Abstract
Patients suffering from acromegaly and Cushing’s Disease (CD) face the risk of several clinical complications. The onset of diabetes mellitus (DM) is among the most important: exposure to elevated growth hormone or cortisol levels is associated with insulin resistance (IR). DM contributes to increasing cardiovascular risk for these subjects, which is higher compared to healthy individuals. Hyperglycemia may also be caused by pasireotide, a second-generation somatostatin receptor ligand (SRLs), currently used for the treatment of these diseases. Accordingly, with 2014 medical expert recommendations, the management of hyperglycemia in patients with CD and treated with pasireotide is based on lifestyle changes, metformin, DPP-4 inhibitors (DPP-4i) and, subsequently, GLP-1 Receptor Agonists (GLP-1 RAs). There is no position for SGLT2-inhibitors (SGLT2-i). However, a very recent experts’ consensus regarding the management of pasireotide-induced hyperglycemia in patients with acromegaly suggests the use of GLP-1 RAs as first line treatment (in suitable patients) and the use of SGLT2-i as second line treatment in patients with high cardiovascular risk or renal disease. As a matter of fact, beyond the hypoglycemic effect of GLP1-RAs and SGLT2-i, there is increasing evidence regarding their role in the reduction of cardiovascular risk, commonly very high in acromegaly and CD and often tough to improve despite biochemical remission. So, an increasing use of GLP1-RAs and SGLT2-i to control hyperglycemia is desirable in these diseases. Obviously, all of that must be done with due attention in order to minimize the occurrence of adverse events. For this reason, large studies are needed to analyze the presence of potential limitations.
Introduction
Acromegaly and Cushing’s Disease (CD) are rare but weakening endocrine diseases.
Acromegaly is usually caused by a growth hormone (GH)-secreting pituitary adenoma, with subsequent excess of insulin-like growth factor (IGF-1).Citation1 CD is characterized by hyperproduction of cortisol due to an adrenocorticotropic hormone (ACTH)-secreting pituitary adenoma.Citation2 Impaired glucose metabolism and the onset of DM are common clinical conditions resulting from these diseases. The worsening of glycemic control might also be caused by treatment with somatostatin receptor analogs, more specifically with pasireotide.
Pasireotide, a second-generation somatostatin receptor ligand (SRLs), is currently used for the treatment of acromegaly and CD.Citation3,Citation4
In the management of acromegaly, long-acting pasireotide is recommended at a starting dose of 40 mg monthly (potentially up-titrated to 60 mg) in patients with poorly controlled or uncontrolled disease after failure with first generation SRLs. Several Randomized Control Trials (RCTs) have shown better outcomes in achieving biochemical control compared to octreotide and lanreotide, both in parallel arms as well as in a cross-over evaluation.Citation5,Citation6 In CD, pasireotide is approved for the treatment of persistent hypercortisolism after a surgical procedure or when surgery is not feasible or refused, at a start dose of 0.6 mg twice daily (potentially up titrated to 0.9 mg twice daily).Citation7,Citation8
Hyperglycemia and Increased Cardiovascular Risk in Acromegaly and CD
Impaired glucose metabolism is one of the comorbidities associated with acromegaly and CD, uniquely linked to the pathophysiology of the diseases. As a matter of fact, in acromegaly, the prevalence of altered basal glucose ranges between 7 and 22%, of altered glucose tolerance between 6 and 45%, and of diabetes between 19 and 56%.Citation9 Additionally, disorders of carbohydrate metabolism occur in 14–74% of the patients among the various forms of hypercortisolism while the prevalence of diabetes varies between 21 and 47%.Citation10
The pathogenesis of insulin resistance (IR) in acromegaly is due to multiple factors: GH exerts its effects both directly by inducing gluconeogenesis, glycogenolysis and lipolysis and promoting IR in the liver and peripheral tissues, as well as indirectly through IGF-1.Citation11 GH stimulates the hydrolysis of triglycerides and the production of free fatty acids from adipose tissue, and this increased synthesis of free fatty acids leads to a decrease in insulin-mediated glucose uptake by inhibiting glucose transporters GLUT-1 and GLUT-4.Citation12,Citation13 Moreover, GH suppresses key insulin signaling pathways involved in stimulating glucose transport in muscle and adipose tissue and inhibiting glucose production in the liver.Citation14
The effects of IR secondary to the excess of GH are initially compensated by the increased secretion of insulin from the pancreatic beta cells, which, however, diminishes over time, favoring the onset of prediabetes and diabetes.Citation15,Citation16 Once the beta cell function is affected, the glucose metabolism disorders persist even after the acromegaly is cured.Citation17 Although physiologically IGF-1 improves glucose homeostasis, the chronic excess of GH in acromegaly that causes IR greatly exceeds the possible beneficial effects of IGF-1 on insulin sensitivity.Citation18
Similar to the excess of GH, hypercortisolism affects carbohydrate metabolism mainly in liver, skeletal muscles, and adipose tissue.Citation19 In the liver, excess glucocorticoids stimulate gluconeogenesis by activating numerous genes involved in the hepatic gluconeogenesis, stimulating lipolysis and proteolysis with increasing substrates for gluconeogenesis, potentiating the action of glucagon and inhibiting glycogenogenesis.Citation20
In the muscle, hypercortisolism induces IR by interfering with different components of the insulin-signaling cascade, as well as by stimulating proteolysis and loss of muscle mass. All this reduces the capacity of the muscle to synthesize glycogen and uptake most of the postprandial glucose from circulation.Citation21
Additionally, hypercortisolism causes an increase in visceral obesity and a relative reduction in peripheral adipose tissue, and this “shift” is closely associated with metabolic syndrome and worsens IR. Moreover, the excess of cortisol influences the synthesis and release of hormones from adipose tissue, mainly adipokines, further contributing to the development of IR.Citation21
Glucocorticoids inhibit the synthesis and secretion of insulin. Also in CD, there is an initial transient phase characterized by the increase in insulin secretion as an adaptive mechanism to IR, but later the chronic exposure to higher levels of cortisol induces pancreatic beta cell apoptosis, loss of beta cell function and the subsequent development of diabetes.Citation20,Citation22
The involvement of the bone system in affecting glucose homeostasis has also been found: in fact, long-term exposure to glucocorticoids causes a reduction in circulating osteocalcin that can increase IR.Citation23
Furthermore, two studies in humansCitation24,Citation25 suggested that secretion of incretins (glucagon-like peptide-1, GLP-1 and glucose dependent insulinotropic peptide, GIP) was unaffected by dexamethasone administration, but their insulinotropic effects of on beta-cells were reduced.
The worsening of glycemic control and the onset of DM are also important limitations in the management of some patients treated with pasireotide.Citation26,Citation27 This topic will be further explored in a subsequent paragraph.
As is well known, hyperglycemia contributes to increasing cardiovascular risk, which is already very high in patients with acromegaly or CD.Citation28,Citation29
Cardiovascular disease is the leading cause of death in 23–50% of patients with acromegaly in different studies.Citation9 Hypertension affects about 33% of the patients, ranging from 11 to 54.7%,Citation30 and it is strongly related with typical cardiac implications of acromegaly as valvulopathy, arrhythmias and cardiomyopathy.
In the large Liege Acromegaly Survey database of 3173 acromegalic patients from 10 European countries,Citation31 left ventricular hypertrophy was present in 15.5% at time of diagnosis. The most common manifestations of cardiopathy are biventricular hypertrophy, diastolic-systolic dysfunction, and valvular regurgitation.Citation32 Certainly, the severity of cardiac disease is correlated with age, duration of acromegaly, GH and IGF-1 levels (both vascular growth factors which stimulate collagen deposition) and long-standing hypertension.Citation33 In the worst cases, hypertrophic cardiopathy can evolve into Left Ventricular Systolic Dysfunction (LVSD), the last stage of cardiac disease, with recurring hospitalizations and very high mortality rates.Citation34 Acromegaly is also associated with sleep apnea (ranging from 45 to 80% of the cases).Citation35
Similarly, in CD cardiovascular disease is the leading cause of death: a retrospective study involving 502 patients (83% in remission) with a median follow-up of 13 yearsCitation36 demonstrated a standardized mortality ratio (SMR) of 3.3 (95% CI 2.6–4.3) for CV disease, in particular 3.6 (95% CI 2.5–5.1) for ischemic cardiac disease and 3.0 (95% CI 1.4–5.7) for stroke. SMR related cardiovascular disease remained higher also after biochemical remission (2.5, 95% CI 1.8–3.4).Citation36 Cardiovascular remodeling caused by hypercortisolism is frequently irreversible: at 5 years post-remission, coronary artery plaques persisted in 27% of subjects vs 3% of control.Citation37 As a result, the risk for ischemic events remains above that of the general population.Citation38
Hypertension is highly prevalent in patients with hypercortisolism: the majority (80–85%) of patients have hypertension at diagnosis and 9% may have required hospital admission because of the hypertension crisis before the diagnosis of hypercortisolism.Citation39 Also, after remission, hypertension results are highly prevalent, as shown in two different studies (50% and 40%, respectively).Citation40,Citation41 Up to 70% of the patients with active CD present abnormal left ventricular mass parameters, whereas systolic and diastolic function were usually normal. Rarely, patients present dilatative cardiopathy and severe HF.Citation42 Moreover, greater incidence of hypokalemia exposes patients to fatal arrhythmias.
Finally, both obesity and dyslipidemia, frequently occurring in these diseases, do not normalize despite biochemical remission.
Mechanisms of Pasireotide-Induced Hyperglycemia
Pasireotide is a multi-receptor targeted SRL, with action on different somatostatin receptors (SSTR). Pasireotide binds with high affinity to SSTR-1, 3 and 5 and lower to SSTR-2 than first generation SSA. More specifically, the affinity for SSTR-5, several times greater than those of octreotide and lanreotide, explains the efficacy of pasireotide: this binding causes the suppression of ACTH and GH, accompanied by tumor volume reduction.Citation43,Citation44
However, this mechanism causes the alteration of glucose metabolism because the binding is not specific to pituitary cells. Stimulation of pancreatic SSTR-5, expressed more in Langerhans islet beta cells than alfa cells (87% vs 44%), suppresses insulin secretion much more than glucagon secretion.Citation45
Pasireotide appears to inhibit the secretion of incretin hormones GIP (glucose-dependent insulinotropic polypeptide) and GLP-1 (glucagon-like peptide-1) in health volunteers after oral glucose tolerance test (OGTT),Citation46 even if a recent study showed no differences in incretin levels and their response to mixed meal tolerance test (MMTT) in CD patients,Citation47 suggesting a main role of direct inhibition of beta-cells activity. However, a reduced intra-islet paracrine effect of GLP-1 cannot be excluded whereas an increased IL-6 mediated GLP-1 secretion in CD may disguise pasireotide inhibitory effect.Citation47,Citation48 Furthermore, pasireotide has no effect on hepatic and peripheral insulin sensitivity.Citation46
Pasireotide-induced hyperglycemia is less pronounced following multiple dosing, and it appears even reversible upon discontinuation of the drug,Citation49 as shown in a pharmacokinetic analysis of single-dose administration, in which mean glucose levels increased to 200 mg/dL (11.1 mmol/L) and returned to euglycemia approximately 23 hours later.Citation50
Not all patients treated with pasireotide develop impaired glucose tolerance or DM: the prevalence of these conditions in CD is respectively 21–64% and 20–47%,Citation51 whereas in acromegaly it is 6–45% and 16–65%.Citation9 This suggests that glycemic control prior to the treatment and a preceding DM, could be predictive of the extent of hyperglycemia.
In the PAOLA studyCitation6 a fasting blood glucose (FBG) > 100 mg/dL (5.5 mmol/L) at baseline correlated with higher FBG and higher HbA1c during treatment with pasireotide, while patients with acromegaly < 40 years of age were less likely to experience hyperglycemia than older patients.
Moreover, in acromegalic patients, the up-titration to a dose of 60 mg was associated with a 21–36% increased risk of hyperglycemia.Citation52,Citation53 Other factors that could increase the risk of hyperglycemia were a Body Mass Index > 30 kg/m², hypertension and dyslipidemia at baseline.Citation54
Superimposable results were obtained in another Phase III study,Citation55 always performed in subjects with acromegaly: it was reported that up to 45% of patients with baseline FBG between 100 (5.5 mmol/L) and 126 mg/dL (7.0 mmol/L) had FBG levels ≥126 mg/dL (7.0 mmol/L) after 26 months of pasireotide.Citation55
Also, in CD, preexisting DM or impaired glucose tolerance increased the risk of hyperglycemia-related adverse events (AEs) with pasireotide, although severe AEs were not reported.Citation7
A meta-analysis showed a lower frequency of hyperglycemia-related AEs in acromegalic patients treated with pasireotide monthly (57.3–67.0%) in comparison to those who received it twice daily for CD (68.4–73.0%).Citation27 Also, the rate of discontinuation due to hyperglycemia was higher in CD trials (6.0% and 5.3%) than that in acromegaly trials (3.4% and 4.0%).Citation5–7,Citation56 The reasons for these findings are unknown.
On the other hand, it has been acknowledged that other drugs, commonly used for the treatment of acromegaly or CD, may affect glucose metabolism leading to clinical benefits, even during pasireotide therapy. In fact, in acromegalic subjects, cabergoline can improve glucose tolerance,Citation57 whereas pegvisomant reduces fasting glucose levels and improves insulin sensitivity.Citation58,Citation59 Similar results have been highlighted for ketoconazole,Citation60 metyraponeCitation61 and osilodostratCitation62 in studies involving patients with CD.
Antidiabetic Drugs with Proven Cardiovascular Benefits
The evidence from Cardio Vascular Outcome Trials with GLP-1 RAs and SGLT2-i have revolutionized the management of Type 2 Diabetes Mellitus (T2DM). As reaffirmed in the recent American Diabetes Association-European Association for the Study of Diabetes (ADA-EASD) Consensus, the treatment approach must be holistic and person-centered, with four main areas of interest: glycemic control, weight loss, CV risk reduction and renal protection.Citation63
In a network meta-analysis of 453 trials assessing glucose-lowering medications from nine drug classes, the greatest reductions in HbA1c were seen with GLP-1 RAs.Citation64 Another meta-analysis comparing the effects of glucose-lowering drugs on body weight and blood pressure indicated the greatest efficacy for reducing body weight with GLP-1 RAs, whereas the greatest reduction in blood pressure is seen with the SGLT2-i.Citation65
Among GLP-1 RAs, liraglutide (at a dose of 1.8 mg daily),Citation66 dulaglutide (at a dose 1.5 mg weekly)Citation67 and injectable semaglutide (at a dose of 0.5 and 1 mg weekly)Citation68 reduced the incidence of three point-MACE (Major Adverse Cardiovascular Events) and the progression of CKD (Chronic Kidney Disease) through the reduction of albuminuria.
With regard to SGLT2-i, empagliflozin and canagliflozin reduced the incidence of three point-MACE.Citation69,Citation70 Empagliflozin, dapagliflozin and canagliflozin demonstrated improvement of CKD in trials with specific renal outcomes, and the first two also demonstrated this benefit in patients without T2DM.Citation71–73 Another significant clinical benefit is the reduction of hospitalization for heart failure (HF), demonstrated also in patients without T2DM for empagliflozin and dapagliflozin, both with reduced ejection fraction (HFrEF)Citation74,Citation75 and preserved ejection fraction (HFpEF).Citation76,Citation77
The Current Management of Pasireotide-Induced Hyperglycemia
Several studies, performed with different designs, evaluated the impact of pasireotide on glucose metabolism. The principal results are summarized in .Citation5–8,Citation78–85
Table 1 Main Studies Regarding the Use of Pasireotide in Acromegaly and in Cushing’s Disease
It’s undeniable that impairment of glucose metabolism occurred: generally, in all studies the number of subjects with diabetes and prediabetes increased, HbA1c levels were higher and anti-hyperglycemic treatments were required. Metformin, DPP-4i and insulin were commonly used to treat hyperglycemia, whereas GLP-1 RAs and SGLT2-i were given only in a small number of cases.
Nevertheless, a recent randomized multicenter study involving 81 patients with acromegaly or CD receiving pasireotideCitation86 and uncontrolled hyperglycemia with metformin or other oral antidiabetic medications (acarbose or sulfonylureas), evaluated the effects of two different regimens of treatment (incretin-based therapy vs insulin). All 38 patients randomized to an incretin-based therapy (acromegaly, n = 26; CD, n =12) received sitagliptin; 28 of them switched to liraglutide. Twelve patients (31.6% [CD, n = 6; acromegaly, n = 6]) randomized to incretin-based therapy received insulin as rescue therapy. The results have shown a trend for better control of HbA1c with incretin-based therapy. Furthermore, in the same study, 109 patients who received pasireotide did not develop hyperglycemia requiring antidiabetic treatment.Citation86 These findings suggest that impaired glucose metabolism or onset of DM during pasireotide therapy are manageable in most patients, without the need for treatment discontinuation.
Accordingly, given the above-mentioned evidence, glycemia should be monitored in all patients treated with pasireotide in order to intercept an initial alteration of glucose metabolism which could be either prediabetes or DM, according to the indications of ADA.Citation87 In patients treated with pasireotide, FBG and HbA1c levels tend to increase during the first 1–3 months of treatment and stabilize thereafter.Citation88
Regarding CD, in 2014, a medical expert recommendation on pasireotide-induced hyperglycemia was published.Citation89 In this, an HbA1c target value less than 7.0–7.5% (53–58 mmol/L) is established, avoiding as much as possible the risk of hypoglycemia. Patients in euglycemia prior to therapy must be monitored: they should self-check FBG and postprandial glucose (PPG) levels during the day, precisely twice in the first week and once weekly later. Instead, patients with prediabetes and DM must be monitored closely (after 1, 2 and 4 weeks), and they should self-check blood glucose values up to six times per day during the first week, and at least four times per day thereafter.Citation26,Citation89
Medical treatment should always include dietary modification and exercise. Metformin is the first line-therapy, unless contraindicated or not tolerated. If glycemic control is not reached or maintained with monotherapy, combination therapy with drugs targeting the incretinic axis is recommended:Citation89 a Phase I studyCitation90 in 19 healthy volunteers randomized to pasireotide 600 μg sc bid alone or co-administered with antidiabetic drugs (metformin 500 mg bid, nateglinide 60 mg tid, vildagliptin 50 mg bid and liraglutide 0.6 daily) demonstrated greater effects of vildagliptin and liraglutide in minimizing hyperglycemia.
Therefore, therapy with a DDP-4i is suggested in a first step combination. Only in the case of failure to reach the HbA1c target, the replace of DDP-4i with a GLP-1 RAs is recommended. If pasireotide-induced hyperglycemia remains uncontrolled with combinations containing metformin and DPP-4i or GLP-1 RAs, experts’ recommendations suggest the beginning of basal insulin therapy. If the individual HbA1c targets are not achieved or the postprandial glucose levels remains elevated, prandial insulin can be added.Citation89
Instead, in acromegaly, a very interesting experts’ consensus statement regarding the management of pasireotide-induced hyperglycemia has been recently published.Citation91 It suggests monitoring blood glucose prior to initiation of pasireotide treatment, through the determination of HbA1c or FBG or the execution of OGTT. Patients are divided into three risk categories related to glycemic status: normal glucose tolerance (NGT) patients at low risk, NGT patients at high risk and prediabetic or diabetic patients. In low-risk patients with no worsening of glycemic control, self-measurement of blood glucose (FBG and PPG) once every week is considered sufficient. In high-risk patients who do not have elevated blood glucose levels, weekly self-monitoring (FBG and PPG) is recommended in the first three months. In patients with pre-existing hyperglycemia, daily self-monitoring in recommended with at least one FBG and one PPG, ideally as multiple-point profiles.Citation91 Further, when possible and economically feasible, high-risk patients should temporarily be equipped with continuous glucose monitors (CGMs) to detect elevated blood glucose levels early and determine deviations from the time in range precisely. During treatment with pasireotide, HbA1c measurements should be routinely performed every three months and at least with each IGF-1measurement.Citation91
For the treatment of hyperglycemia, this recent experts’ consensus statement represents an important leap forward from a conceptual point of view. As a matter of fact, glycemic targets are not strictly fixed but an individualized approach for each patient is suggested. Moreover, CV risk is introduced as a factor influencing the choice of antidiabetic drugs.
Obviously, lifestyle intervention (physical activity, healthy sleep, high-quality nutrition) is always suggested. Metformin is indicated as a first-line medication but, considering the high CV risk of acromegalic subjects, GLP-1 RAs with proven CV benefits could also be considered as a first-line treatment. DPP-4i are considered a viable alternative to GLP-1 RAs in case of gastrointestinal side-effects.Citation91
However, studies demonstrated that 10–30% of acromegalic patients show a paradoxical increase in GH (PI-GH) during 75-g OGTT.Citation3 This is probably due to the action of GIP, which is higher in acromegalic patients, particularly in those with hyperglycemia, and that is likely able to increase the secretion of GH.Citation92,Citation93 As is well known, DPP-4i reduce the incretin-degrading enzyme DPP-4 and thus increase the concentration of active incretins, including GIP. Accordingly, a recent study showed that sitagliptin, administered one hour before 75-g OGTT, increase GH in acromegalic patients, especially in those with PI-GH.Citation94 For this reason, acromegalic patients should be carefully monitored for a potential worsening of the underlying disease during treatment with a DPP- 4i.
The use of SGLT2-i is recommended only as second-line treatment for patients with high CV risk and/or renal disease, despite their high prevalence in acromegaly.Citation91 This is justified by the increased risk of diabetic ketoacidosis (DKA), a severe condition related to treatment with SGLT2-i, in acromegalic subjects.Citation95–97 However, patients safely treated with pasireotide and SGLT2-i are reported.Citation98
The addition of insulin may be considered, but it should ideally be used as an adjunct to metformin and at least one other therapeutic agent.
Obviously, in case of poor glycemic control despite treatment with several anti-hyperglycemic drugs, the dose reduction or even the discontinuation of pasireotide should be considered.
A Potential Change of Perspective and Open Issues
Considering the complex cardiovascular profile of patients with acromegaly and CD, a much greater use of GLP-1 RAs and SGLT2-i might be necessary if DM occurs. There are at least three important aspects that support this consideration: glycemic control, cardiovascular protection, and weight loss.
Accordingly, both in acromegaly and CD, the use of GLP-1 RAs contributes to the achievement of these three main goals, providing an important possibility to enhance the quality of life and to decrease the mortality of patients, with evident advantages compared to DDP-4i and insulin.Citation86,Citation91,Citation99 In this regard, co-agonists of GLP-1 and GIP, such as tirzepatide, with their extraordinary impact in terms of HbA1c reduction and weight loss, represent a theoretically intriguing therapeutic option for the future, despite the current lack of data in acromegaly and CD.
SGLT2-i are not included in the expert recommendations for the patients with CD.Citation89 Currently, there is not enough evidence to support their use, even if their impact on cardiorenal risk might be valuable.
The same reasoning could apply to the acromegalic subjects. In particular, the very favorable benefit of SGLT2-i on HF risk could be extremely crucial.
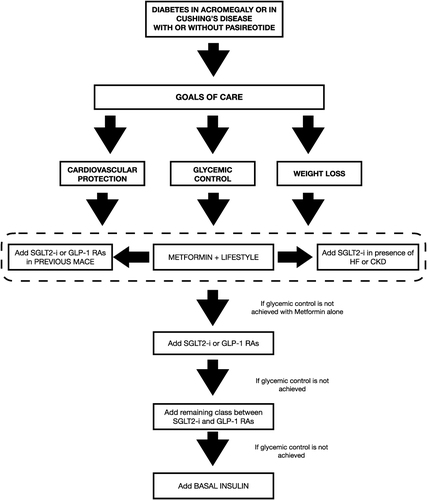
A proposal for an approach to contrasting hyperglycemia, also taking into account the higher cardio-renal risk, in acromegaly and CD is depicted in .
Figure 1 Proposal for a new approach to treat hyperglycemia in patients with acromegaly or Cushing’s Disease, with or without pasireotide treatment. The restoration of euglycemia should be achieved with concomitant reduction in terms of weight and cardiovascular risk, improving quality of life and decreasing mortality.
Potential limits are higher costs and the risk of AEs. It is well known that the most common AEs of GLP-1 RAs are gastrointestinal (nausea, vomiting, and diarrhea) and tend to occur during initiation and dose escalation, diminishing over time.Citation100 Same AEs are noted with pasireotide, even if described as non-severe.
Another AE common to both treatments (pasireotide and GLP-1 RAs) are cholelithiasis and gallbladder disease. Different meta-analysis of RCTs confirmed that GLP1-RAs are associated with an increased risk of cholelithiasis, in the absence of any relevant increase in the risk of pancreatitis and pancreatic cancer.Citation101,Citation102 It is notable that in the study which compared incretin-based and insulin therapy, patients in the latter group had a higher incidence of gallbladder or biliary-related AEs (23.3% vs 13.2%).Citation86
Instead, as reported in the recent consensus about the management of hyperglycemia in acromegaly, a potential limit for the use of SGLT2-i is the risk of DKA, a condition characterized by hyperglycemia, metabolic acidosis and ketosis (pH ≤ 7.3, bicarbonate ≤ 15 mmol/L, anion gap > 12 mmol/L), fortunately rare in acromegaly, considering it concerns only 1% of all cases and it often occurs only in the initial disease manifestation.Citation103 During treatment with SGLT2-i, DKA occurs in the absence of hyperglycemia, and so it also known as euglycemic diabetic ketoacidosis (EuDKA).Citation104 The suggested mechanism behind the EuDKA is the reduction of insulin requirement in patient treated with SGLT2-i due to massive glycosuria, with concomitant increased gluconeogenesis (driven by an increase of glucagon), release of free fatty acid and subsequent propensity to ketone production.Citation105
It is noteworthy that GH and cortisol themselves increase lipolysis, the lipid oxidation rate and so ketone bodies. Moreover, the shift in the insulin/glucagon ratio as observed in pasireotide treatment is thought to be especially prone to this metabolic complication, warranting greater caution.Citation103
It’s essential to consider the higher risk of DKA or EuDKA during treatment with SGLT2-i, but it’s equally necessary to specify that their incidence appears significantly lower compared to that of a fatal cardiovascular event, both in acromegaly and CD. As a matter of fact, a multicenter retrospective study, during 2015–2020, in 9940 persons with T2DM treated with SGLT2-i has shown that the overall prevalence of DKA is around 0.43% (with 0.25% for EuDKA).Citation106 Furthermore, even some real-life evaluations conducted in subjects with Type 1 Diabetes, a clinical condition with a well-known high risk of DKA and in which the use of SGLT2-i is actually contraindicated, have shown similar data: Stougard et alCitation107 have observed an incidence of DKA equal to 0% in patients treated with SGLT2-i whereas Anson et alCitation108 have observed a lower risk of DKA and associated hospitalization in subjects treated with SGLT-2i compared to those treated with GLP-1 RAs (obviously, as an adjunct to insulin therapy).
Additionally, in acromegalic subjects treated with pegvisomant, in monotherapy or in combination with pasireotide, the incidence of the EuDKA should be reduced. In fact, a reciprocal positive interaction could be achieved because SGLT2-i attenuate the hyperglycemic effect by decreased insulin secretion, meanwhile pasireotide in combination with pegvisomant mitigates the hyperglucagonemia induced by SGLT2-i. Also, pegvisomant decreases lipid oxidation via extrahepatic suppression of Growth Hormone Receptor in different tissues.Citation109
Hence, it seems reasonable to encourage the use of SGLT2-i even in acromegalic patients treated with pasireotide, especially in those with well-controlled disease, modest hyperglycemia and undergoing combined treatment with pegvisomant. It should be helpful to advise them to discontinue therapy with SGLT2-i in case of intercurrent illnesses that may cause a reduction in carbohydrates intake and dehydration (eg, infections and gastroenteritis), and to not skip doses in the case of contextual insulin therapy. SGLT2-i should be avoided in patients with poorly controlled disease.
The same considerations could also be applied to patients with poorly controlled CD.
Another potential limit for the use of SGLT2-i, especially in CD patients for the overall increased risk of infection in this disease, is the higher prevalence of genitourinary infections, reported in both clinical trials and real world evidence. These infectious events are usually mild, and their prevalence is related to sex and a prior positive history of genital infections. In fact, the risk appears higher in females, and among them, in those with previous infections.Citation110 Moreover, it is interesting to underline that in the study of McGovern et alCitation110 the use of corticosteroids, a clinical condition similar to CD, higher values of HbA1c were not associated with significant additional infection risk in subjects treated with SGLT2-i.
Therefore, it is good clinical practice to suggest meticulous intimate hygiene to patients treated with SGLT-2i, avoiding the use of this class of drugs in those with positive anamnesis for genitourinary infections, especially for females.
It is also worth noting that neither GLP-1 RAs nor SGLT2-i cause hypoglycemia, another condition that significantly increases cardiovascular risk and mortality, as demonstrated in the ACCORD trial.Citation111
Finally, a recent case reportCitation112 showed the positive effect of a combined therapy of GLP-1 RAs and SGLT2-i on pasireotide-induced hyperglycemia in a patient with CD. After the failure of metformin and DPP-4i, multiple daily insulin injections and, after two days, dulaglutide 0.75 mg were initiated. After improvement of glycemic control, 10 mg of empagliflozin was started and insulin discontinued. After 3 months, hypercortisolemia and glucose impairment were well-regulated, and the patient’s health improved overall.Citation112
Despite several limits (not optimal use of insulin, short follow-up, lack of data regarding other parameters), this is an example of a treatment that is not glycemic-centered but focused to prevent and improve hypercortisolemia-related complications.
Needless to say, further investigations are needed to analyze the above-mentioned considerations and to overcome the limited findings available.
Ethics Statement
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution and considerations, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that they have no competing interests in this work.
Additional information
Funding
References
- Sanno N, Teramoto A, Osamura RY, et al. Pathology of pituitary tumors. Neurosurg Clin N Am. 2003;14(1):25–39. doi:10.1016/s1042-3680(02)00035-9
- Tritos NA, Miller KK. Diagnosis and management of pituitary adenomas: a review. JAMA. 2023;239(16):1386–1389. doi:10.1001/jama.2023.5444
- Katznelson L, Laws ER, Helmed S, et al. Acromegaly: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2014;99(11):3933–3951. doi:10.1210/jc.2014-2700
- Nieman LK, Biller BMK, Findling JW, et al. Treatment of Cushing’s syndrome: an Endocrine Society clinical practice guidelines. J Clin Endocrinol Metab. 2015;100(8):2807–2831. doi:10.1210/jc.2015-1818
- Colao A, Bronstein MD, Freda P, et al. Pasireotide versus octreotide in acromegaly: a head-to-head superiority study. J Clin Endocrinol Metab. 2014;99(3):791–799. doi:10.1210/jc.2013-2480
- Gadelha MR, Bronstein MD, Brue T, et al. Pasireotide versus continued treatment with octreotide or lanreotide in patients with inadequately controlled acromegaly (PAOLA): a randomised, Phase 3 trial. Lancet Diabetes Endocrinol. 2014;2(11):875–884. doi:10.1016/S2213-8587(14)70169-X
- Colao A, Petersenn S, Newell-Price J, et al. A 12-month Phase 3 study of pasireotide in Cushing’s disease. N Engl J Med. 2012;366(10):914–924. doi:10.1016/S2213-8587(14)70169-X
- Lacroix A, Gu F, Gallardo W, et al. Efficacy and safety of once-monthly pasireotide in Cushing’s disease: a 12 month clinical trial. Lancet Diabetes Endocrinol. 2018;6(1):17–26. doi:10.1016/S2213-8587(17)30326-1
- Pivonello R, Auriemma RS, Grasso LF, et al. Complications of acromegaly, cardiovascular, respiratory and metabolic comorbidities. Pituitary. 2017;20:46–62. doi:10.1007/s11102-017-0797-7
- Li D, El Kawkgi OM, Henriquez AF, Bancos I. Cardiovascular risk and mortality in patients with active and treated hypercortisolism. Gland Surg. 2020;9(1):43–58. doi:10.21037/gs.2019.11.03
- Ershadinia N, Tritos NA. Diagnosis and treatment of acromegaly: an update. Mayo Clin Proc. 2022;97:333–346. doi:10.1016/j.mayocp.2021.11.007
- Ferraù F, Albani A, Ciresi A, Giordano C, Cannavò S. Diabetes secondary to acromegaly, physiopathology, clinical features and effects of treatment. Front Endocrinol. 2018;9:358. doi:10.3389/fendo.2018.00358
- Dal J, List EO, Jørgensen JOL, Berryman DE. Glucose and fat metabolism in acromegaly: from mice models to patient care. Neuroendocrinology. 2015;103:96–105. doi:10.1159/000430819
- Del Rincon JP, Iida K, Gaylinn BD, et al. Growth hormone regulation of p85α expression and phosphoinositide 3-kinase activity in adipose tissue. Diabetes. 2007;56:1638–1646. doi:10.2337/db06-0299
- Moustaki M, Paschou SA, Xekouki P, et al. Secondary diabetes mellitus in acromegaly. Endocrine. 2023;81(1):1–15. doi:10.1007/s12020-023-03339-1
- Kasayama S, Otsuki M, Takagi M, et al. Impaired β-cell function in the presence of reduced insulin sensitivity determines glucose tolerance status in acromegalic patients. Clin Endocrinol. 2000;52:549–555. doi:10.1046/j.1365-2265.2000.00986.x
- Kinoshita Y, Fujii H, Takeshita A, et al. Impaired glucose metabolism in Japanese patients with acromegaly is restored after successful pituitary surgery if pancreatic β-cell function is preserved. Eur J Endocrinol. 2011;164:467–473. doi:10.1530/EJE-10-1096
- Frara S, Maffezzoni F, Mazziotti G, Giustina A. Current and emerging aspects of diabetes mellitus in acromegaly. Trends Endocrinol Metab. 2016;27:470–483. doi:10.1016/j.tem.2016.04.014
- Popovicu MS, Paduraru L, Nutas RM, et al. Diabetes mellitus secondary to endocrine diseases: an update of diagnostic and treatment particularities. Int J Mol Sci. 2023;24(16):12676. doi:10.3390/ijms241612676
- Scaroni C, Zilio M, Foti M, Boscaro M. Glucose metabolism abnormalities in Cushing syndrome: from molecular basis to clinical management. Endocr Rev. 2017;38(3):189–219. doi:10.1210/er.2016-1105
- Barbot M, Ceccato F, Scaroni C. Diabetes mellitus secondary to Cushing’s disease. Front Endocrinol. 2018;5(9):284. doi:10.3389/fendo.2018.00284
- Pivonello R, De Leo M, Vitale P, et al. Pathophysiology of diabetes mellitus in Cushing’s syndrome. Neuroendocrinology. 2010;92(Suppl 1):77–81. doi:10.1159/000314319
- Brennan-Speranza TC, Henneicke H, Gasparini SJ, et al. Osteoblasts mediate the adverse effects of glucocorticoids on fuel metabolism. J Clin Investig. 2012;122:4172–4189. doi:10.1172/JCI63377
- Eriksen M, Jensen DH, Tribler S, Holst JJ, Madsbad S, Krarup T. Reduction of insulinotropic properties of GLP-1 and GIP after glucocorticoid-induced insulin resistance. Diabetologia. 2015;58(5):920–928. doi:10.1007/s00125-015-3522-y
- Jensen DH, Aaboe K, Herniksen JE, et al. Steroid-induced insulin resistance and impaired glucose tolerance are both associated with a progressive decline of incretin effect in first-degree relatives of patients with type 2 diabetes mellitus. Diabetologia. 2012;55(5):1406–1416. doi:10.1007/s00125-012-2459-7
- Vergès B. Effects of anti-somatostatin agents on glucose metabolism. Diabetes Metab. 2017;43(5):411–415. doi:10.1016/j.diabet.2017.05.003
- Silvertstein JM. Hyperglycemia induced by Pasireotide in patients with Cushing’s disease or acromegaly. Pituitary. 2016;19:536–543. doi:10.1007/s11102-016-0734-1
- Puglisi S, Ferraù F, Ragonese M, Spagnolo F, Cannavò S. Cardiometabolic risk in acromegaly: a review with a focus on pasireotide. Front Endocrinol. 2020;11:28. doi:10.3389/fendo.2020.00028
- Coulden A, Hamblin R, Wass J, Karavitaki N. Cardiovascular health and mortality in Cushing’s disease. Pituitary. 2022;25(5):750–753. doi:10.1007/s11102-022-01258-4
- Puglisi S, Terzolo M. Hypertension and acromegaly. Endocrinol Metab Clin North Am. 2019;48:779–793. doi:10.1016/j.ecl.2019.08.008
- Petrossians P, Daly AF, Natchev E, et al. Acromegaly at diagnosis in 3173 patients from the Liege Acromegaly Survey (LAS) Database. Endocr Relat Cancer. 2017;24(10):505–518. doi:10.1530/ERC-17-0253
- Sharma AN, Tan M, Amsterdam EA, Singh GD. Acromegalic cardiomyopathy: epidemiology, diagnosis and management. Clin Cardiol. 2018;41(3):419–425.
- Colao A, Marzullo P, Di Somma C, Lombardi G. Growth hormone and the heart. Clin Endocrinol. 2001;54(2):137–154. doi:10.1046/j.1365-2265.2001.01218.x
- Marstrand P, Han L, Day SM, et al. Hypertrophic cardiomyopathy with left ventricular systolic dysfunction: insights from the SHaRe Registry. Circulation. 2020;141(17):1371–1383. doi:10.1161/CIRCULATIONAHA.119.044366
- Davì MV, Giustina A. Sleep apnea in acromegaly, a review on prevalence, pathogenetic aspects and treatment. Expert Rev Endocrinol Metab. 2012;7:55–62. doi:10.1586/eem.11.82
- Ragnarsson O, Olsson DS, Papakokkinou E, et al. Overall and disease-specific mortality in patients with Cushing disease: a Swedish nationwide study. J Clin Endocrinol Metab. 2019;104(6):2375–2384. doi:10.1210/jc.2018-02524
- Colao A, Pivonello R, Spiezia S, et al. Persistence of increased cardiovascular risk in patients with Cushing’s disease after five years of successful care. J Clin Endocrinol Metab. 1999;84(8):2664–2672. doi:10.1210/jcem.84.8.5896
- Varlamov EV, Langlois F, Vila G, Fleseriu M. Management of endocrine disease: cardiovascular risk assessment, thromboembolism, and infection prevention in Cushing’s syndrome: a practical approach. Eur J Endocrinol. 2021;184(5):R207–R224. doi:10.1530/EJE-20-1309
- Fallo F, Di Dalmazi G, Beuschlein F, et al. Diagnosis and management of hypertension in patients with Cushing’s syndrome: a position statement and consensus of the Working Group on Endocrine Hypertension of the European Society of Hypertension. J Hypertens. 2022;40(11):2085–2101. doi:10.1097/HJH.0000000000003252
- Giordano R, Picu A, Marinazzo E, et al. Metabolic and cardiovascular outcomes in patients with Cushing's syndrome of different aetiologies during active disease and 1 year after remission. Clin Endocrinol. 2011;75(3):354–360. doi:10.1111/j.1365-2265.2011.04055.x
- Faggiano A, Pivonello R, Spiezia S, et al. Cardiovascular risk factors and common carotid artery caliber and stiffness in patients with Cushing’s disease during active disease and 1 year after disease remission. J Clin Endocrinol Metab. 2003;88(6):2527–2533. doi:10.1210/jc.2002-021558
- Toja PM, Branzi G, Ciambellotti F, et al. Clinical relevance of cardiac structure and function abnormalities in patients with Cushing’s syndrome before and after cure. Clin Endocrinol. 2012;76(3):332–338. doi:10.1111/j.1365-2265.2011.04206.x
- Bruns C, Lewis I, Briner U, Meno-Tetang G, Weckbecker G. SOM230: a novel somatostatin peptidomimetic with broad somatotropin release inhibiting factor (SRIF) receptor binding and a unique antisecretory profile. Eur J Endocrinol. 2002;146(5):707–716. doi:10.1530/eje.0.1460707
- Moloney KJ, Mercado JU, Ludlam WH, Mayberg MR. Pasireotide (SOM230): a novel pituitary-targeted medical therapy for the treatment of patients with Cushing’s disease. Expert Rev Endocrinol Metab. 2012;7(5):491–502. doi:10.1586/eem.12.49
- Singh V, Brendel MD, Zacharias S, et al. Characterization of somatostatin receptor subtype-specific regulation of insulin and glucagon secretion: an in vitro study on isolated human pancreatic islets. J Clin Endocrinol Metab. 2007;92:673–680. doi:10.1210/jc.2006-1578
- Henry RR, Ciaraldi TP, Armstrong D, Burke P, Ligueros-Saylan M, Mudaliar S. Hyperglycaemia associated with pasireotide: results from a mechanistic study in healthy volunteers. J Clin Endocrinol Metab. 2013;98:3446–3453. doi:10.1210/jc.2013-1771
- Barbot M, Mondin A, Regazzo D, et al. Incretin response to mixed meal challenge in active Cushing’s disease and after pasireotide therapy. Int J Mol Sci. 2022;23:5217. doi:10.3390/ijms23095217
- Schmid AH, Brueggen J. Effects of somatostatin analogs on glucose homeostasis in rats. J Endocrinol. 2012;212:49–60. doi:10.1530/JOE-11-0224
- MacKenzie Feder J, Bourdeau I, Vallette S, Beauregard H, Marie LG S, Lacroix A. Pasireotide monotherapy in Cushing’s disease: a single-centre experience with 5-year extension of phase III trial. Pituitary. 2014;17(6):519–529. doi:10.1007/s11102-013-0539-4
- Golor G, Hu K, Ruffin M, et al. A first-in-man study to evaluate the safety, tolerability, and pharmacokinetics of pasireotide (SOM230), a multireceptor-targeted somatostatin analog, in healthy volunteers. Drug Des Devel Ther. 2012;6:71–79. doi:10.2147/DDDT.S29125
- Feelders RA, Pulgar SJ, Kempel A, Pereira AM. The burden of Cushing’s disease: clinical and health-related quality of life aspects. Eur J Endocrinol. 2012;167(3):311–326. doi:10.1530/EJE-11-1095
- Shen G, Darstein C, Hermosillo Resendiz K, Hu K. Pharmacokinetic and pharmacodynamic analyses of pasireotide LAR and octreotide LAR: randomized, double-blind, phase III study in patients with medically naïve acromegaly. Poster presented at: European congress of endocrinology; May 3–7; 2014; Wroclaw, Poland.
- Shen G, Darstein C, Hermosillo Resendiz K, Hu K. Analysis of pharmacokinetic (PK) and pharmacodynamic (PD) data for efficacy and safety from a randomized phase III study of pasireotide LAR in patients with acromegaly inadequately controlled on first-generation somatostatin analogs (SSA). Poster presented at: Endocrine society annual meeting; March 5–8; 2015; San Diego, CA.
- Gadelha MR, Gu F, Bronstein MD, et al. Risk factors and management of pasireotide-associated hyperglycemia in acromegaly. Endocr Connect. 2020;9(12):1178–1190. doi:10.1530/EC-20-0361
- Sheppard M, Bronstein MD, Freda P, et al. Pasireotide LAR maintains inhibition of GH and IGF-1 in patients with acromegaly for up to 25 months: results from the blinded extension phase of a randomized, double- blind, multicenter, phase III study. Pituitary. 2015;18(3):385–394. doi:10.1007/s11102-014-0585-6
- Boscaro M, Bertherat J, Findling J, et al. Extended treatment of Cushing’s disease with pasireotide: results from a 2-year, Phase II study. Pituitary. 2014;17(4):320–326. doi:10.1007/s11102-013-0503-3
- Higham CE, Atkinson AB, Aylwin S, et al. Effective combination treatment with cabergoline and low-dose pegvisomant in active acromegaly: a prospective clinical trial. J Clin Endocrinol Metab. 2012;97:1187–1193. doi:10.1210/jc.2011-2603
- van der Lely AJ, Hutson RK, Trainer PJ, et al. Long-term treatment of acromegaly with pegvisomant, a growth hormone receptor antagonist. Lancet. 2001;358:1754–1759. doi:10.1016/s0140-6736(01)06844-1
- Schreiber I, Buchfelder M, Droste M, et al. Treatment of acromegaly with the GH receptor antagonist pegvisomant in clinical practice: safety and efficacy evaluation from the German Pegvisomant Observational Study. Eur J Endocrinol. 2007;156:75–82. doi:10.1530/eje.1.02312
- Castinetti F, Guignat L, Giraud P, et al. Ketoconazole in Cushing’s disease: is it worth a try? J Clin Endocrinol Metab. 2014;99(5):1623–1630. doi:10.1210/jc.2013-3628
- Valassi E, Crespo I, Gich I, Rodrìguez J, Webb SM. A reappraisal of the medical therapy with steroidogenesis inhibitors in Cushing’s syndrome. Clin Endocrinol. 2012;77:735–742. doi:10.1111/j.1365-2265.2012.04424.x
- Gadelha M, Bex M, Feelders RA, et al. Randomized trial of osilodrostat for the treatment of Cushing’s disease. J Clin Endocrinol Metab. 2022;107(7):e2882–e2895. doi:10.1210/clinem/dgac178
- Davies MJ, Aroda VR, Collins BS, et al. Management of hyperglycemia in type 2 diabetes. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2022;45(11):2753–2786. doi:10.2337/dci22-0034
- Tsapas A, Avgerinos I, Karagiannis T, et al. Comparative effectiveness of glucose-lowering drugs for type 2 diabetes: a systematic review and network meta-analysis. Ann Intern Med. 2020;173:278–286. doi:10.7326/M20-0864
- Tsapas A, Karagiannis T, Kakotrichi P, et al. Comparative efficacy of glucose-lowering medications on body weight and blood pressure in patients with type 2 diabetes: a systematic review and network meta-analysis. Diabetes Obes Metab. 2021;23:2116–2124. doi:10.1111/dom.14451
- Marso SP, Daniels GH, Brown-Frandsen K, et al.; for the LEADER Steering Committe on behalf of the LEADER Trial Investigators. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375:311–322. doi:10.1056/NEJMoa1603827
- Gerstein HC, Colhoun HM, Dagenais GR, et al.; for the REWIND Investigators. Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): a double-blind, randomised placebo-controlled trial. Lancet. 2019;394:121–130. doi:10.1016/S0140-6736(19)31149-3
- Marso SP, Bain CS, Consoli A, et al.; for the SUSTAIN-6 Investigators. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Eng J Med. 2016;375:1834–1844. doi:10.1056/NEJMoa1607141
- Zinman B, Wanner C, Lachin JM, et al.; for the EMPA-REG OUTCOME Investigators. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;375:2117–2128. doi:10.1056/NEJMoa1504720
- Neal B, Perkovic V, Mahaffey KW, et al.; for the CANVAS Program Collaborative group. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377:644–657. doi:10.1056/NEJMc1712572
- The EMPA-KIDNEY Collaborative group. Empagliflozin in patients with chronic kidney disease. N Engl J Med. 2023;388:117–127. doi:10.1056/NEJMoa2204233
- Heerspink HJL, Stefánsson BV, Correa-Rotter R, et al.; for the DAPA-CKD Trial committees and investigators.. Dapagliflozin in patients with chronic kidney disease. N Engl J Med. 2020;383:1436–1446. doi:10.1056/NEJMoa2024816
- Perkovic V, Jardine MJ, Neal B, et al.; for the CREDENCE Trial investigators. Canagliflozin and renal outcome in type 2 diabetes and nephropathy. N Engl J Med. 2019;380:2295–2306. doi:10.1056/NEJMoa1811744
- Packer M, Anker SD, Butler J, et al.; for the EMPEROR-Reduced Trial investigators. Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med. 2020;383:1413–1424. doi:10.1056/NEJMoa2022190
- McCurray JJV, Solomon SD, Inzucchi SE, et al.; for the DAPA-HF Trial committees and investigators. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med. 2019;381:1995–2008. doi:10.1056/NEJMoa1911303
- Anker SD, Butler J, Filippatos G, et al.; fort the EMPEROR-Preserved Trial investigators. Empagliflozin in heart failure with a preserved ejection fraction. N Engl J Med. 2021;385:1451–1461. doi:10.1056/NEJMoa2107038
- Solomon SD, McMurray JJV, Clagget B, et al.; for the DELIVER Trial committees and investigators. Dapagliflozin in heart failure with mildly reduced of preserved ejection fraction. N Engl J Med. 2022;387:1089–1098. doi:10.1056/NEJMoa2206286
- Fleseriu M, Rusch E, Geer EB; on behalf of the ACCESS Study Investigators. Safety and tolerability of pasireotide long-acting release in acromegaly-results from the acromegaly, open-label, multicenter, safety monitoring program for treating patients who have a need to receive medical therapy (ACCESS) study. Endocrine. 2017;55:247–255. doi:10.1007/s12020-016-1182-4
- Lasolle H, Ferriere A, Vasilijevic A, Eimer S, Nunes ML, Tabarin A. Pasireotide-LAR in acromegaly patients treated with a combination therapy: a real-life study. Endocr Connect. 2019;8:1383–1394. doi:10.1530/EC-19-0332
- Witek P, Bolanowski M, Szamotulska K, Wojciechowska-Luzniak A, Jawiarczyk-Przybylowska A, Kaluzny M. The effect of 6 month’s treatment with pasireotide LAR on glucose metabolism in patients with resistant acromegaly in Real-World clinical settings. Front Endocrinol. 2021;10(12):633944. doi:10.3389/fendo.2021.633944
- Wolf P, Dormoy A, Maione L, et al. Impairment in insulin secretion without changes in insulin resistance explains hyperglycemia in patients with acromegaly treated with pasireotide LAR. Endocr Connect. 2022;11:e220296. doi:10.1530/EC-22-0296
- Fleseriu M, Petersenn S, Biller BMK, et al. Long-term efficacy and safety of once-monthly pasireotide in Cushing’s disease: a phase III extension study. Clin Endocrinol. 2019;91:776–785. doi:10.1111/cen.14081
- Pivonello R, Arnaldi G, Scaroni C, et al. The medical treatment with pasireotide in Cushing’s disease: an Italian multicentre experience based on “real-world experience”. Endocrine. 2019;64:657–672. doi:10.3389/fendo.2020.00648
- Simeoli C, Ferrigno R, De Martino MC, et al. The treatment with pasireotide in Cushing’s disease: effect of long-term treatment on clinical picture and metabolic profile and management of adverse events in the experience of a single center. J Endocrinol Invest. 2020;43:57–73. doi:10.1007/s12020-015-0557-2
- Sahin S, Karimova G, Özcan SG, Durcan E, Özkaya HM, Kadıoğlu P. Pasireotide treatment in Cushing’s Disease: a single tertiary center’s experience. Turk J Med Sci. 2022;52:467–476. doi:10.55730/1300-0144.5335
- Samson SL, Gu F, Feldt-Rasmussen U, et al. Managing pasireotide-associated hyperglycemia: a randomized, open-label, Phase IV study. Pituitary. 2021;24:887–903. doi:10.1007/s11102-021-01161-4
- ElSayed NA, Aleppo G, Aroda VR, et al. Classification and diagnosis of diabetes: standard of Care in Diabetes- 2023. Diabetes Care. 2023;46(Suppl.1):S19–S40. doi:10.2337/dc23-S002
- Samson SL. Management of hyperglycemia in patients with acromegaly treated with pasireotide LAR. Drugs. 2016;76(13):1235–1243. doi:10.1007/s40265-016-0615-y
- Colao A, De Block C, Gaztambide MS, Kumar S, Seufert J, Casanueva FF. Managing hyperglycemia in patients with Cushing’s disease treated with pasireotide: medical expert recommendations. Pituitary. 2014;17:180–186. doi:10.1007/s11102-013-0483-3
- Breitschaft A, Hu K, Hermosillo Resendiz K, Darstein C, Golor G. Management of hyperglycemia associated with pasireotide (SOM230): healthy volunteer study. Diabet Res Clin Pract. 2014;103:458–465. doi:10.1016/j.diabres.2013.12.011
- Störmann S, Meyhöfer SM, Groener JB, et al. Management of pasireotide-induced hyperglycemia in patients with acromegaly: an experts’ consensus statement. Front Endocrinol. 2024;15:1348990. doi:10.3389/fendo.2024.1348990
- Peracchi M, Porretti S, Gebbia C, et al. Increased glucose-dependent insulinotropic polypeptide (GIP) secretion in acromegaly. Eur J Endocrinol. 2001;145:R1–R4. doi:10.1530/eje.0.145r001
- Shekhawat VS, Bhansali S, Dutta P, et al. Glucose-dependent insulinotropic polypeptide (GIP) resistance and β-cell dysfunction contribute to hyperglycaemia in acromegaly. Sci Rep. 2019;9:5646. doi:10.1038/s41598-019-41887-7
- Oba-Yamamoto C, Kameda H, Miyoshi H, et al. Acromegaly cases exhibiting increased Growth Hormone levels during oral glucose loading with preadministration of dipeptidyl peptidase-4 inhibitor. Intern Med. 2021;60(15):2375–2383. doi:10.2169/internalmedicine.4755-20
- Quarella M, Walser D, Brandle M, Fournier JY, Bilz S. Rapid onset of diabetic ketoacidosis after SGLT2 inhibition in a patient with unrecognized acromegaly. J Clin Endocrinol Metab. 2017;102(5):1451–1453. doi:10.1210/jc.2017-00082
- Yoshida N, Goto H, Suzuki H, et al. Ketoacidosis as the initial clinical condition in nine patients with acromegaly: a review of 860 cases at a single institute. Eur J Endocrinol. 2013;169(1):127–132. doi:10.1530/eje-13-0060
- Prencipe N, Bioletto F, Bona C, Gatti F, Grottoli S. Diabetic ketoacidosis in acromegaly: a case study-somatostatin analogs adverse event or disease complication? Acta Diabetol. 2020;57(4):491–493. doi:10.1007/s00592-019-01437-z
- Zaina A, Grober Y, Abid A, Arad E, Golden E, Badarny S. Sodium glucose cotransporter 2 inhibitors treatment in acromegalic patients with diabetes-a case series and literature review. Endocrine. 2021;73(1):65–70. doi:10.1007/s12020-021-02718-w
- Mehlich A, Bolanowski M, Mehlich D, Witek P. Medical treatment of Cushing’s disease with concurrent diabetes mellitus. Front Endocrinol. 2023;14:1174119. doi:10.3389/fendo.2023.1174119
- Wharton S, Davies M, Dicker D, et al. Managing the gastrointestinal side effects of GLP-1 receptor agonists in obesity: recommendations for clinical practice. Postgrad Med. 2022;134(1):14–19. doi:10.1080/00325481.2021.2002616
- He L, Wang J, Ping F, et al. Association of Glucagon-Like Peptide-1 Receptor Agonist use with risk of gallbladder and biliary disease: a systematic review and meta-analysis of Randomized Clinical Trials. JAMA. 2022;182(5):513.519. doi:10.1001/jamainternmed.2022.0338
- Monami M, Nreu B, Scatena A, et al. Safety issues with glucagon-like peptide-1 receptor agonists (pancreatitis, pancreatic cancer and cholelithiasis): data from Randomized Controlled Trials. Diabetes Obes Metab. 2017;19(9):1233–1241. doi:10.1111/dom.12926
- Zaina A, Prencipe N, Golden E, et al. How to position sodium-glucose co-transporter 2 inhibitors in the management of diabetes in acromegaly patients. Endocrine. 2023;80(3):491–499. doi:10.1007/s12020-023-03352-4
- Peteres AL, Buschur EO, Buse JB, Cohan P, Diner JC, Hirsch IB. Euglycemic Diabetic Ketoacidosis: a potential complication of treatment with Sodium-Glucose Cotransporter 2 Inhibition. Diabetes Care. 2015;38(9):1687–1693. doi:10.2337/dc15-0843
- Sampani E, Sarafidis P, Papagianni A. Euglycaemic diabetic ketoacidosis as a complication of SGLT-2 inhibitors: epidemiology, pathophysiology, and treatment. Expert Opin Drug Saf. 2020;19(6):673–682. doi:10.1080/14740338.2020.1764532
- Ata F, Yousaf Z, Khan AA, et al. SGLT-2 inhibitors associated euglycemic and hyperglycemic DKA in multicentric cohort. Sci Rep. 2021;11:10293. doi:10.1038/s41598-021-89752-w
- Stougaard EB, Kristensen PL, Kielgast U, et al. Real life evaluation of sodium-glucose cotransporter 2 inhibition in type 1 diabetes and the risk of diabetic ketoacidosis. Diab Vasc Dis Res. 2022;19:14791641221130043.
- Anson M, Zhao SS, Austin P, Ibarburu GH, Malik RA, Alam U. SGLT2i and GLP-1 RA therapy in type 1 diabetes and Reno-vascular outcomes: a real-world study. Diabetologia. 2023;66:1869–1881.
- Adnan Z. Sodium Glucose Co-transporter Inhibitors in patients with acromegaly and diabetes. Trends Endocrinol Metab. 2019;30(2):77–79. doi:10.1016/j.tem.2018.11.007
- McGovern AP, Hogg M, Shields BM, et al.; BM MASTERMIND consortium. Risk factors for genital infections in people initiating SGLT2 inhibitors and their impact on discontinuation. BMJ Open Diabetes Res Care. 2020;8(1):e001238. doi:10.1136/bmjdrc-2020-001238
- The Action to Control Cardiovascular Risk in Diabetes Study Group. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358:2545–2559. doi:10.1056/NEJMoa0802743
- Shikata M, Ashida K, Goto Y, et al. Pasireotide-induced hyperglycemia in a patient with Cushing’s disease: potential use of sodium-glucose cotransporter 2 inhibitor and glucagon-like peptide-1 receptor agonist for treatment. Clin Case Rep. 2020;8(12):2613–2618. doi:10.1002/ccr3.3230
