Abstract
Purpose
Elevated urine albumin-to-creatinine ratio (UACR) is an established risk factor for microvascular disease in the general population. However, it is unclear whether UACR is associated with arterial stiffness in diabetes. We aimed to assess the relationship between UACR levels and the risk of arterial stiffness in patients with diabetes.
Methods
From July 2021 to February 2023, a total of 1039 participants were assessed for the risk of arterial stiffness, which was evaluated by brachial-ankle pulse wave velocity (baPWV). The value of UACR≥30 mg/g was defined as high UACR. The UACR level had an abnormal distribution and was log2-transformed for analyses to reduce skewness and volatility. High baPWV was evaluated as categorical variables divided by the highest quartile of the values by sex. The relationship between UACR and arterial stiffness was analyzed by linear curve fitting analyses. Multiple logistic regression models were used to analyze the crude and adjusted odds ratio (OR) of UACR for high baPWV with 95% confidence interval (CI). In addition to applying non-adjusted and multivariate-adjusted models, interaction and stratified analyses were also carried out.
Results
The baPWV level was significantly higher in the high UACR group compared with that in the normal UACR group (1861.84 ± 439.12 cm/s vs 1723.13 ± 399.63 cm/s, p<0.001). Adjusted smoothed plots suggested that there are linear relationships between log2-transformed UACR and high baPWV, and Spearman correlation coefficient was 0.226 (0.176–0.276, p<0.001). The OR (95% CI) between log2-transformed UACR and high baPWV were 1.26 (1.19–1.33, p<0.001), and 1.16 (1.08–1.25, p<0.001) respectively in diabetic patients before and after adjusting for potential confounders.
Conclusion
The elevated UACR was associated with arterial stiffness in Chinese patients with diabetes.
Plain Language Summary
1. The mean baPWV level was significantly higher in the high UACR group compared with that in the normal UACR group.
2. The sex-specific hierarchical analysis revealed that baPWV levels and the incidence of high baPWV were significantly elevated with increased UACR.
3. Curvilinear relationships between log2-transformed UACR and the risk of high baPWV.
4. Positive association between UACR and high baPWV in patients with diabetes.
Introduction
Diabetes is a systemic disease that can cause widespread organ damage, encompassing cardiovascular events, kidney disease, and cerebrovascular disorders.Citation1 Consequently, early identification of individuals at high risk is crucial for the prevention and mitigation of diabetes.
Arterial stiffness is a pathological process resulting from functional and structural changes within the arterial wall. As the artery stiffens, its cushioning function is impaired, leading to increased flow pulsatility that is transmitted to the microvasculature of vascular beds with low-resistance.Citation2 Indeed, increased arterial stiffness has been linked to both renal dysfunctionCitation3 and cardiovascular disease (CVD).Citation4 Numerous studies and meta-analyses have demonstrated the prognostic value of arterial stiffness beyond established CVD risk factors, including age and blood pressure.Citation4,Citation5 Nevertheless, clinical practice guidelines rarely recommend the routine assessment of arterial stiffness in daily care. Recently, emerging evidence strongly indicates that arterial stiffness is a potential risk factor for the onset of diabetes.Citation6,Citation7 However, the precise mechanisms by which arterial stiffness is linked to diabetes are not yet fully understood and warrant further investigation. Chronic low-grade inflammation and oxidative stress may also be shared risk factors for both diabetes and arterial stiffness.Citation8
Brachial- ankle pulse wave velocity (baPWV) is usually the feasible way to measure the pulse wave propagation velocity from the point of the brachial artery propagating to the ankle. Although carotid-femoral pulse wave velocity (cfPWV) is regarded as the gold standard for non-invasive assessment of large arterial stiffness,Citation9 baPWV is widely accepted, particularly in Eastern Asia, due to its cost-effectiveness and convenience.Citation10 Numerous studies have found that baPWV exhibits a moderate correlation with cfPWV and serves as a predictor of CVD risk and prognosis.Citation11 However, baPWV may not accurately reflect early pathological changes in atherosclerosis, and results can vary based on ethnic differences and assessment methods.
Albuminuria, defined as urinary albumin excretion exceeding 30 mg/L, is a well-recognized risk factor for both CVD and chronic kidney disease (CKD). CVD and CKD share several convergent pathological pathways, with albuminuria being a common feature in conditions like hypertension and diabetes, which are also CVD risk factors. Inflammation plays a crucial role in atherogenesis, involving various inflammatory biomarkers.Citation12,Citation13 Recently, novel inflammatory predictors such as C-reactive protein to serum albumin ratio have emerged as an independent risk factor for diabetic nephropathy and diabetic neuropathy.Citation14,Citation15 Clinical trials have also demonstrated that reducing plasma C-reactive protein levels is associated with significantly improved coronary artery disease outcomes and reduced cardiovascular mortality.Citation16,Citation17 Elevated inflammatory markers are also linked to the presence of albuminuria, further strengthening the association between albuminuria and CVD.Citation18 Additionally, besides structural changes in large arteries,Citation19 vascular endothelial dysfunction or injury may contribute to atherosclerosis associated with arterial stiffness.Citation20 In fact, endothelial damage occurs simultaneously in both large and small arteries. It is thus reasonable to hypothesize that there might be a close association between albuminuria levels and arterial stiffness. Even though the precise mechanism underlying this association is still unclear, previous research suggests that increased arterial stiffness can lead to glomerular hypertension and inflammation, ultimately compromising the functional incapacitation of the glomerular filtration barrier.Citation21
There are several methods to detect albuminuria in clinical practice. The gold standard is the 24‐hour measurement of albumin excretion rate, which accounts for the high short‐term variability in albuminuria excretion within individuals. However, this test is cumbersome and impractical for routine clinical practice. The urine albumin-to-creatinine ratio (UACR), a ratio between two measured substances, is commonly used to estimate 24-hour urine albumin excretion.Citation22 Unlike a dipstick test for albuminuria, the UACR remains unaffected by variations in urine concentration. eGFR is another marker of renal function, but it is unreliable to diagnose renal injury in the early stage of CKD, primarily due to the absence of significant reduction. Furthermore, eGFR is not reliable for patients with rapidly changing creatinine levels, extremes in muscle mass and body size, or altered diet patterns. In addition, while pulse pressure > 60 mmHg was associated with an increase in albuminuria, it was not associated with a reduction of eGFR in the Veterans Affairs Diabetes Trial (VADT).Citation23 Thus, as a sensitive indicator of early renal damage, UACR may serve as a superior risk marker for predicting the progression of diabetic kidney disease (DKD) and the future development of CVD. Changes in UACR may also reflect responses to therapy and the risk of disease progression. However, previous study results regarding renal-related markers for CVD are inconsistent. Some studies have reported a positive association between high UACR levels and all-cause mortality or major cardiovascular events,Citation24,Citation25 while others have not.Citation26 Otherwise, no studies have yet explored the association between the degree of renal impairment and arterial stiffness.
Based on current evidence, further investigation is needed to determine whether UACR is associated with arterial stiffness in diabetes. We hypothesized that there is a correlation between UACR and high baPWV in patients with diabetes, but the exact nature of this relationship remains unclear. Thus, the aim of our study is to assess the association between UACR and arterial stiffness in the diabetic population.
Materials and Methods
Ethics
This study was carried out in accordance with the principles of the Declaration of Helsinki and was approved by the ethics committee of Yangpu Hospital, School of medicine, Tongji University, Shanghai, China. Prior to collecting samples, we sought the informed consent of all patients. If the potential participant lacks the capacity to understand the information provided due to age, disability, illness, or other reasons, a family member may act as an interpreter or proxy for consent.
Design
The purpose of this cross-sectional study was to explore the correlation between UACR levels and arterial stiffness among patients with diabetes. From July 17, 2021, to February 19, 2023, we enrolled patients with diabetes from Yangpu Hospital, School of Medicine, Tongji University in China. The data of all participants were documented in the hospital’s database.
Study Subjects
Patients were diagnosed with diabetes according to the criteria defined by the World Health Organization published in 2018.Citation27 The inclusion criteria were as follows: (i) aged≥18 years, (ii) random venous plasma glucose concentration≥11.1mmol/l or fasting blood glucose concentration≥7.0mmol/l, (iii) hemoglobin A1c (HbA1c)≥6.5%, (iv) patients previously diagnosed diabetes taking glucose-lowering treatment, or antidiabetic therapy.
Patients were excluded based on the exclusion criteria listed below: (i) pregnant diabetes or other special types of diabetes, (ii) active inflammation (eg, systemic lupus erythematosus, urinary tract infection, acute fever), (iii) acute complications of diabetes (eg, diabetic ketoacidosis, nonketotic diabetic hyperosmolar coma), (iv) recent trauma, surgery, or use of nephrotoxic drugs, (v) difficult to calculate baPWV, and (vi) subjects with the presence of any serious concomitant disease limiting life expectancy.
Clinical and Laboratory Data
All participants were given a standardized questionnaire interview to gather information about their age, gender, occupation, family and personal history of genetic diseases, medical history, past and current medication use, and personal habits such as smoking, drinking, and dieting. Additionally, systolic blood pressure (SBP) was also measured using an automatic blood pressure cuff, and the value was calculated by averaging three consecutive readings. Weight and height measurements were used to calculate body mass index (BMI).
Blood and urine samples were collected prior to treatment in the early morning after admission and fasting for 8 hours. The level of UACR was detected through urine specimens which were measured with a commercially available quantitative test kit obtained from Biotechnology Co., Ltd (Shanghai, China). The UACR test is a two-step experiment. Initially, the fluorescent probe was used to detect urinary albumin content (Ex/Em = 600/630 nm). In the second step, creatinine was generated by enzymatic reaction to form intermediate products and react with chromogenic groups to form colored substrates (570 nm). The detection values ranged from 0.02 to 2.5 mg/mL and 0.002 to 0.5 mg/mL for urine albumin and urine creatinine respectively. The value of UACR≥30 mg/g was defined as the high UACR, and accordingly the value of UACR<30mg/g was defined as the normal UACR. Additionally, blood samples were gathered to assess routine blood indicators, blood biochemical indicators, and blood lipid index.
The baPWV was automatically measured using form PWV/ABI instruments (PWV/ABI, BP-203RPE; Omron-Colin, Japan) by trained doctors from our hospital. The patients who were included in the study lay supine for at least 10 minutes. After that, a skilled researcher put the cuffs around the arms and legs and simultaneously recorded the pulse waveforms from the cuffs. The mean baPWV value was calculated by averaging the baPWV readings taken from the arm and ankle on both sides. High baPWV was defined as the highest percentile of values among the research participants.Citation28
Statistical Analysis
Baseline characteristics of participants are presented by UACR level and quartiles of baPWV. Categorical variables were reported as counts and percentages and were analyzed by Fisher’s exact test or Chi-square test. Continuous variables were presented as the means and standard deviations for data of normal distribution, which were analyzed by t-test and One-Way ANOVA analysis, and they were reported as medians and interquartile ranges for data of abnormal distribution, which were analyzed by Mann–Whitney U-test and Kruskal–Wallis tests. The UACR level had an abnormal distribution and was log2-transformed for analyses to reduce skewness and volatility. High baPWV was evaluated as a categorical variable divided by the highest quartile of the values by sex. The association between log2-transformed UACR and high baPWV was assessed by linear curve fitting analyses and multivariate logistic regression analysis. Baseline variables that were considered relevant to UACR or that showed a univariate relationship with high baPWV were entered into the multivariate logistic regression model. Model 1 was unadjusted. Model 2 was adjusted for age, education level, hypertension history, SBP, heart rate, hemoglobin, albumin, antihypertensive drugs, oral antidiabetic drugs, and drug adjustment. Odds ratio (OR) and 95% confidence interval (CI) were reported. In addition to applying non-adjusted and multivariate-adjusted models, interaction and stratified analyses were also carried out. Statistical Product Service Solutions version 24.0 (SPSS, Chicago, IL, USA) and R (version 3.4) was used to conduct statistical analyses. The level of significance was set at a two-tailed p-value of <0.05.
Results
Baseline Characteristics
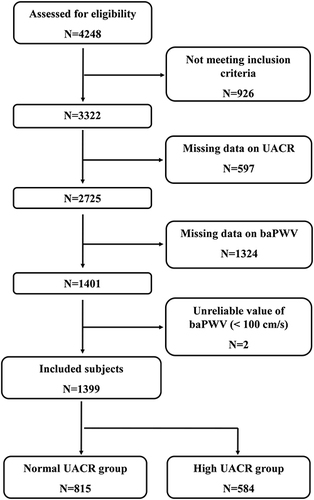
At the time of the final survey in February 2023, a total of 4248 consecutive patients had been recruited for the study. Among these candidates, those who had met at least one of the exclusion criteria were excluded (n=926), as were those who had missing data for UACR (n=597) and baPWV (n=1324). Those with unreliable baPWV values (<100 cm/s) (n=2) were also excluded from the eligible candidates for this study. As a result, the final analyses included 1399 patients in total. A flowchart of the study is shown in .
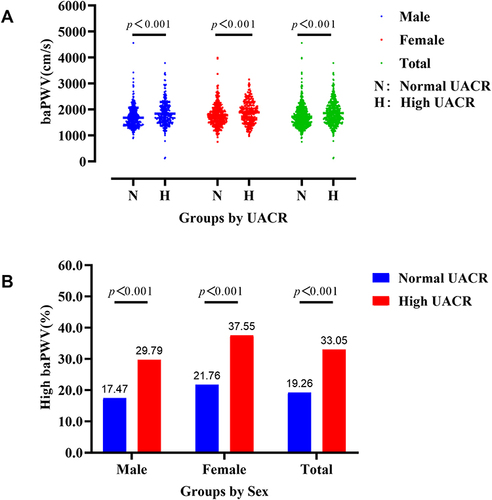
Among the 1399 study subjects, women accounted for 41.82% (n=585) and men for 58.18% (n=814). The age of the enrolled subjects ranged from 23 to 87 years (women, 23–87 years; men, 23–85 years) with a mean age of 59.16±10.99 years (women, 61.04±10.39 years; men, 57.81±11.21 years). The duration of the disease before admission ranged from 1.0 to 487 months with a median and interquartile range of 43 (1–122) months. The UACR ranged from 3.27 to 8459.88 mg/g, and the baPWV ranged from 121 to 4563 cm/s. and Supplement Table 1 list the demographic characteristics of the study patients in both groups at baseline. The mean baPWV in the high UACR group was significantly higher than in the normal UACR group (1861.84 ± 439.12 cm/s vs 1723.13 ± 399.63 cm/s, p<0.001). Hierarchical analysis stratified by sex revealed a significant difference in both women (p<0.001) and men (p<0.001) (see , Supplement Table 2). The prevalence of high baPWV was greater in the high UACR group compared to the normal UACR group (33.05% vs 19.26%, p<0.001). Additionally, the sex-specific hierarchical analysis indicated that baPWV levels and the prevalence of high baPWV were significantly elevated with increased UACR (see , Supplement Table 2).
Table 1 Baseline Characteristics of Participants by UACR Level
Figure 2 Comparison of baPWV by UACR levels and sex. (A) The baPWV level was significantly elevated in the high UACR group in male, female, and total patients (p<0.001). (B) The prevalence of high baPWV were significantly elevated in the high UACR group in male, female, and total patients (p<0.001).
Linear Curve Fitting of the Relationship Between UACR and High baPWV
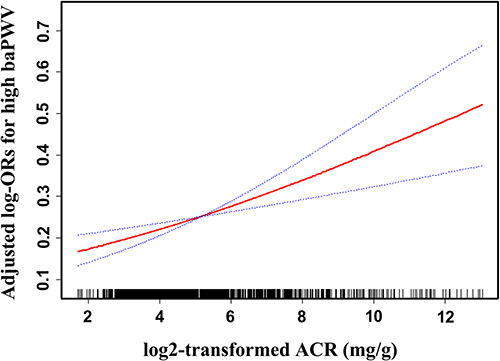
After adjusting for age, sex, education level, hypertension history, SBP, heart rate, hemoglobin, albumin, oral antidiabetic drugs, antihypertensive drugs, and drug adjustment, smoothed plots suggested that there were linear relationships between log2-transformed UACR and high baPWV (see ). For the connection between UACR and high baPWV, Spearman correlation coefficient (95% CI) was 0.226 (0.176–0.276, p<0.001) in all patients.
Figure 3 Linear curve fitting of the relationship between log2-transformed UACR and risk of high baPWV. A linear relationship between log2-transformed UACR and risk of high baPWV was detected after adjusting for sex, age, education level, hypertension history, SBP, heart rate, hemoglobin, ALB, antihypertensive drugs, oral antidiabetic drugs, and drug adjustment. Solid lines represent the fitting curve and dotted lines represent the corresponding 95% CI.
Multiple Logistic Regression Analyses of the Relationship Between UACR and High baPWV
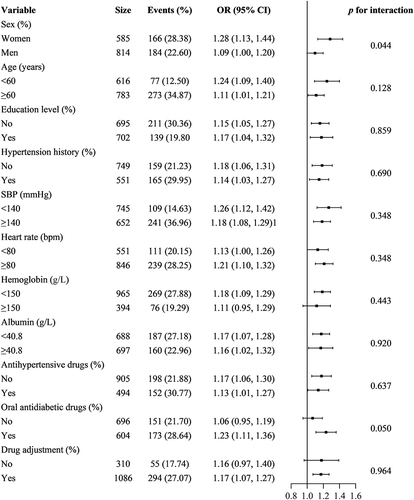
Age, sex, education level, hypertension history, SBP, heart rate, hemoglobin, albumin, oral antidiabetic drugs, antihypertensive drugs, and drug adjustment were identified as potential interacting or confounding factors related to high baPWV by univariate analysis (p<0.05) (see Supplement Table 3). According to multiple logistic regression analysis, the OR (95% CI) for the association between log2-transformed UACR and high baPWV was 1.26 (1.19–1.33, p<0.001) in all patients. When UACR was categorized into normal and high groups, the OR (95% CI) for the relationship between UACR and high baPWV was 2.07 (1.62–2.65, p<0.001) in comparison to normal UACR in all patients. When the multivariate analysis was performed after adjusting for age, sex, education level, hypertension history, SBP, heart rate, hemoglobin, albumin, antihypertensive drugs, oral antidiabetic agents, and drug adjustment, the OR (95% CI) for the relationship between log2-transformed UACR and high baPWV was 1.16 (1.08–1.25, p<0.001) in all patients and 1.55 (1.15–2.11, p=0.004) in the high UACR group, respectively. Additionally, the OR (95% CI) showed a gradual increase according to the tertiles of log2-transformed UACR (see ), indicating that the statistical significance was maintained. When multivariate logistic regression for the effects of UACR on high baPWV was performed by sex and tertiles, the association between log2-transformed UACR and high baPWV was statistically significant in both women and men except for the tertile 3 group in men (see Supplement Table 4). A hierarchical analysis based on age, sex, education level, hypertension history, SBP, heart rate, hemoglobin, albumin, oral antidiabetic drugs, antihypertensive drugs, and drug adjustment, also showed a statistically significant association between log2-transformed UACR and high baPWV (see , Supplement Table 5).
Table 2 Multivariate Logistic Regression for Effects of UACR on High baPWV
Figure 4 Hierarchical analysis of the relationship of log2-transformed UACR and high baPWV. Each stratification adjusted for all the factors (sex, age, education level, hypertension history, SBP, heart rate, hemoglobin, ALB, antihypertensive drugs, oral antidiabetic drugs, and drug adjustment) except the stratification factor itself.
Discussion
In our current investigation, we found that elevated UACR levels were independently associated with high baPWV in diabetic individuals. This positive correlation was observed across all considered subgroups and persisted even after comprehensive adjustments for confounding factors. Accordingly, our findings showed that UACR was linked to arterial stiffness in patients with diabetes, suggesting a progressive increase in the risk of cardiovascular and cerebrovascular events, as well as all-cause mortality, with escalating levels of microalbuminuria.
Among the microvascular complications of diabetes mellitus, DKD is the most seriously detrimental. In addition to ESDR and death, patients with DKD face a significantly elevated risk of cardiovascular and cerebrovascular disease compared to those without DKD.Citation29 The presence of microalbuminuria often indicates the onset of pathophysiological process of systemic atherosclerosis. Clinical studies have found that patients with massive proteinuria have a much higher mortality risk than those experiencing a gradual decline in eGFR or initiating renal replacement therapy, primarily due to a significantly increased risk of cardiovascular events. Due to the delayed diagnosis of diabetic macroangiopathy, angiography and ultrasound are still the primary diagnostic methods. However, some patients are usually seen in critical care when the disease is detected. In this context, UACR could potentially serve as a new non-invasive diagnostic method to screen for atherosclerosis in diabetic patients.
BaPWV is a metric to estimate arterial stiffness and reflects the stiffness of the aorta and peripheral arteries. The faster the BaPWV, the less compliant and stiffer the artery. Since its automated and simplified measurement compared to cfPWV, it is more easily applicable in the clinic. Previous studies have documented that baPWV values over 1400 cm/s for females and 1600 cm/s for males could be a workable cutoff point to evaluate aortic stiffness and predict cardiovascular mortality by the Framingham risk score. According to our findings, the high UACR group’s mean baPWV values were 1861.84±439.12 cm/s. This result is similar to the recently published observational study conducted with a community-based cohort in China involving participants over 40 years of age,Citation30 but it is lower than the findings reported in Xu’s study.Citation31 Because age is a significant contributor of arterial stiffness, the variations in baPWV values may partly be attributable to the older participant population in Xu’s study. An individual participant data meta-analysis, based on 14,673 Japanese participants without a history of CVD, was conducted to investigate the association between baPWV and the risk of developing CVD. The results suggested that baPWV and CVD risk remain significantly positively correlated even after excluding various influencing factors.Citation29 Thus, measurement of the baPWV could enhance the efficacy of prediction of the risk of developing CVD compared to the Framingham risk score, which is based on the traditional cardiovascular risk factors.
Our study has shown that higher UACR levels are significantly associated with increased baPWV, indicative of a dose-response effect. In addition, these associations were stronger in patients with macroalbuminuria, hypertension, and diabetes compared to those without these disorders.Citation32 Besides, Smith et al discovered that in patients with diabetes, the reduction of arterial stiffness reflected by baPWV was associated with the improvement of kidney injury or persistent renal dysfunction calculated by UACR changes, and this effect was independent of blood glucose reduction.Citation33 As a result, lowering albuminuria may be a useful strategy for preventing the progression of arterial stiffness and cardiovascular events in diabetes.
The emergence of novel therapeutic agents has revolutionized diabetes treatment, significantly reducing albuminuria and CVD risks. Sodium-glucose cotransporter 2 (SGLT2) inhibitors have exhibited renal benefits, decreasing the risk of albuminuria and mitigating the progression of end-stage renal disease.Citation34 These agents also prevent heart failure and cardiovascular events in patients with or without type 2 diabetes mellitus.Citation35 Glucagon‐like peptide‐1 (GLP-1) receptor agonists, commonly prescribed for diabetes, have been found to slow the progression of albuminuria and correlate with a reduced risk of major adverse cardiovascular events (MACE) regardless of their glucose-lowering effects. These benefits are associated with their anti-inflammatory, antioxidant, antifibrotic, and renal natriuretic effects.Citation36 Additionally, they contribute to the prevention of non-fatal and fatal strokes, as well as myocardial infarctions, possibly due to GLP-1’s impact on platelet activation and atherosclerotic plaque stability.Citation37 Tirzepatide, a dual GIP/GLP-1 receptor agonist, has shown promise in attenuating albuminuria, although its effects on eGFR loss are still being assessed.Citation38 Recently approved Finerenone effectively reduces renal and cardiovascular risks in patients with CKD and type 2 diabetes mellitus, with mediation analysis revealing that its beneficial effects are primarily attributed to the reduction of albuminuria.Citation39
Uncertainty surrounds the pathophysiological mechanism underlying the connection between UACR and arterial stiffness in diabetes. According to the study by Nikolaidou B et al, hyperglycemia in early-stage diabetes appeared to be the common factor in microvascular injury and was the cause of the interaction between UACR and arterial stiffness.Citation40 Chronic inflammation and endothelial dysfunction caused by growth factors, inflammatory cytokines, and oxidative stress contribute to the complications associated with microalbuminuria in the hyperglycemic state.Citation41 The formation of advanced glycation end products (AGEs) from the glycation of structural proteins like collagen and elastin during aging and long-term diabetes affects arterial compliance. This process has been proposed as a further mechanism for microalbuminuria. Furthermore, because glomerular capillaries are located between the arterioles transporting blood into and out of the glomerulus, renal microvessels make the kidneys vulnerable to damage during arterial stiffness. Prolonged exposure to elevated pulse pressure over extended periods disrupts the renal adaptive mechanism. This mechanism was mediated by tubuloglomerular feedback and response to changes in the blood pressure of afferent arterioles. Arterial stiffness may potentially transmit pulsatile energy to the microvasculature. Consequently, the renal microvasculature may undergo remodeling or sustain the glomerular injury, further damaging the vascular endothelium. Multiple homeostatic systems involved in the secretion of vasoactive substances by endothelial cells are disrupted. These systems regulated the tension of vascular bed, the permeability of microvascular wall, and the balance between coagulation and fibrinolysis. Their disruption directly increases the tension of vascular bed and the permeability of microvascular wall, leading to arterial stiffness. Moreover, glomerular leakage of albumin triggered proinflammatory and profibrotic responses, which might also play a role in kidney injury or the atherosclerotic process.Citation42,Citation43
The interpretation of our results needs to take into account a number of limitations. Firstly, since this research was cross-sectional in nature, despite conducting multiple logistic regression analyses, a causal relationship still needs to be established in further prospective studies with larger sample sizes. Secondly, the study focused on hospitalized patients with diabetes in China, the majority of whom were older with a mean age of approximately 60 years. Thirdly, the association between UACR and high baPWV was statistically significant in both women and men, except for the tertile 3 group in men. With the increment of samples, statistical significance may appear in the tertile 3 group in men. Finally, the evidence of potential mechanisms for the interaction between UACR and high baPWV was gathered from pertinent literature and will need to be validated through experimental studies. These merit further study in other populations to verify that the findings are generalizable.
Conclusions
In conclusion, the present study suggests that elevated UACR was associated with arterial stiffness in Chinese diabetes. Whether the elevated UACR might be served as a risk factor for arterial stiffness in diabetes remains to be identified through prospective studies.
Consent for Publication
All authors read and approved the final manuscript and had agreed to submit this manuscript for publication.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
Thanks to all who contributed to this study. We gratefully acknowledge Desheng Zhu for his guide in data analysis.
Data Sharing Statement
The raw data used to support the findings of this study are available from the corresponding author (Zunhai Zhou) upon request without undue reservation.
Additional information
Funding
References
- Sukkar L, Kang A, Hockham C, et al. Incidence and associations of chronic kidney disease in community participants with diabetes: a 5-year prospective analysis of the EXTEND45 study. Diabetes Care. 2020;43(5):982–990. doi:10.2337/dc19-1803
- O’Rourke MF, Hashimoto J. Mechanical factors in arterial aging: a clinical perspective. J Am Coll Cardiol. 2007;50(1):1–13. doi:10.1016/J.JACC.2006.12.050
- Ford ML, Tomlinson LA, Chapman TPE, Rajkumar C, Holt SG. Aortic stiffness is independently associated with rate of renal function decline in chronic kidney disease stages 3 and 4. Hypertension. 2010;55(5):1110–1115. doi:10.1161/HYPERTENSIONAHA.109.143024
- Vlachopoulos C, Aznaouridis K, Stefanadis C. Prediction of cardiovascular events and all-cause mortality with arterial stiffness: a systematic review and meta-analysis. J Am Coll Cardiol. 2010;55(13):1318–1327. doi:10.1016/j.jacc.2009.10.061
- Ben-Shlomo Y, Spears M, Boustred C, et al. Aortic pulse wave velocity improves cardiovascular event prediction: an individual participant meta-analysis of prospective observational data from 17,635 subjects. J Am Coll Cardiol. 2014;63(7):636–646. doi:10.1016/J.JACC.2013.09.063
- Muhammad IF, Borné Y, Östling G, et al. Arterial stiffness and incidence of diabetes: a population-based cohort study. Diabetes Care. 2017;40(12):1739–1745. doi:10.2337/dc17-1071
- Zheng M, Zhang X, Chen S, et al. Arterial stiffness preceding diabetes: a longitudinal study. Circ Res. 2020;127(12):1491–1498. doi:10.1161/CIRCRESAHA.120.317950
- Wang X, Bao W, Liu J, et al. Inflammatory markers and risk of type 2 diabetes: a systematic review and meta-analysis. Diabetes Care. 2013;36(1):166–175. doi:10.2337/dc12-0702
- Del Giorno R, Troiani C, Gabutti S, Stefanelli K, Gabutti L. Comparing oscillometric and tonometric methods to assess pulse wave velocity: a population-based study. Ann Med. 2021;53(1):1–16. doi:10.1080/07853890.2020.1794538
- Munakata M. Brachial-ankle pulse wave velocity: background, method, and clinical evidence. Pulse. 2016;3(3–4):195–204. doi:10.1159/000443740
- Kimoto E, Shoji T, Shinohara K, et al. Preferential stiffening of central over peripheral arteries in type 2 diabetes. Diabetes. 2003;52(2):448–452. doi:10.2337/diabetes.52.2.448
- Sincer I, Gunes Y, Mansiroglu AK, Cosgun M, Aktas G. Association of mean platelet volume and red blood cell distribution width with coronary collateral development in stable coronary artery disease. Adv Interv Cardiol. 2018;14(3):263–269. doi:10.5114/aic.2018.78329
- Jain S, Khera R, Corrales-Medina VF, Townsend RR, Chirinos JA. Inflammation and arterial stiffness in humans. Atherosclerosis. 2014;237(2):381–390. doi:10.1016/j.atherosclerosis.2014.09.011
- Bilgin S, Kurtkulagi O, Atak Tel BM, et al. Does C-reactive protein to serum Albumin Ratio correlate with diabetic nephropathy in patients with type 2 diabetes mellitus? The CARE TIME study. Prim Care Diabetes. 2021;15(6):1071–1074. doi:10.1016/j.pcd.2021.08.015
- Aktas G. Serum C-reactive protein to albumin ratio as a reliable marker of diabetic neuropathy in type 2 diabetes mellitus. Biomol Biomed. Epub 2024 April 14.
- Ridker PM. A test in context: high-sensitivity C-reactive protein. J Am Coll Cardiol. 2016;67(6):712–723. doi:10.1016/j.jacc.2015.11.037
- Lyngbakken MN, Myhre PL, Røsjø H, Omland T. Novel biomarkers of cardiovascular disease: applications in clinical practice. Crit Rev Clin Lab Sci. 2019;56(1):33–60. doi:10.1080/10408363.2018.1525335
- Upadhyay A, Larson MG, Guo CY, et al. Inflammation, kidney function and albuminuria in the Framingham Offspring cohort. Nephrol Dial Transplant. 2011;26(3):920–926. doi:10.1093/ndt/gfq471
- Zhou T, Huang X, Cai X, Xie L. Combined treatment of irbesartan and diltiazem ameliorates endothelium dependent vasodilatation in hypertensives. Clin Exp Hypertens. 2017;39(7):612–618. doi:10.1080/10641963.2017.1306537
- Sun Z. Aging, arterial stiffness, and hypertension. Hypertension. 2015;65(2):252–256. doi:10.1161/HYPERTENSIONAHA.114.03617
- Chen SC, Chang JM, Liu WC, et al. Brachial-ankle pulse wave velocity and rate of renal function decline and mortality in chronic kidney disease. Clin J Am Soc Nephrol. 2011;6(4):724–732. doi:10.2215/CJN.07700910
- McFarlane PA. Testing for albuminuria in 2014. Can J Diabetes. 2014;38(5):372–375. doi:10.1016/j.jcjd.2014.07.221
- Anderson RJ, Bahn GD, Emanuele NV, Marks JB, Duckworth WC. Blood pressure and pulse pressure effects on renal outcomes in the Veterans Affairs Diabetes Trial (VADT). Diabetes Care. 2014;37(10):2782–2788. doi:10.2337/DC14-0284
- Wang J, Wang F, Liu S, Zhou M, Zhang L, Zhao M. Reduced kidney function, albuminuria, and risks for all-cause and cardiovascular mortality in China: a population-based cohort study. BMC Nephrol. 2017;18(1):188. doi:10.1186/s12882-017-0603-9
- Lees JS, Welsh CE, Celis-Morales CA, et al. Glomerular filtration rate by differing measures, albuminuria and prediction of cardiovascular disease, mortality and end-stage kidney disease. Nat Med. 2019;25(11):1753–1760. doi:10.1038/s41591-019-0627-8
- Sung KC, Ryu S, Lee JY, et al. Urine albumin/creatinine ratio below 30 mg/g is a predictor of incident hypertension and cardiovascular mortality. J Am Heart Assoc. 2016;5(9):e003245. doi:10.1161/JAHA.116.003245
- World Health Organization. Guidelines on second-and third-line medicines and type of insulin for the control of blood glucose levels in non-pregnant adults with diabetes mellitus. Br J Psychiatry. 2018.
- Guo W, Li XN, Li J, et al. Increased plasma miR-146a levels are associated with subclinical atherosclerosis in newly diagnosed type 2 diabetes mellitus. J Diabetes Complications. 2020;34(12):107725. doi:10.1016/j.jdiacomp.2020.107725
- Ohkuma T, Ninomiya T, Tomiyama H, et al. Brachial-ankle pulse wave velocity and the risk prediction of cardiovascular disease: an individual participant data meta-analysis. Hypertens. 2017;69(6):1045–1052. doi:10.1161/HYPERTENSIONAHA.117.09097
- Jiang Y, Fan F, Jia J, et al. Brachial–ankle pulse wave velocity is independently associated with urine albumin-to-creatinine ratio in a Chinese community-based cohort. Int Urol Nephrol. 2020;52(4):713–720. doi:10.1007/s11255-020-02404-2
- Xu X, He J, Wang S, et al. Ankle-brachial index and brachial-ankle pulse wave velocity are associated with albuminuria in community-based Han Chinese. Clin Exp Hypertens. 2016;38(7):618–623. doi:10.1080/10641963.2016.1182177
- Liu CS, Pi-Sunyer FX, Li CI, et al. Albuminuria is strongly associated with arterial stiffness, especially in diabetic or hypertensive subjects--a population-based study (Taichung Community Health Study, TCHS). Atherosclerosis. 2010;211(1):315–321. doi:10.1016/J.ATHEROSCLEROSIS.2010.02.015
- Smith A, Karalliedde J, De Angelis L, Goldsmith D, Viberti G. Aortic pulse wave velocity and albuminuria in patients with type 2 diabetes. J Am Soc Nephrol. 2005;16(4):1069–1075. doi:10.1681/ASN.2004090769
- Bae JH, Park EG, Kim S, Kim SG, Hahn S, Kim NH. Effects of sodium-glucose cotransporter 2 inhibitors on renal outcomes in patients with type 2 diabetes: a systematic review and meta-analysis of randomized controlled trials. Sci Rep. 2019;9(1):13009. doi:10.1038/s41598-019-49525-y
- Cardoso R, Graffunder FP, Ternes CMP, et al. SGLT2 inhibitors decrease cardiovascular death and heart failure hospitalizations in patients with heart failure: a systematic review and meta-analysis. eClinicalMedicine. 2021;36:100933. doi:10.1016/j.eclinm.2021.100933
- Kristensen SL, Rørth R, Jhund PS, et al. Cardiovascular, mortality, and kidney outcomes with GLP-1 receptor agonists in patients with type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet Diabetes Endocrinol. 2019;7(10):776–785. doi:10.1016/S2213-8587(19)30249-9
- Gilbert RE, Connelly KA. Reduction in the incidence of myocardial infarction with sodium-glucose linked cotransporter-2 inhibitors: evident and plausible. Cardiovasc Diabetol. 2019;18(1):6. doi:10.1186/s12933-019-0812-6
- Karakasis P, Patoulias D, Fragakis N, Klisic A, Rizzo M. Effect of tirzepatide on albuminuria levels and renal function in patients with type 2 diabetes mellitus: a systematic review and multilevel meta-analysis. Diabetes Obes Metab. 2024;26(3):1090–1104. doi:10.1111/dom.15410
- Agarwal R, Tu W, Farjat AE, et al. Impact of finerenone-induced albuminuria reduction on chronic kidney disease outcomes in type 2 diabetes. Ann Intern Med. 2023;176(12):1606–1616. doi:10.7326/M23-1023
- Nikolaidou B, Gkaliagkousi E, Anyfanti P, et al. The impact of hyperglycemia on urinary albumin excretion in recent onset diabetes mellitus type II. BMC Nephrol. 2020;21(1):119. doi:10.1186/S12882-020-01774-0
- Satchell SC, Tooke JE. What is the mechanism of microalbuminuria in diabetes: a role for the glomerular endothelium? Diabetologia. 2008;51(5):714–725. doi:10.1007/S00125-008-0961-8
- Yan X, Sano M. God gives IL-19 with both hands: anti-inflammatory but pro-angiogenic. J Mol Cell Cardiol. 2015;80:20–22. doi:10.1016/J.YJMCC.2014.12.012
- Heerspink HJL, Gansevoort RT. Albuminuria is an appropriate therapeutic target in patients with CKD: the pro view. Clin J Am Soc Nephrol. 2015;10(6):1079–1088. doi:10.2215/CJN.11511114