Abstract
Introduction
This study aimed to investigate the association of Nicotinamide phosphoribosyl transferase (NAMPT) rs61330082 polymorphism with co-morbid hypertension (HTN) and the progression of hypertension in Chinese patients with type 2 diabetes mellitus (T2DM).
Methods
A total of 453 T2DM patients were genotyped for the polymorphism of rs61330082 using SNP-scan high-throughput technology. These patients were divided into T2DM group (261 patients) and T2DM combined with hypertension group (T2MH, 192 patients). The T2MH group was further categorized into Grade I, Grade II, and Grade III based on the results of the Hypertension Grade Score. Peripheral blood plasma urea, plasma creatinine, renin-angiotensin system (RAS) indexes, and lipid biochemistry indexes were measured in patients and analyzed in relation to NAMTP polymorphisms.
Results
We found that the presence of the NAMPT rs61330082-AA genotype was associated with a significantly increased risk of developing higher-grade hypertension in patients with T2MH. In addition, the A allele of the NAMPT rs61330082 gene displayed more associated in developing a higher grade of hypertension compared to the G allele. Also, the level of total cholesterol (TC) and low-density lipoprotein cholesterol (LDL-c) increased with hypertension grade in the NAMPT rs61330082-GG genotype.
Conclusion
NAMPT rs61330082 polymorphism was significantly associated with the progression of hypertension grade in T2MH patients and also affected plasma creatinine and LDL-c levels.
Introduction
Globally, the number of people with diabetes has increased exponentially over the past three decades and 90% of them have type 2 diabetes (T2DM).Citation1 The prevalence of diabetes mellitus in China increased from 10.9% to 12.4% between 2013 and 2018,Citation2 with approximately 60% of T2DM patients having comorbid hypertension,Citation3 and the combination of diabetes mellitus and hypertension produces a synergistic effect that significantly increases the risk of occurrence and progression of macrovascular and microvascular complications.Citation4 The prevalence is increasing in all age groups, but the trend is more severe in the younger age groups and there is substantial evidence of a strong correlation between morbidity and mortality from complications such as hypertension and cardiovascular disease.Citation5,Citation6 Insulin resistance is a common pathway in the development of T2DM and hypertensive disease.Citation7 Nicotinamide phosphoribosyl transferase (NAMPT), an adipokine secreted primarily by macrophages and adipocytes, also known as visfatin and pre-B cell colony-enhancing factor, is considered to be an insulin mimetic factor and a potential regulator in inflammatory and immune processes,Citation8,Citation9 and contributed to the onset of insulin resistance, a typical characteristic of T2DM.Citation10 Then, the NAMPT gene is located between human chromosome 7q22.2 and q31.33 and contains 10 introns and 11 exons,Citation11 which was found to be associated with insulin resistance syndrome-related phenotypes in nondiabetic Mexican-Americans.Citation12
Many studies have reported the association between NAMPT gene polymorphisms and hypertensionCitation13,Citation14 and its resulting cardiovascular diseasesCitation15,Citation16 and diabetes,Citation17,Citation18 however, the conclusions are inconsistent. There are also no studies analyzing its association with T2DM combined with hypertension. Therefore, we explored the association of NAMPT gene polymorphisms with Chinese patients with T2DM combined with hypertension and their hypertension grading and analyzed its relationship with lipids.
Materials and Methods
Study Subjects
All subjects were 453 patients with T2DM diagnosed between September 2021 and January 2022 at Guilin Medical University Hospital. Patients included in the study were consecutive. Exclusion criteria included severe cardiovascular diseases such as severe heart failure, stroke all types of embolism, and other diabetic complications. Special conditions such as type 1 diabetes mellitus, abnormal liver and renal function, malignant tumors, autoimmune diseases, and pregnancy in women were also excluded. All subjects did not take any medication 12h prior to participation in the experiment. There were no statistically significant differences in age and gender between the T2DM and T2MH groups (p>all 0.05). All subjects were independent individuals without any genetic relationship. The study protocol was reviewed and approved by the Medical Ethics Committee (2023QTLL-37) of our institution and is in accordance with the Declaration of Helsinki. In addition, the diagnosis of type 2 diabetes mellitus in the included study population was based on American Diabetes Association (ADA) criteria of fasting glucose (FPG) ≥126 mg/dL (7.0 mmol/L) or 2-hour blood glucose (2-hour PG) ≥200 mg/dL (11.1 mmol/L) or Hemoglobin A1C (HbA1C) ≥6.5% (48 mmol/mol),Citation19 and the diagnosis of hypertension was based on the report of the Subcommittee of Professional and Public Education of the American Heart Association Council with a systolic blood pressure (SBP) ≥130 mmHg and/or diastolic blood pressure (DBP) ≥80 mmHg.Citation20 Hypertension grading criteria were based on the Eighth Joint National Committee’s evidence-based management guidelines.Citation21 To determine the grading of high blood pressure without taking antihypertensive medication. Measurements need to be taken 2–3 times at 1~4 week intervals to determine elevated blood pressure and its grading. Grade I is SBP 140–159 mmHg and/or DBP 90–99 mmHg; Grade II is SBP 160–179 mmHg and/or DBP 100–109 mmHg; Grade III is SBP ≥ 180 mmHg and/or DBP ≥ 110 mmHg. The final hypertension classification was 144 patients due to missing data on blood pressure levels in some T2MH patients.
Testing Indicators
Baseline information was collected from the clinical medical records of all participants, including data on age, sex, weight, blood pressure, blood glucose, plasma creatinine, plasma urea, lipids, adrenocorticotropic hormone (ACTH), and RAS biochemical indices. Blood biochemical tests were performed in the Laboratory Department of Guilin Medical University Hospital (ISO15189 NO.ML00036). The biochemical indices of ACTH and RAS were gauged using the AutoLumo A6000 automatic chemiluminescence immunoassay analyzer, while the remaining indices were assessed with the Cobas 8000 biochemical analyzer. The levels of glycated hemoglobin were measured using automated ion exchange chromatography, with a reference range of 4.0–6.0% (Bio-Rad, CA). For HbA1c, the variation coefficient (CV) for both inter-assay and intra-assay instances was less than or equal to 3.1%, with values below 6.5%. The Friedewald equation was employed to calculate low-density lipoprotein cholesterol (LDL-C).Citation22
NAMPT Gene Polymorphism Analysis
The reagents and methods used for DNA isolation, primer design and genotyping were consistent with our previous studies. The PCR primer sequence information is as follows: NAMPT rs61330082 A/G (Forward: 5′-TTGTAAGTGTACTAGTATTTACTACATT GGTGAGTTCC-3′, Reverse: 5′-TTTTGTAAGTGTACTAGTATTTACTACATTGGT GAGTCCT-3′).
Statistical Analysis
All statistical assessments were performed using SPSS 26.0 software (IBM Corp., Armonk, NY, USA). Independent samples t-test and Mann–Whitney U-test were selected based on whether continuous variables conformed to a normal distribution. Three or more measurements were compared using the Kruskal–Wallis test or one-way ANOVA. Categorical variables and Hardy-Weinberg genetic balance were tested using the χ2 test. Logistic regression analysis was used to assess the effects of known or potential risk factors on the emergence of T2MH and the analysis of interactions. P-values of less than 0.05 were considered statistically significant differences.
Results
Comparison of Clinical and Biochemical Characteristics
Patients with T2DM combined with hypertension had higher levels of Postprandial Blood Glucose (PBG) (P=0.028), ACTH (P= 0.009), and blood pressure levels (SBP<0.001, DBP=0.055) than patients with T2DM. At the same time we performed binary regression analysis of the indicators with significant differences showing that SBP was an independent risk factor (P =0.002) whereas PBG, ACTH and DBP (P >0.05) were not. The rest of the clinical characteristics and biochemical indices were not statistically different, proving that our selected cohort was comparable ().
Table 1 Clinical Characteristics of the Study Subjects
The Relation Between NAMPT Gene Polymorphisms and T2MH
All genotypes and allele frequencies conformed to Hardy-Weinberg equilibrium (all P> 0.05). The genotype and allele distributions of NAMPT rs61330082 are shown in , and . The results showed that the distribution of genotypes of NAMPT was not significant between the T2DM group and the comorbidity group but differed significantly among the different hypertension classifications of T2MH (χ2=10.675, P=0.030), with the AA genotype frequency being lowest in the Grade I group and the GG genotype frequency being lowest in the Grade III group. There was also a significant difference in A and G allele frequencies between the two groups (χ2 = 7.110, P = 0.029), with the Grade III group having a higher A allele frequency than the Grade I and II group.
Table 2 NAMPT Gene Polymorphism Relating in Chinese Patients with Type 2 Diabetes
Table 3 NAMPT Gene Polymorphism Relating to Hypertension Classification in Chinese Patients with Type 2 Diabetes
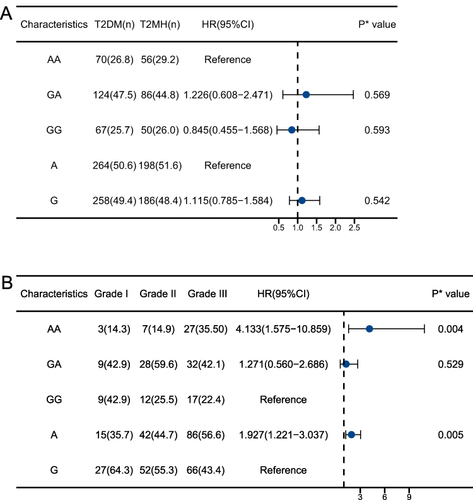
Considering confounding risk factors such as age, sex, and dyslipidemia, binary logistic regression analyses were used to estimate the dominance odds ratios (ORs) for the prevalence of hypertension in diabetic patients, and ordered logistic regression analyses were used to estimate the ORs for the progression of hypertension grading in patients with T2MH. The results of the binary and ordered logistic regression analyses (expressed as [T2DM vs T2MH] as dependent variables, and blood pressure, FBG, ACTH and NAMPT genotype as covariates, respectively; and [Grade I vs Grade II vs Grade III] as dependent variables, and age, plasma creatinine and NAMPT genotype as covariates) showed that after adjusting for confounders, NAMPT gene polymorphisms and diabetes mellitus combined with hypertension were not significantly correlation () but significant correlation with the progression of hypertension grading in T2MH patients (). The risk of graded progression of hypertension was 3.609 times higher in the AA genotype than in the GG genotype (95% CI: 1.099, 11.857; P=0.034). The risk of graded progression of hypertension was 1.903 times higher in the A allele than in the G allele (95% CI, 1.063, 3.404; P=0.030.) The risk of graded progression of hypertension was 1.903 times higher in the G genotype (95% CI, 1.063, 3.404; P=0.030).
The Relation Between Levels of Lipid Metabolism Indexes, ACTH, and NAMPT Gene Polymorphism in the T2DM Population
The results in showed that all ACTH in the T2MH group was higher than that in the T2DM group, in which the difference in expression of GA genotype was statistically significant (P=0.008). However, the differences in other lipid metabolism indexes (TG, TC, HDL-c, LDL-c) between the two were not statistically significant.
Table 4 Association Between NAMPT (rs61330082) Polymorphism and Lipid Metabolism Index and ACTH
Comparison of Baseline Data Between Different Hypertension Class Groups in the T2MH Group
summarises the clinical and biochemical characteristics of T2DM patients with different hypertensive grades (21 Grade I, 47 Grade II, 76 Grade III). The differences in gender, body weight, blood glucose index, lipid metabolism index, TG, TC, ACTH, and RAAS system index were not statistically significant (P>0.05). The mean age of the Grade I group was less than that of the Grade II and III groups (P=0.017). We also found that the level of plasma creatinine Grade I< Grade II< Grade III (P=0.021). The difference in blood pressure levels, although not statistically significant, also showed a stepwise increase (SBP, P=0.379; DBP, P=0.241).
Table 5 Clinical Characteristics of the T2MH Patients
Association of NAMPT Gene Polymorphisms with Serum TG, TC, and Lipid Levels in T2DM Patients with Different Hypertension Grades
demonstrates the comparison of NAMPT gene polymorphisms with serum TG, TC, and lipid levels in T2DM patients with different hypertension classifications. Both plasma creatinine and LDL-c showed an increase with increasing grade in T2MH patients expressing the GG genotype (P=0.014; P=0.018). This finding was absent in T2MH patients expressing AA and GA genotypes. Plasma urea, TG, TC, and HDL-c were not statistically significant in all three genotypes of T2MH patients with different hypertension grades (P>0.05).
Table 6 Association Between NAMPT (rs61330082) Polymorphism and Lipid Metabolism Index in Different Grades T2MH Patients
Discussion
To the best of our knowledge, this is the first epidemiological study to investigate the association of NAMPT gene polymorphisms with susceptibility and hypertension grade in Chinese patients with type 2 diabetes and hypertension. We found no statistically significant association between NAMPT gene polymorphisms and T2DM combined hypertension, but a significant correlation with its hypertension grade progression.
T2DM is a multifactorial metabolic disease characterized by insulin resistance and progressive decline in islet cell function. T2DM and HTN are related to each other, and their coexistence is linked to various diabetes-associated complications.Citation23 Although the underlying pathophysiological mechanisms remain elusive, genetic susceptibility as a key to T2DM and diabetes-related diseases is increasingly recognized. NAMPT as a nicotinamide adenine dinucleotide biosynthetic enzyme impacts multiple metabolic and stress responses and also plays a major role in regulating insulin secretion in pancreatic β-cells. Several NAMPT gene polymorphisms are related to T2DM and lipid profiles in different ethnic groups.Citation24–27 Therefore, it is crucial to investigate the link of NAMPT rs61330082 polymorphism with susceptibility to T2DM, HTN complication, and lipid profile in the Chinese population as it has never been evaluated and reported yet.
In the present study, we reported that NAMPT -rs61330082 genotype and allele frequencies were not significantly different between T2DM and controls but were significantly different in T2MH patients with different grades. The NAMPT rs61330082 polymorphism was also found to be unrelated to hypertension susceptibility in T2DM patients but associated with hypertension grade in T2MH patients, in which the AA genotype and the A allele were significant risk factors for the progression of hypertension grade. Although there are no previous studies on the correlation of NAMPT gene polymorphisms with T2MH and its hypertensive grade, some studies are showing its association with the progression of the risk of hypertension and related diseases. S QCitation28 and DeliciaCitation29 found that rs61330082 led to elevated triglyceride levels, severe systolic blood pressure, and severe hypertension in obese children. In patients with gestational hypertension, MarcelCitation14,Citation30 found that rs61330082 affected circulating levels of visfatin while reducing the effectiveness of antihypertensive therapy. Meanwhile, NAMPT gene polymorphisms have been associated with susceptibility to other complications in patients with T2DM. Tarek’sCitation18 study showed that the NAMPT- 948G /T polymorphism was associated with a predisposition to cardiovascular disease (CVD) in patients with T2DM, and PedroCitation15 showed that the NAMPT-rs9770242 polymorphism may increase the risk of coronary artery disease (CAD) in patients with T2DM. Therefore, we hypothesized that the potential mechanism by which rs61330082, located in the promoter region of the NAMPT gene, is associated with susceptibility to hypertension grade progression in patients with T2MH may be that the AA genotype or A allele of rs61330082 results in altered transcriptional activity, which affects the structure, function, or expression of NAMPT, and that this SNP may be a useful biomarker for assessing the progression of hypertension grades in T2MH in national populations.
Serum creatinine levels are strongly associated with T2DMCitation31 and hypertension.Citation32 We found that plasma creatinine levels were significantly different and increased with hypertension grade in patients with different grades of T2MH. Meanwhile, we found the same results in T2MH expressing the GG genotype. Therefore, we suspected that the NAMPT-GG genotype might contribute to the increase in hypertension grade by affecting plasma creatinine. Meanwhile, JiaCitation33 found in a previous study that genetic variants of NAMPT may affect lipid parameters in Chinese patients with T2DM, and TokunagaCitation34 found that the NAMPT −1535 C/T polymorphism affected certain lipid levels in patients with T2DM, including reduced HDL-c levels and increased TG levels in patients with the TT genotype. Our study also found significant differences in LDL-c levels in the T2MH population expressing the GG genotype and rising with blood pressure class.
There are some limitations of our study and analyses that need to be pointed out. First, our research concentrated on the NAMPT polymorphic site, and future investigations should delve deeper into NAMPT’s mechanisms in NOD mice and its impact on patients suffering from T2DM and hypertension. Additionally, the count of instances for certain indicators decreased owing to the absence of foundational clinical data. Thirdly, additional collection and analysis will be conducted on environmental risk elements linked to NAMPT gene variations.
Conclusion
NAMPT rs61330082 polymorphism was significantly associated with the progression of hypertension grade in T2MH patients and also affected plasma creatinine and LDL-c levels.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare no conflicts of interest in this work.
Acknowledgments
The author of this article expresses gratitude for the collaborative efforts and contributions made by all members involved.
Data Sharing Statement
Data are available by the corresponding author upon reasonable request.
Additional information
Funding
References
- Zheng Y, Ley SH, Hu FB. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat Rev Endocrinol. 2018;14(2):88–98. doi:10.1038/nrendo.2017.151
- Wang L, Peng W, Zhao Z, et al. Prevalence and treatment of diabetes in China, 2013–2018. JAMA. 2021;326(24):2498–2506. doi:10.1001/jama.2021.22208
- Zhang Y-Q, Li Y, Dong Y-G, et al. A nationwide assessment of blood pressure control and the associated factors in Chinese type 2 diabetes mellitus patients. J Clin Hypertens. 2019;21(11):1654–1663. doi:10.1111/jch.13675
- Zhang Y, Nie J, Zhang Y, et al. Degree of blood pressure control and incident diabetes mellitus in Chinese adults with hypertension. J Am Heart Assoc. 2020;9(16):e017015. doi:10.1161/JAHA.120.017015
- Magliano DJ, Sacre JW, Harding JL, et al. Young-onset type 2 diabetes mellitus - implications for morbidity and mortality. Nat Rev Endocrinol. 2020;16(6):321–331. doi:10.1038/s41574-020-0334-z
- Lonardo A, Nascimbeni F, Mantovani A, et al. Hypertension, diabetes, atherosclerosis and NASH: cause or consequence? J Hepatol. 2018;68(2):335–352. doi:10.1016/j.jhep.2017.09.021
- DeFronzo RA, Ferrannini E, Groop L, et al. Type 2 diabetes mellitus. Nat Rev Dis Primers. 2015;1(15019). doi:10.1038/nrdp.2015.19
- Friebe D, Neef M, Kratzsch J, et al. Leucocytes are a major source of circulating nicotinamide phosphoribosyltransferase (NAMPT)/pre-B cell colony (PBEF)/visfatin linking obesity and inflammation in humans. Diabetologia. 2011;54(5):1200–1211. doi:10.1007/s00125-010-2042-z
- Sommer G, Garten A, Petzold S, et al. Visfatin/PBEF/Nampt: structure, regulation and potential function of a novel adipokine. Clin Sci. 2008;115(1):13–23. doi:10.1042/CS20070226
- Bilir BE, Güldiken S, Tunçbilek N, et al. The effects of fat distribution and some adipokines on insulin resistance. Endokrynol Pol. 2016;67(3):277–282. doi:10.5603/EP.a2016.0023
- Javanmard SH, Dehghananzadeh R, Rafiee L, et al. Genetic associations of the visfatin G-948T polymorphism with obesity-related metabolic traits in an Iranian population. J Res Med Sci. 2016;21(105). doi:10.4103/1735-1995.193177
- Arya R, Blangero J, Williams K, et al. Factors of insulin resistance syndrome--related phenotypes are linked to genetic locations on chromosomes 6 and 7 in nondiabetic Mexican-Americans. Diabetes. 2002;51(3):841–847. doi:10.2337/diabetes.51.3.841
- Pereira DA, Sandrim VC, Palei AC, et al. NAMPT single-nucleotide polymorphism rs1319501 and visfatin/NAMPT affect nitric oxide formation, sFlt-1 and antihypertensive therapy response in preeclampsia. Pharmacogenomics. 2021;22(8):451–464. doi:10.2217/pgs-2021-0006
- Luizon MR, Belo VA, Palei AC, et al. Effects of NAMPT polymorphisms and haplotypes on circulating visfatin/NAMPT levels in hypertensive disorders of pregnancy. Hypertens Res. 2015;38(5):361–366. doi:10.1038/hr.2015.15
- Saddi-Rosa P, Oliveira C, Crispim F, et al. Association of circulating levels of nicotinamide phosphoribosyltransferase (NAMPT/Visfatin) and of a frequent polymorphism in the promoter of the NAMPT gene with coronary artery disease in diabetic and non-diabetic subjects. Cardiovasc Diabetol. 2013;12(119). doi:10.1186/1475-2840-12-119
- Leander K, Gigante B, Silveira A, et al. NAMPT (visfatin) and AKT1 genetic variants associate with myocardial infarction. Clin Chim Acta. 2012;413(7–8):727–732. doi:10.1016/j.cca.2012.01.002
- Zhou Q, Chen B, Ji T, et al. Association of genetic variants in RETN, NAMPT and ADIPOQ gene with glycemic, metabolic traits and diabetes risk in a Chinese population. Gene. 2018;642:439–446. doi:10.1016/j.gene.2017.10.084
- Motawi TMK, Shaker OG, El-Sawalhi MM, et al. Visfatin −948G/T and resistin −420C/G polymorphisms in Egyptian type 2 diabetic patients with and without cardiovascular diseases. Genome. 2014;57(5):259–266. doi:10.1139/gen-2014-0022
- American Diabetes Association. 2. classification and diagnosis of diabetes: standards of medical care in diabetes-2021. Diabetes Care. 2021;44(Suppl. 1):S15–S33. doi:10.2337/dc21-ad09
- American Diabetes Association. (2) Classification and diagnosis of diabetes. Diabetes Care. 2015;38(Suppl 1):S8–S16. doi:10.2337/dc15-S005
- James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA. 2014;311(5):507–520. doi:10.1001/jama.2013.284427
- Wilson PW, Abbott RD, Garrison RJ, et al. Estimation of very-low-density lipoprotein cholesterol from data on triglyceride concentration in plasma. Clin Chem. 1981;27(12):2008–2010.
- Zhao K-Y, Yuan M-L, Y-n W, et al. Association of rs1137101 with hypertension and type 2 diabetes mellitus of Mongolian and Han Chinese. World J Diabetes. 2022;13(8):643–653. doi:10.4239/wjd.v13.i8.643
- Zhang -Y-Y, Gottardo L, Thompson R, et al. A visfatin promoter polymorphism is associated with low-grade inflammation and type 2 diabetes. Obesity. 2006;14(12):2119–2126. doi:10.1038/oby.2006.247
- Bailey SD, Loredo-Osti JC, Lepage P, et al. Common polymorphisms in the promoter of the visfatin gene (PBEF1) influence plasma insulin levels in a French-Canadian population. Diabetes. 2006;55(10):2896–2902. doi:10.2337/db06-0189
- Böttcher Y, Teupser D, Enigk B, et al. Genetic variation in the visfatin gene (PBEF1) and its relation to glucose metabolism and fat-depot-specific messenger ribonucleic acid expression in humans. J Clin Endocrinol Metab. 2006;91(7):2725–2731. doi:10.1210/jc.2006-0149
- Blakemore AIF, Meyre D, Delplanque J, et al. A rare variant in the visfatin gene (NAMPT/PBEF1) is associated with protection from obesity. Obesity. 2009;17(8):1549–1553. doi:10.1038/oby.2009.75
- Ooi SQ, Chan RME, Poh LKS, et al. Visfatin and its genetic variants are associated with obesity-related morbidities and cardiometabolic risk in severely obese children. Pediatr Obes. 2014;9(2):81–91. doi:10.1111/j.2047-6310.2013.00149.x
- Ooi DSQ, Ong SG, Heng CK, et al. In-vitro function of upstream visfatin polymorphisms that are associated with adverse cardiometabolic parameters in obese children. BMC Genomics. 2016;17(1):974. doi:10.1186/s12864-016-3315-9
- Luizon MR, Palei ACT, Belo VA, et al. Gene-gene interactions in the NAMPT pathway, plasma visfatin/NAMPT levels, and antihypertensive therapy responsiveness in hypertensive disorders of pregnancy. Pharmacogenomics J. 2017;17(5):427–434. doi:10.1038/tpj.2016.35
- Qin P, Lou Y, Cao L, et al. Dose-response associations between serum creatinine and type 2 diabetes mellitus risk: a Chinese cohort study and meta-analysis of cohort studies. J Diabetes. 2020;12(8):594–604. doi:10.1111/1753-0407.13038
- Chen X, Jin H, Wang D, et al. Serum creatinine levels, traditional cardiovascular risk factors and 10-year cardiovascular risk in Chinese patients with hypertension. Front Endocrinol. 2023;14(1140093). doi:10.3389/fendo.2023.1140093
- Jian W-X, Luo T-H, Y-y G, et al. The visfatin gene is associated with glucose and lipid metabolism in a Chinese population. Diabet Med. 2006;23(9):967–973. doi:10.1111/j.1464-5491.2006.01909.x
- Tokunaga A, Miura A, Okauchi Y, et al. The −1535 promoter variant of the visfatin gene is associated with serum triglyceride and HDL-cholesterol levels in Japanese subjects. Endocr J. 2008;55(1):205–212. doi:10.1507/endocrj.k07e-039