Abstract
Purpose
The clinical background and prognostic impact of diabetes mellitus (DM) on vasospastic angina (VSA) are unclear; thus, in this retrospective study, we investigated whether they differ based on the presence or absence of DM in patients with VSA.
Patients and Methods
We included 272 Japanese patients with VSA diagnosed by coronary angiography (CAG) and the spasm provocation test (SPT). The diagnosis of DM was determined by measuring fasting blood glucose and hemoglobin A1C and by the patient’s current oral medications. On CAG, the presence of atherosclerotic lesions (20%–50%) was checked. On SPT, the coronary spasm was defined as transient coronary vasoconstriction >90% on CAG, accompanied by chest symptoms and/or ST-T changes. Focal spasm was defined as coronary spasm occurring within one segment of the American Heart Association classification on CAG. Blood and urine tests and vascular endothelial function were also evaluated when possible. A major adverse cardiovascular event (MACE), which is defined as cardiac mortality and rehospitalization due to cardiovascular illness, was the basis for determining the prognosis.
Results
There were 49 patients (18%) in the DM group and 223 (82%) in the non-DM group. No significant differences in urinary albumin levels and peripheral vascular function were between groups. On CAG, atherosclerotic lesions were observed significantly more frequently in the DM group (63% vs 46%; P = 0.028). Results of SPT showed a trend toward fewer focal spasms in the DM group (24% vs 39%; P = 0.072). No significant differences in MACE were noted between groups in the primary analysis of DM, whereas sub-analyses of focal spasms showed lower MACE-free survival in the DM group (P = 0.042).
Conclusion
The study results support the hypothesis that DM associated with VSA should be treated appropriately, especially in cases of focal spasm, which may require more attention in treatment.
Introduction
Transient vasoconstriction of the epicardial coronary arteries causes myocardial ischemia in patients with vasospastic angina (VSA).Citation1–3 Like exertional or resting angina, acute coronary syndromes, heart failure, and sudden cardiac death are all largely attributed to coronary spasm.Citation4–6 Recently, more studies have focused on both angina with non-obstructive coronary artery disease (ANOCA) and/or myocardial infarction with non-obstructive coronary arteries (MINOCA), which causes chest pain and myocardial damage without significant coronary artery stenosis. One of the main causes of these diseases is VSA, for which the etiology, diagnosis, and treatment are increasingly being investigated. VSA prognosis is relatively good,Citation7 and several prognostic factors have been reported, including smoking, oral β-blocker use, the non-use of calcium channel blockers, alcohol intake, variant angina, and coronary angiography (CAG) findings such as the presence of atherosclerotic lesions, multivessel spasm (MVS), and focal spasm.Citation7–10
In many primary and secondary prevention studies, diabetes mellitus (DM) is listed as a significant risk factor for atherosclerotic coronary artery disease (CAD),Citation11–13 and CAD guidelines clearly describe the management of DM.Citation14 The role of DM has been reported in the pathogenesis of VSA through factors such as increased insulin resistance.Citation15–17 However, the impact of DM on VSA prognosis is variable,Citation7,Citation8,Citation10,Citation18–21 although DM is addressed in the guideline of the Japanese Circulation Society (JCS).Citation2 Of the several factors associated with VSA prognosis, we considered focal spasm an important factor;Citation9,Citation22 the presence of unstable plaques at the site of the focal spasm has also been reported.Citation23–25 We hypothesized that DM might have a prognostic impact on VSA, especially in the presence of focal spasm and atherosclerotic lesions.
This study examined the clinical characteristics of DM in patients with VSA at the authors’ hospital and investigated whether the prognostic impact of DM varies based on the presence or absence of atherosclerotic lesions and focal spasms.
Material and Methods
Study Patients
This retrospective study assessed 323 Japanese patients diagnosed with VSA based on CAG and spasm provocation test (SPT) results at the authors’ hospital from January 2011 to March 2018. Of these 323 patients, 51 were excluded per the following criteria: significant stenosis of ≥50% on CAG or lesions with a fractional flow reserve (FFR) of ≤0.8 assessed by a pressure wire (21 patients); a history of percutaneous coronary intervention (PCI, 17 patients), and a history of heart failure or hypertrophic cardiomyopathy (5 patients); we also excluded eight patients with missing hemoglobin (Hb) A1C data (). The remaining 272 patients (122 men, 150 women; mean age: 67 years) were assessed. This study was performed in full compliance with the Declaration of Helsinki. The ethics committee of our institution approved the study protocol (No. 2023–4, JR Hiroshima Hospital). For SPT, written informed consent was obtained from each participant. To verify the final consent to participate, an opt-out approach (http://www.jrhh.sakura.ne.jp/annnai/torikumi.html) was employed.
CAG and SPT
The CAG and SPT methods are well documented,Citation26 and the typical approach at the authors’ institution is as follows: The right coronary artery (RCA) was assessed by administering 20, 50, and 80 µg of acetylcholine (ACh) at twenty-second and three-minute intervals; for the left coronary artery (LCA), 50, 100, or 200 µg of ACh was administered every three minutes for 20 seconds; if VSA activity was high, then a smaller dose was administered. As previously reported,Citation26 SPT was started in the RCA at the authors’ institution until April 2016; thereafter, SPT was started in the LCA. If ACh does not induce coronary spasms, then either methylergometrine maleate or additional ACh provocation was performed as needed.Citation27 After each drug provocation, CAG was performed. Nitroglycerin (NTG) was administered at 0.3 mg after the last provocation if coronary spasm was not induced or if it improved quickly after induction; a final CAG was performed just after NTG administration. If chest pain was prolonged or hemodynamic instability occurred during the provoked coronary spasm, NTG was unavoidably administered. After NTG was administered to one coronary artery, the SPT was still performed in the contralateral coronary artery. If a coronary spasm was induced, the diagnosis of VSA was positive, whereas the diagnosis was undetermined if no spasm was induced. Whether such lesions would have been positive for coronary spasm was not included in the analysis.
Factors Assessed on CAG and SPT
Quantitative CAG has previously been described.Citation26 An atherosclerotic lesion was defined as coronary artery stenosis >20% and <50%. Significant stenosis was defined as stenosis >50% and an FFR of ≤0.8; these patients were excluded from the study as previously noted. Myocardial bridging (MB) was defined as a contraction of systolic coronary artery diameter by >20% compared with the diastolic diameter.Citation28
For SPT in this study, positive coronary spasm was defined as transient epicardial coronary artery constriction >90% on drug challenge, with electrocardiographic ST-T changes and/or usual chest symptoms.Citation4 A focal spasm was defined as coronary spasm within one segment of the American Heart Association (AHA) classification, and a diffuse spasm was defined as coronary spasm over one segment of the AHA classification, as previously reported.Citation9 For this study, focal spasm was evaluated in both RCA and LCA, and patients diagnosed with focal spasm had a focal spasm in at least one of the coronary arteries, and patients diagnosed with diffuse spasm had only diffuse spasm. Multivessel spasm (MVS) was defined as coronary spasm in ≥2 of the major coronary arteries; however, MVS could not be determined if it was negative after NTG administration.Citation26 A low dose of ACh (LDA) was defined as coronary spasm with a provocative dose of ≤20 µg ACh in the RCA or ≤50 µg ACh in the LCA. Other causes of focal spasm were transient occlusion (TOC) of a coronary artery caused by coronary spasm, ST-segment elevation during SPT, and unavoidable NTG administration. It was also determined how frequently coronary spasms occurred in the left anterior descending coronary artery (LAD), left circumflex coronary artery (LCX), and right coronary artery (RCA). During the SPT, ventricular fibrillation and hemodynamic instability requiring treatment, such as prolonged atrioventricular block (AVB) or hypotension requiring catecholamines, were defined as serious complications.
Chest symptoms of coronary spasm were described, whether they occurred at rest, on exertion, or both. In addition, the number of anginal attacks was presented as a monthly average. For the number of anginal attacks during follow-up, the monthly average of the number of anginal attacks during the three months prior to the visit is shown. ST-segment elevation on electrocardiography during spontaneous attacks was the definition of variant angina (VA). Cases in which the coronary spasm was accompanied by severe symptoms (such as cold sweats or fainting) were documented. Electronic medical records and copies of medicine notebooks were reviewed to determine the medications at the time of admission. As a rule, coronary dilators were withdrawn 48 hours before the SPT but any medications taken before that time were noted. The number of coronary dilators at the time of discharge and during follow-up was listed. Rehospitalization due to cardiovascular illness and cardiac mortality were considered major adverse cardiovascular events (MACEs). This information is gathered from electronic medical records, phone calls to patients, and the content of referrals from family doctors.
Other Clinical Characteristics Measured
Other parameters were measured as previously reported.Citation26 Smoking, alcohol status, and family CAD history were investigated based on data from the patient, family members, and electronic medical records. Blood and urine tests, such as those to assess lipids, DM, C-reactive protein (CRP), brain natriuretic peptide (BNP), and urinary albumin–creatinine ratio (UACR, mg/gCr), were performed in the fasting state on the same day of CAG. Hypertension and dyslipidemia were defined per the appropriate guidelines.Citation29,Citation30 For DM, patients were evaluated for fasting blood glucose (FBS) and HbA1C. Patients with FBS of ≥126 mg/dL and HbA1C of ≥6.5% and those who were on DM medication were classified as having DM.Citation14 As previously reported,Citation26 the estimated glomerular filtration rate (eGFR) and the presence of chronic kidney disease (CKD) were determined, and the presence of metabolic syndrome (MtS) was also checked. As previously reported,Citation28 the left ventricular ejection fraction (LVEF) was determined by echocardiography (UCG) using the modified Simpson method, and flow-mediated dilation (FMD) and nitroglycerin-induced dilation (NID) of the brachial artery were determined.
Finally, study participants were classified into DM or non-DM groups to determine any between-group differences in clinical backgrounds, CAG/SPT results, and prognosis.
Statistical Analyses
Continuous variables having values that were non-normally distributed were expressed as the median (interquartile range), while normally distributed continuous variables were expressed as the mean ± standard deviation. Between-group comparisons were performed using Student’s unpaired t-test, the Wilcoxon signed-rank test, and the χ2 test. The prognosis of MACE was analyzed using the Kaplan–Meier survival curve and tested using the Log rank test. These analyses were performed based on the presence of DM (DM group: n = 49, non-DM group: n = 223), atherosclerotic lesions (presence: n = 133, absence: n = 139), focal spasm (focal spasms: n = 98, diffuse spasm: n = 174), MVS (presence: n = 158, single vessel spasm: n = 80), LDA (presence: n = 59, absence: n = 213), and ST elevation during SPT (presence: n = 31, absence: n = 241). In addition, these analyses performed using the Kaplan–Meier survival curve were based on the presence of focal spasms (n = 98, DM group: n = 12, non-DM: n = 86) and atherosclerotic lesions (n = 133, DM group: n = 31, non-DM group: n = 102). The factors influencing MACE were assessed using the Cox proportional hazards model with the hazard ratio (HR) and its confidence interval (CIs). JMP version 17 (SAS Institute, Cary, NC, USA) was used for all statistical analyses, and P < 0.05 was considered statistically significant.
Results
Clinical Characteristics
Patient characteristics are shown in . We found no significant differences in sex and mean age; however, the body mass index was significantly higher in the DM group than in the non-DM group (P < 0.001). Conventional coronary risk factors, family CAD history, and frequency of CKD did not differ significantly between the two groups; however, the frequency of MtS was significantly higher in the DM than in the non-DM group (P < 0.001).
Table 1 Patients’ Characteristics
Blood biochemistry, urinalysis, and echocardiographic parameters are shown in . Lipid-related indices did not differ significantly between the two groups. Both FBS and HbA1C were significantly higher in the DM than in the non-DM group (P < 0.001). The CRP, BNP, and eGFR values did not differ significantly between groups. The UACR could be measured in 205 patients, and the results did not differ significantly between groups. As echocardiographic indices, the LVEF on UCG did not differ significantly between groups. Brachial artery ultrasonography was available in 244 patients, and we found no significant differences in FMD or NID between groups.
Table 2 Blood Biochemical, Urine, and Ultrasonographic Parameters
Medications and factors associated with VSA are shown in . Regarding medications administered on admission, the rate of use of long-acting nitrate and renin–angiotensin system (RAS) inhibitors was significantly greater in the DM than in the non-DM group. The following table shows the specific DM medications in the DM group (). Seventeen patients (35%) engaged in diet and exercise without taking any anti-diabetic medications. The median number of DM medications in the DM group was 1 (0, 2). The frequency of coronary dilator use did not differ significantly between groups at admission, discharge, and follow-up. The contents of coronary dilators at discharge are shown in , and no significant differences were found between the two groups. No statistically significant difference was found in the frequency of chest symptoms (whether they occurred at rest, on exertion, or both), severe symptoms (such as cold sweats and syncope), or VA. There was no significant difference in the median number of angina attacks per month at admission or during follow-up.
Table 3 Medications and VSA-Related Parameters
Results of CAG and SPT
Results of CAG and SPT are shown in . In CAG, atherosclerotic lesions were observed in 133 patients (49%). The frequency of atherosclerotic lesions was significantly higher in the DM group than in the non-DM group (P = 0.028), whereas the frequency of MB did not differ significantly between groups. On SPT, the frequency of focal spasms was observed in 98 patients (36%). The frequencies of atherosclerotic lesions and focal spasm were not significantly coincident (P = 0.132). No significant differences in the frequency of coronary spasm induced by LDA, TOC, ST-segment elevation during SPT, and MVS were found between groups. The frequency of focal spasm tended to be lower in the DM group than in the non-DM group (P = 0.072). The number of coronary artery branches in which coronary spasms occurred did not differ between groups. Regarding the serious complications, the frequency of prolonged AVB was higher in the DM group than in the non-DM group (P = 0.032), while the frequencies in Vf and unstable hemodynamics requiring catecholamine use did not differ significantly between the two groups. There was no significant difference in the rate of serious complications that occurred during SPT between groups.
Table 4 Results of CAG and SPT
Prognosis
The median follow-up time was 68 (23, 101) months for the non-DM group and 80 (52, 109) months for the DM group, and this parameter did not differ significantly between the two groups (P = 0.196). The frequency of anginal attacks per month during follow-up and the frequency of consumption of coronary dilators did not differ significantly between groups ().
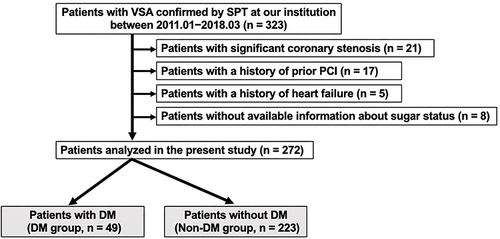
Of the 234 (86%) patients who were eligible for follow-up, 38 (16%) had MACE, including these cases: 2 cardiac deaths, 1 nonfatal myocardial infarction, 1 PCI for worsening angina, 5 heart failures, 23 rehospitalizations because of worsening angina, 3 other nonpharmacological cardiac treatments, 1 revascularization of peripheral artery disease, and 2 cerebral infarctions. The incidence of MACE was observed in the DM group (10 patients, 23%, No. = 43) versus non-DM group (28 patients, 15%, No. = 191, P = 0.174). Noncardiac death was observed in 11 patients (5%, No. = 234), and there was no significant difference in the rate of this outcome between groups (P = 0.986). The Kaplan–Meier MACE-free rate did not differ between the two groups (P = 0.234, ). Furthermore, it did not differ between groups with and without atherosclerotic lesions (P = 0.362, ); however, it was worse in patients with focal spasms compared with those with diffuse spasms (P = 0.028, ). Other factors, such as the presence of MVS (P = 0.155), LDA (P = 0.881), and ST elevations during the SPT (P = 0.341) did not influence MACE (Supplementary File1). Cox proportional hazards model analyses revealed that aging (HR 1.06, CI 1.02–1.11, P = 0.003) and the presence of focal spasm (HR 1.97, CI: 1.03–3.77, P = 0.041) were determinants of MACE. In patients with atherosclerotic lesions (133 patients), the presence of DM did not significantly affect MACE (; P = 0.743); however, in patients with focal spasm (98 patients), the MACE-free survival rate differed between groups (; P = 0.042). Even in patients with focal spasms, the Cox proportional hazards model revealed that aging (HR 1.09, CI: 1.02–1.17, P = 0.013) and the presence of DM (HR 2.82, CI: 1.05–7.60, P = 0.041) were factors influencing MACE. The MACEs in the focal spasm group with follow-up are as follows: in the DM group (n = 12), there were six cases (50%): 1 cardiac death, 1 PCI, 1 heart failure, 2 readmissions for chest pain, and 1 PAD; in the non-DM group (n = 73), there were 13 (18%): 1 cardiac death, 3 heart failures, and 9 readmissions for chest pain. The incidence of MACE was higher in the DM group than in the non-DM group (P = 0.023).
Figure 2 Kaplan–Meier curve for MACE-free survival during the follow-up period for the non-DM and DM groups.
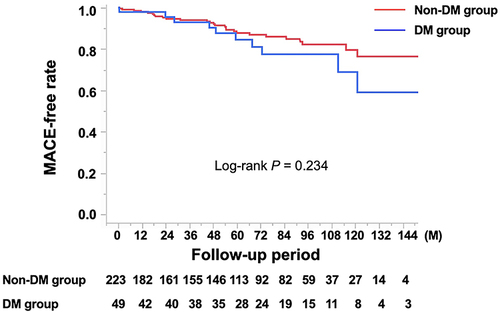
Figure 3 Kaplan–Meier curve for MACE-free survival during the follow-up period: influence of (A) atherosclerotic lesions (B) focal spasms.
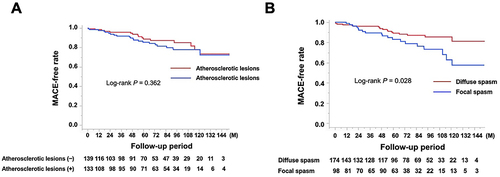
Figure 4 Kaplan–Meier curve for MACE-free survival during the follow-up period for the non-DM and DM groups in (A) patients with atherosclerotic lesions (B) patients with focal spasms.
Discussion
In this retrospective study, we investigated the clinical background and prognostic impact of DM on VSA. The DM group had a greater frequency of atherosclerotic lesions on CAG; however, it did not differ significantly from the non-DM group in any SPT parameters. The prognosis did not differ significantly between the DM and non-DM groups, although the prognosis for patients with focal spasm might be worse; notably, focal spasm was less frequent in the DM group. As indicated in the guidelines,Citation2 DM associated with VSA should be strictly managed, and more attention may be required in treatment when DM is associated with focal spasm.
A hyperglycemic state causes vascular endothelial dysfunction,Citation31 which has been recognized in many clinical studies as an important factor involved in CAD pathogenesis.Citation14,Citation32 Many clinical studies have also reported the relationship between coronary spasm and DM.Citation15–21,Citation33–35 Increased insulin resistance is considered a key mechanism underlying VSA pathogenesis,Citation15–17 and some reports have shown that insulin resistance is associated with the degree of coronary spasm.Citation34 However, other reports document that DM is not associated with the pathogenesis of coronary spasms.Citation33 In addition, some reports indicate that DM is a prognostic factor for VSA,Citation18–21 whereas others do not list it as a prognostic factor.Citation7,Citation8,Citation10
The present study investigated how the clinical context of VSA differs from DM and how it further influences prognosis. We found no significant differences in clinical characteristics, except that DM is associated with a greater frequency of atherosclerotic lesions in the coronary arteries. Considering that patients with DM had an HbA1C of 6.9, 35% of them were on a diet, and the UACR did not significantly differ from that in the non-DM group, it is likely that the patients with DM in this study had mild disease or relatively good glycemic control. Furthermore, in this study, no statistically significant difference in vascular endothelial function was observed between groups, which may also reflect the mild degree of DM in the study participants. In contrast, the non-DM group also had reduced vascular endothelial function, which may indicate the presence of other etiological factors or the presence of VSA-related endothelial dysfunction,Citation36 which is one of the mechanisms of VSA pathogenesis that may have masked some of DM’s influence on VSA. One important reason for the different prognostic impacts of these DM complications may be the influence of differences in patient background, especially in the degree and duration of DM.
One class of coronary spasms, focal versus diffuse spasm, has a worse prognosis than diffuse spasm.Citation9,Citation22 This study’s findings also corroborate the fact that VSA patients with focal spasm have a poorer prognosis than those with diffuse spasm. Possible explanations for poorer prognosis with focal spasm include the fact that the degree of ischemia at the time of spasm is greater in focal spasm than in diffuse spasm,Citation28 as well as the presence or formation of unstable atherosclerotic lesions at the site of focal spasm.Citation23,Citation37 Furthermore, unstable atherosclerotic lesions may exist or form at the site of the focal spasm, possibly with unstable plaques, such as yellowish or thrombosed lesions on coronary angioscopyCitation24,Citation25,Citation38 and thrombus formation or intraplaque hemorrhage on optical coherence tomography.Citation24,Citation39,Citation40
The focus of the present study was on the effect of DM on focal spasm, which may be associated with such unstable plaques; therefore, a sub-analysis of patients with focal spasm was conducted, with the results suggesting that focal spasm may be less likely in the DM group. One report indicates that increased insulin resistance is associated with diffuse spasm,Citation41 partially supporting our results. In contrast, others report no difference in coronary spasm morphology depending on the presence or absence of DM.Citation33 However, the definition of focal spasm employed may lead to differences in interpretation.Citation28 In this study, the frequency of atherosclerotic lesions was increased by DM complications; however, focal spasm was rather unlikely to occur. Furthermore, our results suggest that focal spasm may have a poor prognosis when complicated with DM, even though it tends to be less common, and this finding might be noteworthy.
Although the small number of cases in this study necessitates caution in the interpretation of the results, various atherosclerosis-promoting mechanisms resulting from DMCitation42 may have affected the prognosis of focal spasm, with the possibility of such unstable plaques. In addition, although this is a retrospective study and how the DM treatment changed during the follow-up periods is unknown, the choice of DM medications at the time of admission may differ from the current standard of care.Citation14 For example, the use of sodium-glucose cotransporter 2 inhibitors and glucagon-like peptide 1 agonist has been reported to prevent heart failureCitation43 and atherosclerosis,Citation44 and these anti-diabetic drugs are now the first choice for patients predisposed to atherosclerosis.Citation14 If such drugs had been used in the study participants with DM, the results may have differed from those obtained with other or older drug treatment choices. In any case, the prognosis of focal spasm in DM can be evaluated in the future using the current standard DM medications after increasing the number of cases in a multicenter registry or other methods and specifically defining focal spasm.
The sub-analysis also examined the effect of DM on atherosclerotic lesions in VSA; however, it found little impact of DM on the VSA prognosis of patients with and without atherosclerotic lesions. Patients with lesions considered significantly stenotic were excluded from the current study. Similarly, Hao et al reported that the VSA prognosis of patients with atherosclerotic lesions that were not significantly stenotic was similar to that of VSA patients without atherosclerotic lesions.Citation45 Also, in the authors’ previous study that was conducted using coronary angioscopy,Citation25 intracoronary thrombi were found more frequently in areas with focal spasm and not in areas of atherosclerotic lesions that did not present with spasm. As previously noted, atherosclerotic lesions at focal spasm sites and atherosclerotic lesions without focal spasm may have different plaque characteristics, such as instability. The prognosis of such lesions should be studied in the future by increasing the number of cases in a multicenter registry.
Nevertheless, this study has several limitations. First, the study design was retrospective, with few cases and single-center data. In particular, the small number of patients in the sub-analysis of the prognostic value of focal spasm in DM may have influenced the results. In addition, the study assessed only Japanese patients, and it is unclear whether the results can be applicable globally. Second, the definition of DM in this study was based on FBS only and not the oral glucose tolerance test. In addition, although guidelines suggest the importance of repeated testing,Citation32 patients with DM were mainly evaluated with only one blood test, which may underestimate the frequency of DM. The definition of pre-DM may also be a concern,Citation32 with much evidence that this condition also influences the pathogenesis of atherosclerosis,Citation46 and the inclusion of such patients in the non-DM group to examine DM versus non-DM may have an impact on the prognosis of the patients in the non-DM group. In addition, some DM parameters were not assessed; these parameters include insulin resistanceCitation21,Citation34 and intraday glucose variability,Citation35 and they may affect VSA status. Third, although we followed patients up as much as possible in the prognostic study of this study, the follow-up rate was insufficient. Fourth, coronary atherosclerotic lesions were found only in CAG and were not evaluated in detail using intravascular imaging, such as optical coherence tomography. Fifth, in this study, we defined MACE as cardiac death and readmission due to cardiovascular disease. Again, in addition to the fact that some cases have not been adequately followed up, MACE has been designed to focus on the effects of diabetes rather than the effects of coronary spasms, which may have significantly affected the study’s findings. Finally, the frequency of chest pain was indicated by the number of times it occurred per month, and a recent objective evaluation methodCitation47 was not employed.
Conclusion
This study retrospectively examined the clinical background and prognosis of DM complications in patients with VSA. The degree of vascular endothelial dysfunction and UACR did not differ significantly between DM and non-DM patients. Atherosclerotic disease on CAG was more frequent in patients with DM. Patients with DM tended to have focal spasm less frequently, and patients with focal spasm may have a worse prognosis. While DM patients with VSA should be managed per established guidelines, those with focal spasm may need to be treated more rigorously. Future studies with large numbers of patients should clarify this issue.
Disclosure
The authors report no conflicts of interest in this work.
Acknowledgments
We appreciate Ms. Akemi Seno’s help with secretarial duties. We also applaud the personnel of our institution’s cardiovascular unit, outpatient clinic, and catheterization laboratory.
References
- Yasue H, Nakagawa H, Itoh T, Harada E, Mizuno Y. Coronary artery spasm--clinical features, diagnosis, pathogenesis, and treatment. J Cardiol. 2008;51(1):2–17. doi:10.1016/j.jjcc.2008.01.001
- Group JCSJW. Guidelines for diagnosis and treatment of patients with vasospastic angina (Coronary Spastic Angina) (JCS 2013). Circ J. 2014;78(11):2779–2801. doi:10.1253/circj.cj-66-0098
- Beltrame JF. Management of vasospastic angina. Heart. 2022;109(1):70–77. doi:10.1136/heartjnl-2022-321268
- Hokimoto S, Kaikita K, Yasuda S, et al. JCS/CVIT/JCC 2023 guideline focused update on diagnosis and treatment of vasospastic angina (coronary spastic angina) and coronary microvascular dysfunction. Circ J. 2023;87(6):879–936. doi:10.1253/circj.CJ-22-0779
- Sueda S, Kohno H, Oshita A, Izoe Y, Nomoto T, Fukuda H. Vasospastic heart failure: multiple spasm may cause transient heart failure? J Cardiol. 2009;54(3):452–459. doi:10.1016/j.jjcc.2009.07.007
- Ahn JM, Lee KH, Yoo SY, et al. Prognosis of variant angina manifesting as aborted sudden cardiac death. J Am Coll Cardiol. 2016;68(2):137–145. doi:10.1016/j.jacc.2016.04.050
- Takagi Y, Takahashi J, Yasuda S, et al. Prognostic stratification of patients with vasospastic angina: a comprehensive clinical risk score developed by the Japanese coronary spasm association. J Am Coll Cardiol. 2013;62(13):1144–1153. doi:10.1016/j.jacc.2013.07.018
- Yasue H, Takizawa A, Nagao M, et al. Long-term prognosis for patients with variant angina and influential factors. Circulation. 1988;78(1):1–9. doi:10.1161/01.CIR.78.1.1
- Sato K, Kaikita K, Nakayama N, et al. Coronary vasomotor response to intracoronary acetylcholine injection, clinical features, and long-term prognosis in 873 consecutive patients with coronary spasm: analysis of a single-center study over 20 years. J Am Heart Assoc. 2013;2(4):e000227. doi:10.1161/JAHA.113.000227
- Ishii M, Kaikita K, Sato K, et al. Acetylcholine-provoked coronary spasm at site of significant organic stenosis predicts poor prognosis in patients with coronary vasospastic angina. J Am Coll Cardiol. 2015;66(10):1105–1115. doi:10.1016/j.jacc.2015.06.1324
- Holman RR, Paul SK, Bethel MA, Neil HA, Matthews DR. Long-term follow-up after tight control of blood pressure in type 2 diabetes. N Engl J Med. 2008;359(15):1565–1576. doi:10.1056/NEJMoa0806359
- Ray KK, Seshasai SR, Wijesuriya S, et al. Effect of intensive control of glucose on cardiovascular outcomes and death in patients with diabetes mellitus: a meta-analysis of randomised controlled trials. Lancet. 2009;373(9677):1765–1772. doi:10.1016/S0140-6736(09)60697-8
- Nakatani D, Sakata Y, Suna S, et al. Incidence, predictors, and subsequent mortality risk of recurrent myocardial infarction in patients following discharge for acute myocardial infarction. Circ J. 2013;77(2):439–446. doi:10.1253/circj.cj-11-1059
- Cosentino F, Grant PJ, Aboyans V, et al. ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur Heart J. 2020;41(2):255–323. doi:10.1093/eurheartj/ehz486
- Shimabukuro M, Shinzato T, Higa S, et al. Enhanced insulin response relates to acetylcholine-induced vasoconstriction in vasospastic angina. J Am Coll Cardiol. 1995;25(2):356–361. doi:10.1016/0735-1097(94)00381-y
- Shinozaki K, Suzuki M, Ikebuchi M, et al. Insulin resistance associated with compensatory hyperinsulinemia as an independent risk factor for vasospastic angina. Circulation. 1995;92(7):1749–1757. doi:10.1161/01.cir.92.7.1749
- Suzuki M, Arita M, Arita M, Kakuta T, Numano F, Numano F. Impaired glucose tolerance with late hypersecretion of insulin during oral glucose tolerance test in patients with vasospastic angina. J Am Coll Cardiol. 1996;27(6):1458–1463. doi:10.1016/0735-1097(96)00011-3
- Ito A, Fukumoto Y, Shimokawa H. Changing characteristics of patients with vasospastic angina in the era of new calcium channel blockers. J Cardiovasc Pharmacol. 2004;44(4):480–485. doi:10.1097/01.fjc.0000141473.29254.62
- Io K, Minatoguchi S, Nishigaki K, et al. Effects of benidipine and some other calcium channel blockers on the prognosis of patients with vasospastic angina. Cohort study with evaluation of the ergonovine coronary spasm induction test. Arzneimittelforschung. 2007;57(9):573–581. doi:10.1055/s-0031-1296652
- Nishigaki K, Inoue Y, Yamanouchi Y, et al. Prognostic effects of calcium channel blockers in patients with vasospastic angina--a meta-analysis. Circ J. 2010;74(9):1943–1950. doi:10.1253/circj.CJ-10-0292
- Nakagomi A, Saiki Y, Kosugi M, et al. Effect of insulin resistance associated with compensatory hyperinsulinemia on the long-term prognosis in patients with vasospastic angina. Int J Cardiol. 2013;167(5):2222–2227. doi:10.1016/j.ijcard.2012.06.016
- Nishimiya K, Suda A, Fukui K, et al. Prognostic Links Between OCT-delineated coronary morphologies and coronary functional abnormalities in patients with INOCA. JACC. 2021;14(6):606–618. doi:10.1016/j.jcin.2020.12.025
- Saito S, Yamagishi M, Takayama T, et al. Plaque morphology at coronary sites with focal spasm in variant angina: study using intravascular ultrasound. Circ J. 2003;67(12):1041–1045. doi:10.1253/circj.67.1041
- Kitano D, Takayama T, Sudo M, et al. Angioscopic differences of coronary intima between diffuse and focal coronary vasospasm: comparison of optical coherence tomography findings. J Cardiol. 2018;72(3):200–207. doi:10.1016/j.jjcc.2018.04.013
- Teragawa H, Orita Y, Oshita C, Uchimura Y. Intracoronary thrombogenicity in patients with vasospastic angina: an observation using coronary angioscopy. Diagnostics. 2021;11(9).
- Teragawa H, Oshita C, Uchimura Y. Clinical characteristics and prognosis of patients with multi-vessel coronary spasm in comparison with those in patients with single-vessel coronary spasm. J Cardiovasc Dev Dis. 2022;9(7).
- Sueda S, Miyoshi T, Sasaki Y, Sakaue T, Habara H, Kohno H. Sequential spasm provocation tests might overcome a limitation of the standard spasm provocation tests. Coron Artery Dis. 2015;26(6):490–494. doi:10.1097/MCA.0000000000000267
- Teragawa H, Oshita C, Uchimura Y. Does the intracoronary pressure differ according to two types (diffuse or focal) of coronary spasm? World J Cardiol. 2023;15(1):1–12. doi:10.4330/wjc.v15.i1.1
- Umemura S, Arima H, Arima S, et al. The Japanese society of hypertension guidelines for the management of hypertension (JSH 2019). Hypertens Res. 2019;42(9):1235–1481. doi:10.1038/s41440-019-0284-9
- Mach F, Baigent C, Catapano AL, et al. ESC/EAS Guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J. 2019;40:2700–2709. doi:10.1093/eurheartj/ehz455
- Maruhashi T, Soga J, Fujimura N, et al. Relationship between flow-mediated vasodilation and cardiovascular risk factors in a large community-based study. Heart. 2013;99(24):1837–1842. doi:10.1136/heartjnl-2013-304739
- ElSayed NA, Aleppo G, Aroda VR, et al. 2. classification and diagnosis of diabetes: standards of care in diabetes-2023. Diabetes Care. 2023;46(Suppl 1):S19–S40. doi:10.2337/dc23-S002
- Li YJ, Hyun MH, Rha SW, et al. Diabetes mellitus is not a risk factor for coronary artery spasm as assessed by an intracoronary acetylcholine provocation test: angiographic and clinical characteristics of 986 patients. J Invasive Cardiol. 2014;26(6):234–239.
- Kang KW, Choi BG, Rha SW. Impact of insulin resistance on acetylcholine-induced coronary artery spasm in non-diabetic patients. Yonsei Med J. 2018;59(9):1057–1063. doi:10.3349/ymj.2018.59.9.1057
- Ito T, Ichihashi T, Fujita H, et al. The impact of intraday glucose variability on coronary artery spasm in patients with dysglycemia. Heart Vessels. 2019;34(8):1250–1257. doi:10.1007/s00380-019-01353-w
- Kugiyama K, Ohgushi M, Motoyama T, et al. Nitric oxide-mediated flow-dependent dilation is impaired in coronary arteries in patients with coronary spastic angina. J Am Coll Cardiol. 1997;30(4):920–926. doi:10.1016/S0735-1097(97)00236-2
- Miyao Y, Kugiyama K, Kawano H, et al. Diffuse intimal thickening of coronary arteries in patients with coronary spastic angina. J Am Coll Cardiol. 2000;36(2):432–437. doi:10.1016/S0735-1097(00)00729-4
- Etsuda H, Mizuno K, Arakawa K, Satomura K, Shibuya T, Isojima K. Angioscopy in variant angina: coronary artery spasm and intimal injury. Lancet. 1993;342(8883):1322–1324. doi:10.1016/0140-6736(93)92245-O
- Shin ES, Her AY, Ann SH, et al. Thrombus and plaque erosion characterized by optical coherence tomography in patients with vasospastic angina. Rev Esp Cardiol. 2017;70(6):459–466. doi:10.1016/j.recesp.2016.10.027
- Nishi T, Kume T, Yamada R, et al. Layered Plaque in organic lesions in patients with coronary artery spasm. J Am Heart Assoc. 2022;11(7):e024880. doi:10.1161/JAHA.121.024880
- Park SH, Choi BG, Rha SW, Kang TS. The multi-vessel and diffuse coronary spasm is a risk factor for persistent angina in patients received anti-angina medication. Medicine. 2018;97(47):e13288. doi:10.1097/MD.0000000000013288
- La Sala L, Prattichizzo F, Ceriello A. The link between diabetes and atherosclerosis. Eur J Prev Cardiol. 2019;26(2_suppl):15–24. doi:10.1177/2047487319878373
- Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes. N Engl J Med. 2015;373(22):2117–2128. doi:10.1056/NEJMoa1504720
- Marso SP, Daniels GH, Brown-Frandsen K, et al. Liraglutide and Cardiovascular Outcomes in Type 2 Diabetes. N Engl J Med. 2016;375(4):311–322. doi:10.1056/NEJMoa1603827
- Hao K, Takahashi J, Kikuchi Y, et al. Prognostic impacts of comorbid significant coronary stenosis and coronary artery spasm in patients with stable coronary artery disease. J Am Heart Assoc. 2021;10:e017831. doi:10.1161/JAHA.120.017831
- Tamita K, Katayama M, Takagi T, et al. Impact of newly diagnosed abnormal glucose tolerance on long-term prognosis in patients with acute myocardial infarction. Circ J. 2007;71(6):834–841. doi:10.1253/circj.71.834
- Ford TJ, Stanley B, Good R, et al. Stratified medical therapy using invasive coronary function testing in angina: The CorMicA Trial. J Am Coll Cardiol. 2018;72(23Pt A):2841–2855. doi:10.1016/j.jacc.2018.09.006