross-sectional study was conducted between March and May 2010, located in Caihe Streets of Hangzhou, Zhejiang province, China, as documented previously.Citation20 Stratified cluster random sampling method was used. All residents aged 40–65 (residing for over 5 years) in the 15 communities of Caihe streets were stratified by gender. Within each gender, each community was divided into five groups according to age (40–45, 45–50, 50–55, 55–60, 60–65). Initially, stratified cluster random sampling was conducted in 10 communities. Then, the remaining 5 communities were also included in the sampling due to insufficient samples. A total number of 1181 Chinese Han participants aged 40–65 were enrolled in this study. The followings were excluded: 1) previous cardiovascular events, 2) oral or intravenous corticosteroids, 3) cirrhosis and ascites, 4) known hyperthyroidism or hypothyroidism, 5) malignant tumor, 6) severe disability or mental illness, 7) pregnancy. Written informed consent was signed with participants based on the inclusion and exclusion criteria. Of the 1181 participants, 140 were excluded with missing data on weight, height, waist circumference (WC) and blood pressure; 8 were excluded with missing data on blood measurements; 76 were further excluded due to refusing MRI examination. Finally, 957 participants (386 males and 571 females) with MRI data were included in the primary analysis (). The study protocol was approved by the Ethics Committee of Sir Run Run Shaw Hospital on 14 January 2010 and was conducted in accordance with the Declaration of Helsinki.
Figure 1 Flow chart of study population.
This study was part of the multicenter study on Abdominal Obesity and Metabolic Syndrome in China, which was conducted over a 3-year prospective period in populations from different regions of the country.Citation21 The baseline survey selected urban communities in eastern (Shanghai, Hangzhou), central-western (Chengdu), northern (Shenyang, Beijing), and southern (Guangzhou) cities. The total sample size was approximately 5000, including 1000 each in Shanghai and Hangzhou, and 750 each in Chengdu, Shenyang, Beijing and Guangzhou.
Measurements
Demographic variables and anthropometric data
Before the study began, the project working group established a well-trained research team (including medical investigators, community doctors, nurses and volunteers) and standardized operating procedures and quality control systems. We used questionnaires to collect the information about marital status, education, dietary pattern, physical activity, sitting time, sleep duration, history of alcohol consumption, smoking, tea drinking and disease including diabetes as well as hypertension. All participants were interviewed face-to-face by trained medical investigators. The main socio-demographic factors included: sex (men; women), age (40–65), marital status (married or remarriage; single or divorce/widowhood), education (illiterate or primary, junior high school, high school or university level), dietary pattern (meat-base, half meat half vegetarian or vegetarian).
Participants were informed in writing of the precautions to be taken (including fasting blood sampling, the OGTT test and the collection of urine samples) in advance. The project working group provided the standard instruments for basic anthropometric data, including height and weight measuring instruments, WC meters and blood pressure measurements. For 75-g oral glucose tolerance test (OGTT), participants were instructed to fast overnight for at least 10 h, and then blood samples were taken in central laboratory of Sir Run Run Shaw Hospital. Fasting plasma glucose (FPG), 2-hour post-load plasma glucose (2hPG), TG and HDL-C were measured with enzymatic method by auto analyzer (Aeroset, Chicago, IL, USA). Fasting serum insulin (FINS) levels were measured by chemiluminescent enzyme-linked immunosorbent assay using an insulin detection kit (Beijing North Institute Biological Technology, China). Insulin sensitivity was assessed by homeostatic model assessment for insulin resistance (HOMA-IR), calculated as: [FINS (mU/L) × FBG (mg/dl)/18] / 22.5.Citation22 Body Mass Index (BMI) was calculated by weight (kg) to the square of height (m).
After the demographic survey and the collection of blood and urine samples were completed, MRI examinations were carried out in batches by trained medical investigators. All subjects underwent MRI using a Signa 1.5 T MRI device equipped with an abdominal coil (SMT-100; Shimadzu Corp., Kyoto, Japan). The abdominal subcutaneous fat area (SFA) and abdominal VFA were obtained from the MRI scans at the umbilical level between L4 and L5, measured by two trained investigators using SliceOmatic image analysis software (version 5). The above measurement was based on the 2-D pixels in DICOM images fitting the “adipose shading threshold”.
Physical activity level and sitting time
Exercise data were collected by the International Physical Activity Questionnaire Short Form (IPAQ-SF),Citation23 the validity and reliability of which has been well established.Citation24,Citation25 Three specific types of activity namely walking, moderate and vigorous activity, were measured by the questionnaire. All subjects were asked to declare the duration in minutes per day and the average number of days per week of three physical activities. The volume of physical activity was computed by weighting each type of activity based on its energy requirements defined as metabolic equivalents (MET), and was presented in MET-min/week units. The MET score for each activity was defined as: walking = 3.3 MET, moderate PA = 4.0 MET, vigorous PA = 8.0 MET. And total MET-min/week units for each activity were calculated as: MET score × duration (min) × frequency (days). According to the criteria from IPAQ Research Committee, PA levels were classified into three groups: low, moderate, and high.Citation23 Since individual health benefits from regular physical activity, the grouping criteria consider not only the overall level of PA but also the frequency per week and duration per day. Sitting time during the last week was measured by IPAQ-SF as well. Then, we calculated the average number of hours spent sitting per day. Overall declared sitting time daily was categorized as ≤3 h/day, 3–6 h/day, and >6 h/day.
Definition
Metabolic syndrome was defined according to the Chinese Guidelines for the Prevention and Treatment of Type 2 Diabetes (2020 Edition),Citation26 wherein MetS was diagnosed when meeting three or more criteria. Five criteria were as follows: 1) abdominal obesity: WC ≥ 90 cm for men and ≥85 cm for women; 2) hyperglycemia: FPG ≥ 110 mg/dL or 2hPG ≥ 140 mg/dL and (or) those who have been diagnosed with diabetes and treated; 3) hypertension: blood pressure ≥ 130/85 mmHg and (or) confirmed hypertension and treated; 4) fasting TG ≥ 150 mg/dL; (5) fasting HDL-C < 40 mg/dL.
A history of smoking was defined as currently smoking or ever smoked. Alcohol use and tea drinking were defined as frequent or occasional drinking. Chinese population survey found that the optimal VFA cutoff was near 80 cm2 in identifying the MetS with two or more components (not including overweight/obesity by either of the two definitions).Citation27,Citation28 Therefore, we defined central obesity as VFA ≥ 80 cm2 in our study.
Statistical Analysis
Data were analyzed using SPSS Statistics 25.0 (IBM Corp., Armonk, NY, USA). Normally distributed continuous variables were presented as means ± standard deviation (SD), while the vast majority did not have satisfactory distribution of normality resulting in being presented as median (interquartile range). Categorical variables were presented in absolute and relative frequency. To compare means between subjects with and without MetS or central obesity, the t-test or Mann–Whitney U-test was applied. Differences among normally distributed continuous variables of three categories according to PA or ST were analyzed using one-way analysis of variance (ANOVA). Comparisons between non-normally distributed variables were performed by the non-parametric Kruskal–Wallis test. Categorical variables across different groups were compared using the Chi-squared test. Associations of three levels of PA and ST with the MetS as well as its components were examined using binary multivariable logistic regression analysis: model 1 without adjustment; model 2, adjusted for confounding variables (sex, age, BMI, smoke, alcohol use, tea drinking, sleep duration, education, dietary pattern, marital status, serum uric acid, total cholesterol, low-density lipoprotein cholesterol). Initially, demographic and behavioral risk factors were considered as potential confounders. Then variables associated with the MetS at p < 0.2 were retained as confounders. For avoiding multicollinearity, the risk factors strongly correlating with each other were not included. Three levels of ST and PA levels were also added as a confounder for each other’s exposure. A two-side p value < 0.05 was considered at a statistically significant level.
Results
Both PA and ST Were Associated with the Risk of MetS and Its Components
A total of 957 participants (386 males and 571 females) were included in the primary analysis; the mean age was 53.1 ± 6.8 years. Sociodemographic and lifestyle characteristics are listed in by MetS status. The prevalence rates of MetS in the overall sample, men and women were 16.4%, 25.4% and 10.3%, respectively. MetS patients smoked more, consumed more alcohol and tea, slept longer, and were older than those without MetS. Low PA was more common in those with than without MetS (29.9% vs 20.9%); more participants without MetS engaged in high PA compared to those with MetS (29.9% vs 19.7%). The daily ST was longer in patients with than without MetS; the proportion of individuals with MetS who sat for >6 h/day was much higher than that of those without MetS (32.5% vs 23.4%). Each MetS component occurred at a significantly higher frequency in those with than without MetS.
Table 1 Sociodemographic Characteristics, Lifestyle and Metabolic Parameters of the Total Sample and Across MetS Status
and list parameters of metabolic health according to PA level and ST. Participants declaring high PA exhibited superior health profiles, ie, smaller WC, lower VFA, TG, fasting insulin, HOMA-IR levels, and higher HDL-C concentrations, wherein WC, TG and HDL-C levels showed statistically significant differences with increased activity levels. A significant decrease in HDL-C was apparent in subjects with ST > 6 h/day compared to those with ST ≤ 3 h/day. Notably, the MetS prevalence rates were 22.0%, 16.7%, and 11.5% in those reporting low, moderate, and high PA levels, respectively (p = 0.008). Consistently, the MetS incidence increased with longer ST, being 13.2%, 16.3% and 21.4% among subjects reporting three levels of ST respectively (p = 0.029).
Table 2 Prevalence of Metabolic Parameters and MetS According to Physical Activity Levels
Table 3 Prevalence of Metabolic Parameters and MetS According to Sitting Time
Correlations of PA and ST with MetS Were Stronger When the VFA Level Was Considered
Individuals with VFA ≥ 80 cm2 (ie, with central obesity) had a significantly higher prevalence of MetS (34.5% vs 4.2%) and its components than those without central obesity (Table S1). Subjects of central obesity had fewer performance of high-level activities (25.9% vs 29.8%), comparable moderate activities (49.0% vs 49.7%), and more low-level activities (25.1% vs 20.5%) than those without central obesity. Likewise, there was a trend toward a higher performance of ST > 6 h/day in participants with central obesity (both p for trend were close to 0.1).
The odds ratios for MetS and components thereof after stratification by central obesity status are listed in . In both the total subject population and the central obesity subgroup, high PA was related to a lower risk of MetS compared to those declaring low PA. After adjustment, using low PA as the reference, a lower OR for MetS was found in high-PA individuals in the overall sample (OR = 0.46, 95% CI: 0.25, 0.86, p = 0.014), and this became even more significant in those with central obesity (OR = 0.33, 95% CI: 0.16, 0.70, p = 0.003). Notably, even moderate (compared to low-level) PA was significantly related to a lower MetS risk in the central obesity subgroup (OR = 0.54, 95% CI: 0.29, 0.99, p = 0.046). Moreover, high PA was inversely associated with hyperglycemia, and moderate-to-high PA correlated with a lower risk of decreased HDL-C concentrations in the central obesity subgroup both before and after adjustment.
Table 4 Odds Ratio for the MetS and Its Components Depending on Physical Activity (or, 95% CI)
ST > 6 h/day (compared to ≤3 h/day) was associated with a significantly higher MetS risk in the overall sample (). In adjusted analysis, participants who sat for 3–6 and >6 h/day exhibited 1.8-fold (95% CI: 1.06, 2.99) and almost 3-fold increased odds (95% CI: 1.69, 5.19) of MetS than those who sat for ≤3 h/day, respectively. STs of 3–6 and >6 h/day were even more closely correlated with an increased risk of MetS in individuals with central obesity.
Table 5 Odds Ratio for the MetS and Its Components Depending on Sitting Time (or, 95% CI)
A longer ST (>3 h), both in the total subject population and in the central obesity subgroup, was associated with an increased TG concentration. After adjustment for confounders, the odds of hypertriglyceridemia were even higher for participants in the central obesity subgroup with STs of 3–6 and >6 h/day compared to those in the overall sample. Neither PA nor ST was significantly associated with the risk of MetS or its components in the subgroup without central obesity.
Discussion
We found that high PA may have a role in prevention of MetS and that ST > 3 h/day was associated with a higher risk of MetS in the overall sample; above associations became stronger in those with central obesity.
In this community-based study, the overall prevalence of MetS was 16.4%, with a higher prevalence in males (25.4%) than females (10.3%), consistent with the results of a cross-sectional study on 33,149 employees in northeastern urban China; the rates of MetS in males and females were 24.5% and 15.4%, respectively.Citation29 The incidence of MetS varies by ethnicity, national development levels and diagnostic criteria of MetS. A recent prospective cohort study included 16,209 subjects in Amsterdam, the Netherlands, reported that the prevalence of MetS in Dutch was 20.6% for men and 9% for women; while for participants from African Surinamese descent, it was 15.4% for men and 14.9% for women; further, MetS occurred the most in Turkish, with 32.4% in men and 18% in women, respectively.Citation30 In most studies, the prevalence of MetS was higher in men than women aged <50–60 years despite differences in ethnicity and geography, as the proportion of central obesity in males is higher,Citation30,Citation31 suggesting a close correlation between central obesity and MetS status. Yet in a study among adults aged 18–74 years in rural China, compared to urban male residents, lower prevalence of MetS in rural men (17.5%) was found,Citation32 probably because rural male residents engage in more PA while working, which is partly indicative of the impact of PA on MetS.
Lifestyle is strongly associated with metabolic risk. In our entire study group, we found significant inverse associations of high PA with the risk of MetS. A cross-sectional study of 1000 subjects aged 20–70 years living in an urban area of northern Iran found that high PA (compared to low PA) was inversely associated with MetS,Citation33 similar to a large-scale study of 10,367 participants aged 37–66 years conducted in Poland. Suliga et al also found that the risk of MetS in participants reporting low PA was higher than that of those declaring high PA.Citation34 Moreover, a recent study included 4865 adults in China indicated that higher levels of moderate-to-vigorous PA and total PA were associated with a lower MetS risk.Citation35 Although all of our subjects were middle-aged or older, and they all lived in the same city, we also found that high PA was associated with a reduced risk of MetS. Our study adds to knowledge on the correlation between PA and MetS status in Han Chinese.
However, the relationships between the PA level and MetS components remain controversial. We found that increased PA was inversely associated with the risk of decreased HDL-C concentrations in the overall sample. A cross-sectional study of 750 patients from central rural India found that PA was negatively correlated with TG and TC levels, but not correlated with HDL-C levels.Citation36 An observation of Cardiovascular Risk Factors in Luxembourg study reported that those who undertook a medium level of intense PA had significantly higher HDL-C levels and lower TG levels than those who undertook less than this, but the TC and LDL-C levels were not significantly associated with the PA level.Citation37 In another cross-sectional study, Li et alCitation38 found that the most-active (compared to the least-active) subjects had a lower risk of MetS and a lower risk of decreased HDL-C levels, similar to our study. Although PA is well known to benefit metabolism and overall human health, the diverse results among studies may reflect demographic or racial differences, employment of different MetS diagnostic criteria, and the use of various methods to assess PA status.
VFA accumulation, particularly in elderly individuals, is strongly associated with a higher risk of MetS and components thereof.Citation39 Few studies have explored the relationships among PA, MetS, and its components in middle-aged and older adults stratified by VFA status. We found that the associations between PA and metabolic risk factors were particularly strong in participants with central obesity. In that subgroup, high PA (compared to low PA) was related to a lower risk of MetS components, including hyperglycemia and decreased HDL-C levels. A similar cross-sectional study of adults aged >60 years with BMI ≥ 30 kg/m2 found that those who engaged in high PA were at a lower risk of MetS.Citation40 Another 6-year cohort study in 1046 Mexican adults found a different effect of PA pattern: among the overweight or obesity participants adopting an active PA pattern was associated with lower risk of MetS in comparison with an inactive pattern, while maintaining an active lifestyle did not provide additional protection against developing MetS in lean individuals.Citation41 PA may play a prominent role in central obese populations for the following reasons. First, PA triggers weight loss, particularly VAT loss, and this seems to greatly reduce MetS prevalence.Citation9,Citation42 Second, the MetS incidence in our patients with central obesity was much greater than that in the overall sample.
We found that, among all participants, those sitting for >3 h/day had a higher risk of MetS and hypertriglyceridemia, independent of PA level. A positive correlation between prolonged ST and the risk of MetS was reported in most previous studies.Citation10–12,Citation32,Citation43–45 Although most randomized controlled trials found no significant relationship between ST and TG levels,Citation46,Citation47 a few cross-sectional studies and prospective-observational studies reported a positive association.Citation36,Citation48–50 Many studies have found that prolonged sitting contributes to the loss of local contractile stimulation, in turn decreasing the activity of skeletal muscle lipoprotein lipase (the enzyme responsible for hydrolysis of TG-rich lipoproteins), TG uptake by red skeletal muscle, and glucose uptake.Citation51,Citation52 Another reasonable hypothesis is that long STs may increase energy intake due to greater food consumption.Citation53 This may help explain the positive association between ST and the lipid profile.
Similarly, the associations between ST and the risk of MetS and hypertriglyceridemia were stronger in our participants with central obesity. Few studies have explored the relationship between ST and MetS in such individuals. Wendy C King et alCitation54 reported that objectively measured indices of ST were positively associated with the risk of MetS in large sample of adults with severe obesity. However, two studies that enrolled overweight and obese participants (BMI ≥ 25 kg/m2) found no significant associations between ST and lipid concentration.Citation34,Citation37 The inconsistence may be explained by the fact that BMI does not reliably identify obese individuals with excess VAT on computed tomography (CT) or MRI.Citation42 Peterson et alCitation55 examined the extent of obesity misclassification among US adults. Individuals with high body fat levels who were misclassified as non-obese based on BMI had a significantly higher likelihood of MetS than correctly classified normal-BMI subjects with low body fat. We used MRI to assess VFA status because it accurately identifies individuals with central obesity; such individuals have the highest cardiometabolic risk.
To the best of our knowledge, this is one of the first Chinese studies to examine the associations among PA, ST levels, MetS, and its components in middle-aged and older adults varying in VFA status. One strength of our study was that the VFA data were obtained using MRI, which determines abdominal obesity status more accurately than WC and BMI. We adjusted for many potential confounders, including sex, alcohol and tea consumption, smoking, sleep duration, dietary patterns, and other sociodemographic variables.
This study had some limitations. First, self-report measures were used to assess PA and ST status, which can lead to recall bias. Second, the age range of our subjects was narrow, and the conclusions may not be applicable to the general adult population. Third, the study was cross-sectional, so we could not infer causality. Fourth, the study population in this research is from a specific region in China, thus the generalizability of the conclusions to other populations or regions is limited. Future studies should base on multicentric population and use objective measurements derived from accelerometers or movement sensors to prospectively study the causal associations among PA, ST, and metabolic risk.
Conclusion
Our data suggested that moderate-to-high PA may have a role in prevention of MetS, and ST > 3 h/day was associated with a higher risk of MetS both in the overall sample and in individuals exhibiting central obesity; People with central obesity should be encouraged to reduce ST and increase PA to help improve metabolic outcomes and reduce cardiovascular risk.
Abbreviations
PA, physical activity; ST, sitting time; MetS, metabolic syndrome; VFA, visceral fat area; IPAQ, International Physical Activity Questionnaire; NCD, non-communicable disease; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; TG, triglyceride; WC, waist circumference; FPG, fasting plasma glucose; 2hPG, 2-hour post-load plasma glucose; HOMA-IR, homeostatic model assessment for insulin resistance; BMI, body mass index; VAT, visceral adipose tissue; SAT, subcutaneous adipose tissue.
Ethics Approval and Informed Consent
This study was approved by the Medical Ethics Committee of Sir Run Run Shaw Hospital affiliated to Zhejiang University on 14 January 2010. All participants signed an informed consent prior to participation.
Consent for Publication
All authors approved the publication.
Disclosure
All authors declare no conflicts of interest.
Acknowledgments
This work was supported by the National Key Technology R&D Program of China (Grant nos. 2009BAI80B00). We gratefully acknowledge the commitment and dedication of the participants of this study.
Data Sharing Statement
The datasets are not publicly available due institution’s policy but are available from the corresponding author upon reasonable request.
Additional information
Funding
References
- Alberti KG, Eckel RH, Grundy SM, et al. Harmonizing the metabolic syndrome: a joint interim statement of the international diabetes federation task force on epidemiology and prevention; national heart, lung, and blood institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120(16):1640–1645. doi:10.1161/circulationaha.109.192644
- Åberg F, Byrne CD, Pirola CJ, Männistö V, Sookoian S. Alcohol consumption and metabolic syndrome: clinical and epidemiological impact on liver disease. J Hepatol. 2023;78(1):191–206. doi:10.1016/j.jhep.2022.08.030
- Noubiap JJ, Nansseu JR, Lontchi-Yimagou E, et al. Global, regional, and country estimates of metabolic syndrome burden in children and adolescents in 2020: a systematic review and modelling analysis. Lancet Child Adolesc Health. 2022;6(3):158–170. doi:10.1016/s2352-4642(21)00374-6
- Ekelund U, Griffin SJ, Wareham NJ. Physical activity and metabolic risk in individuals with a family history of type 2 diabetes. Diabetes Care. 2007;30(2):337–342. doi:10.2337/dc06-1883
- Roberts CK, Hevener AL, Barnard RJ. Metabolic syndrome and insulin resistance: underlying causes and modification by exercise training. Compr Physiol. 2013;3(1):1–58. doi:10.1002/cphy.c110062
- Sisson SB, Camhi SM, Church TS, Tudor-Locke C, Johnson WD, Katzmarzyk PT. Accelerometer-determined steps/day and metabolic syndrome. Am J Prev Med. 2010;38(6):575–582. doi:10.1016/j.amepre.2010.02.015
- Berra K, Rippe J, Manson JE. Making physical activity counseling a priority in clinical practice: the time for action is now. JAMA. 2015;314(24):2617–2618. doi:10.1001/jama.2015.16244
- Bull F, Goenka S, Lambert V, Pratt M. Physical Activity for the Prevention of Cardiometabolic Disease. In: Prabhakaran D, Anand S, Gaziano TA, Mbanya JC, Wu Y, Nugent R, editors. Cardiovascular, Respiratory, and Related Disorders. The International Bank for Reconstruction and Development / The World Bank © 2017 International Bank for Reconstruction and Development / The World Bank; 2017.
- Myers J, Kokkinos P, Nyelin E. Physical activity, cardiorespiratory fitness, and the metabolic syndrome. Nutrients. 2019;11(7). doi:10.3390/nu11071652
- Healy GN, Wijndaele K, Dunstan DW, et al. Objectively measured sedentary time, physical activity, and metabolic risk: the Australian Diabetes, Obesity and Lifestyle Study (AusDiab). Diabetes Care. 2008;31(2):369–371. doi:10.2337/dc07-1795
- Bankoski A, Harris TB, McClain JJ, et al. Sedentary activity associated with metabolic syndrome independent of physical activity. Diabetes Care. 2011;34(2):497–503. doi:10.2337/dc10-0987
- Wijndaele K, Duvigneaud N, Matton L, et al. Sedentary behaviour, physical activity and a continuous metabolic syndrome risk score in adults. Eur J Clin Nutr. 2009;63(3):421–429. doi:10.1038/sj.ejcn.1602944
- Health AGDo. Australia’s Physical Activity and Sedentary Behaviour Guidelines: Tips and Ideas for Older Australians (65 Years and Older). Australian Government Department of Health; 2014.
- Colberg SR, Sigal RJ, Yardley JE, et al. Physical activity/exercise and diabetes: a position statement of the American Diabetes Association. Diabetes Care. 2016;39(11):2065–2079. doi:10.2337/dc16-1728
- Health DO. Start Active, Stay Active: A Report on Physical Activity for Health from the Four Home Countries’. Chief Medical Officers; 2011.
- Hiuge-Shimizu A, Kishida K, Funahashi T, et al. Absolute value of visceral fat area measured on computed tomography scans and obesity-related cardiovascular risk factors in large-scale Japanese general population (the VACATION-J study). Ann Med. 2012;44(1):82–92. doi:10.3109/07853890.2010.526138
- Goossens GH. The metabolic phenotype in obesity: fat mass, body fat distribution, and adipose tissue function. Obes Facts. 2017;10(3):207–215. doi:10.1159/000471488
- Bays HE. Adiposopathy is ”sick fat” a cardiovascular disease? J Am Coll Cardiol. 2011;57(25):2461–2473. doi:10.1016/j.jacc.2011.02.038
- Tchernof A, Després JP. Pathophysiology of human visceral obesity: an update. Physiol Rev. 2013;93(1):359–404. doi:10.1152/physrev.00033.2011
- Qiu R, Wu B, He Y, et al. Age-related adiposity and beta-cell function: impact on prediabetes and diabetes prevalence in middle-aged and older Han Chinese adults. J Endocrinol Invest. 2023;46(2):405–413. doi:10.1007/s40618-022-01917-0
- Yuqian B. A multicenter domestic study on abdominal obesity and metabolic syndrome. Presented at: The 15th National Academic Conference Proceedings of the Diabetes Branch of the Chinese Medical Association. Beijing; 2012. Available from: https://d.wanfangdata.com.cn/conference/ChZDb25mZXJlbmNlTmV3UzIwMjQwMTA5Egc4NTkwMzQ0GghxOTJpNGU5dA==. Accessed June 11, 2024.
- Wallace TM, Levy JC, Matthews DR. Use and abuse of HOMA modeling. Diabetes Care. 2004;27(6):1487–1495. doi:10.2337/diacare.27.6.1487
- Committee IR. Guideline for Data Processing and Analysis of the International Physical Activity Questionnaire (IPAQ). short form; 2005.
- Cleland C, Ferguson S, Ellis G, Hunter RF. Validity of the International Physical Activity Questionnaire (IPAQ) for assessing moderate-to-vigorous physical activity and sedentary behaviour of older adults in the United Kingdom. BMC Med Res Methodol. 2018;18(1):176. doi:10.1186/s12874-018-0642-3
- Craig CL, Marshall AL, Sjöström M, et al. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc. 2003;35(8):1381–1395. doi:10.1249/01.Mss.0000078924.61453.Fb
- Prevention D, Treatment of Clinical Guidelines Writing Group. 中国老年2型糖尿病防治临床指南 (2022年版) [Clinical guidelines for prevention and treatment of type 2 diabetes mellitus in the elderly in China (2022 edition)]. Zhonghua Nei Ke Za Zhi. 2022;61(1):12–50. Chinese. doi:10.3760/cma.j.cn112138-20211027-00751
- Bao Y, Lu J, Wang C, et al. Optimal waist circumference cutoffs for abdominal obesity in Chinese. Atherosclerosis. 2008;201(2):378–384. doi:10.1016/j.atherosclerosis.2008.03.001
- Luo Y, Ma X, Hao Y, et al. Association between serum osteocalcin level and visceral obesity in Chinese postmenopausal women. Clin Endocrinol. 2015;83(3):429–434. doi:10.1111/cen.12793
- Wang X, Yang F, Bots ML, et al. Prevalence of the metabolic syndrome among employees in Northeast China. Chin Med J. 2015;128(15):1989–1993. doi:10.4103/0366-6999.161337
- Balvers M, de Goffau M, van Riel N, et al. Ethnic variations in metabolic syndrome components and their associations with the gut microbiota: the HELIUS study. Genome Med. 2024;16(1):41. doi:10.1186/s13073-024-01295-7
- Pucci G, Alcidi R, Tap L, Battista F, Mattace-Raso F, Schillaci G. Sex- and gender-related prevalence, cardiovascular risk and therapeutic approach in metabolic syndrome: a review of the literature. Pharmacol Res. 2017;120:34–42. doi:10.1016/j.phrs.2017.03.008
- Xiao J, Shen C, Chu MJ, et al. Physical activity and sedentary behavior associated with components of metabolic syndrome among people in rural China. PLoS One. 2016;11(1):e0147062. doi:10.1371/journal.pone.0147062
- Hajian-Tilaki K, Heidari B, Firouzjahi A, Bagherzadeh M, Hajian-Tilaki A, Halalkhor S. Prevalence of metabolic syndrome and the association with socio-demographic characteristics and physical activity in urban population of Iranian adults: a population-based study. Diabetes Metab Syndr. 2014;8(3):170–176. doi:10.1016/j.dsx.2014.04.012
- Suliga E, Cieśla E, Rębak D, Kozieł D, Głuszek S. Relationship between sitting time, physical activity, and metabolic syndrome among adults depending on Body Mass Index (BMI). Med Sci Monit. 2018;24:7633–7645. doi:10.12659/msm.907582
- Bai J, Wang Y, Zhang XF, et al. Associations of sedentary time and physical activity with metabolic syndrome among Chinese adults: results from the china health and nutrition survey. Biomed Environ Sci. 2021;34(12):963–975. doi:10.3967/bes2021.132
- Bondge B, Jain J, Warkad M, Joshi M, More S, Janaarthanan S. Association of physical activity with lipid profile in healthy subjects: a cross sectional study in tertiary care hospital from central rural India. Indian J Endocrinol Metab. 2021;25(6):520–526. doi:10.4103/ijem.ijem_327_21
- Crichton GE, Alkerwi A. Physical activity, sedentary behavior time and l id levels in the observation of cardiovascular risk factors in Luxembourg study. Lipids Health Dis. 2015;14:87. doi:10.1186/s12944-015-0085-3
- Li CL, Lin JD, Lee SJ, Tseng RF. Associations between the metabolic syndrome and its components, watching television and physical activity. Public Health. 2007;121(2):83–91. doi:10.1016/j.puhe.2006.08.004
- Kuk JL, Saunders TJ, Davidson LE, Ross R. Age-related changes in total and regional fat distribution. Ageing Res Rev. 2009;8(4):339–348. doi:10.1016/j.arr.2009.06.001
- Xu F, Cohen SA, Lofgren IE, Greene GW, Delmonico MJ, Greaney ML. The association between physical activity and metabolic syndrome in older adults with obesity. J Frailty Aging. 2019;8(1):27–32. doi:10.14283/jfa.2018.34
- Perez-Rodriguez M, Talavera JO, Salmeron J. Diet quality, physical activity, and weight changes and their association with 6-year risk of metabolic syndrome in Mexican adults. Am J Lifestyle Med. 2023;17(3):448–458. doi:10.1177/15598276211017488
- Neeland IJ, Ross R, Després JP, et al. Visceral and ectopic fat, atherosclerosis, and cardiometabolic disease: a position statement. Lancet Diabetes Endocrinol. 2019;7(9):715–725. doi:10.1016/s2213-8587(19)30084-1
- Gao X, Nelson ME, Tucker KL. Television viewing is associated with prevalence of metabolic syndrome in Hispanic elders. Diabetes Care. 2007;30(3):694–700. doi:10.2337/dc06-1835
- Gardiner PA, Healy GN, Eakin EG, et al. Associations between television viewing time and overall sitting time with the metabolic syndrome in older men and women: the Australian Diabetes, Obesity and Lifestyle study. J Am Geriatr Soc. 2011;59(5):788–796. doi:10.1111/j.1532-5415.2011.03390.x
- Yeo Y, Cho IY, Sim MS, Song HG, Song YM. Relationship between daily sedentary behaviors and metabolic syndrome in middle-aged adults: results from a health survey in Taean-Gun, Republic of Korea. Metab Syndr Relat Disord. 2021;19(1):48–55. doi:10.1089/met.2020.0021
- Aadahl M, Linneberg A, Møller TC, et al. Motivational counseling to reduce sitting time: a community-based randomized controlled trial in adults. Am J Prev Med. 2014;47(5):576–586. doi:10.1016/j.amepre.2014.06.020
- Suboc TB, Strath SJ, Dharmashankar K, et al. Relative importance of step count, intensity, and duration on physical activity’s impact on vascular structure and function in previously sedentary older adults. J Am Heart Assoc. 2014;3(1):e000702. doi:10.1161/jaha.113.000702
- Dickie K, Micklesfield LK, Chantler S, Lambert EV, Goedecke JH. Cardiorespiratory fitness and light-intensity physical activity are independently associated with reduced cardiovascular disease risk in urban black south African women: a cross-sectional study. Metab Syndr Relat Disord. 2016;14(1):23–32. doi:10.1089/met.2015.0064
- Yin N, Yu X, Wang F, et al. Self-reported sedentary behavior and metabolic syndrome among children aged 6–14 years in Beijing, China. Nutrients. 2022;14(9). doi:10.3390/nu14091869
- Zhou Z, Xi Y, Zhang F, et al. Sedentary behavior predicts changes in cardiometabolic risk in professional workers: a one-year prospective study. J Occup Environ Med. 2016;58(4):e117–23. doi:10.1097/jom.0000000000000673
- Hamilton MT, Hamilton DG, Zderic TW. Exercise physiology versus inactivity physiology: an essential concept for understanding lipoprotein lipase regulation. Exerc Sport Sci Rev. 2004;32(4):161–166. doi:10.1097/00003677-200410000-00007
- Bey L, Hamilton MT. Suppression of skeletal muscle lipoprotein lipase activity during physical inactivity: a molecular reason to maintain daily low-intensity activity. J Physiol. 2003;551(Pt 2):673–682. doi:10.1113/jphysiol.2003.045591
- Owen N, Healy GN, Matthews CE, Dunstan DW. Too much sitting: the population health science of sedentary behavior. Exerc Sport Sci Rev. 2010;38(3):105–113. doi:10.1097/JES.0b013e3181e373a2
- King WC, Chen JY, Courcoulas AP, et al. Objectively-measured sedentary time and cardiometabolic health in adults with severe obesity. Prev Med. 2016;84:12–18. doi:10.1016/j.ypmed.2015.12.007
- Peterson MD, Al Snih S, Stoddard J, Shekar A, Hurvitz EA. Obesity misclassification and the metabolic syndrome in adults with functional mobility impairments: nutrition examination survey 2003–2006. Prev Med. 2014;60:71–76. doi:10.1016/j.ypmed.2013.12.0
