Abstract
Purpose
To explore the correlation between hemoglobin, albumin, lymphocyte count, platelet count (HALP) score and type 2 diabetic retinopathy (DR).
Methods
The study was conducted on 674 patients with type 2 diabetes (T2DM). According to the results of the fundus examination, they were divided into non-diabetic retinopathy group (NDR, n=388) and diabetic retinopathy group (DR, n=286). Collected patients baseline data, calculated HALP score, analyzed the correlation between HALP score and DR.
Results
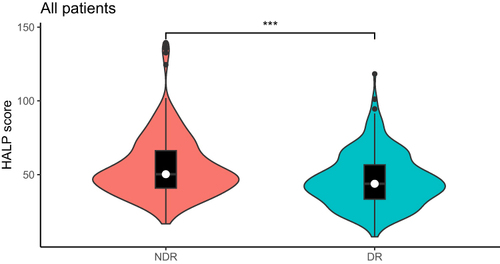
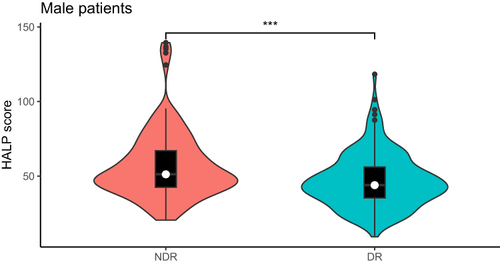
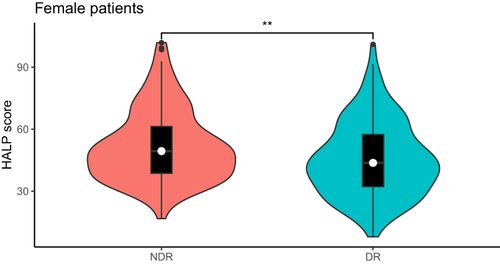
In all patients, male patients and female patients, the HALP score of the DR group was lower than that of the NDR group (P<0.001), and the HALP score was negatively correlated with the incidence of DR (P<0.05). HALP score was independent risk factors for DR, regardless of gender. In male patients, HALP score and DR had a linear relationship, but in female patients, HALP score and DR showed a nonlinear relationship, and HALP score was more sensitive to the onset of DR in male patients. The combined diagnostic model of HALP score, course of disease, SBP and BUN was used to diagnose DR, and it was found that the diagnostic value was the highest among male patients, with AUC of 0.761, sensitivity of 58.3% and specificity of 80.3%.
Conclusion
HALP score was an independent risk factor for DR, attention should be paid to monitoring HALP score, especially in male T2DM patients. The accuracy of HALP score, disease course, SBP and BUN combined model diagnosis of DR was high, which can become a biological indicator for early screening of DR.
Introduction
Diabetic retinopathy (DR) is a serious fundus disease caused by diabetes. According to statistics, after 15 years of diabetes, 3/4 of diabetics will be affected by DR.Citation1 DR mainly causes patients with low vision, which can lead to blindness in severe cases. In recent years, with the in-depth study of DR, it has been found that DR is closely related to the patient’s cerebrovascular accident, myocardial infarction, heart failure and all-cause mortality. DR not only affects the patient’s vision, but also increases the risk of acute vascular accident and death.Citation2 However, what is worrying is that there are still many patients who do not know the severity of DR, and in some areas where medical resources are scarce, fundus examination for diagnostic DR has not been carried out, resulting in a low detection rate of DR, delaying the timing of treatment, and seriously affecting the health of patients.Citation3 Therefore, finding more easily obtained biological indicators to assist in the diagnosis of DR has become the focus of our work.
DR is a well-known diabetic inflammatory neurovascular complication. The inflammatory changes found in the cornea of diabetic patients support the important role of inflammation in the development of DR, and the elevation of some inflammatory indicators in DR patients also confirms that DR is an inflammatory reaction.Citation4,Citation5 At the same time, nutrition cannot be ignored in the development of DR. In a large-scale healthy nutrition screening, it was found that serum albumin levels were significantly negatively correlated with DR.Citation6 In order to comprehensively assess the impact of inflammation and nutrition on DR, we introduced the HALP score. HALP score integrates four indicators of hemoglobin, albumin, lymphocyte count and platelet in serum. It is calculated from the formula and has many advantages such as simple, easy to obtain and economical. Clinically, it has been confirmed that HALP score can be used as an evaluation tool for cancer, blood lipid metabolism disorders, acute heart failure and stroke,Citation7–10 but the relationship between HALP score and DR is not clear. Therefore, this study analyzed the correlation between HALP score and DR to explore whether HALP score can be used to help diagnose the occurrence of DR.
Methods
Experimental design: This study was a cross-sectional study. The clinical data of 836 T2DM patients diagnosed in Hebei General Hospital from January 2021 to December 2023 were selected, and the patients were screened according to the following criteria. Included criteria: 1) Aged 18–80 years old; 2) T2DM patients diagnosed per the 2011 World Health Organization Diagnostic Standards for Diabetes. Exclusion criteria: 1) Acute complications of diabetes and acute stress status; 2) Patients with severe heart, liver and renal insufficiency;3) Patients with cancer, recent infection, immune system or blood system diseases;4) Patients with hyperthyroidism, glaucoma and other patients who cannot cooperate with fundus examination. In the end, 674 T2DM patients were included in this study. All patients underwent fundus examination. According to the DR classification criteria formulated by the International Society of Ophthalmology in 2002, they were divided into non-diabetic retinopathy group (NDR, n=388) and diabetic retinopathy group (DR, n=286). The HALP scores of the two groups were compared. Considering the differences between hemoglobin between male and female, patients were classified by gender again to explore the impact of HALP scores on DR of different genders.
Data collection and laboratory analysis: The baseline data of the patients were collected, including age, gender, course of disease, smoking history, drinking history, systolic blood pressure (SBP), diastolic blood pressure (DBP), height and weight. Laboratory examinations included hemoglobin (HGB), lymphocyte count (LYM), platelet count (PLT), glycated hemoglobin (HbA1c), fasting blood glucose (FBG), aspartate aminotransferase (AST), alanine transaminase (ALT), albumin (ALB), blood creatinine (Cr), blood uric acid (UA), blood urea nitrogen (BUN), glomerular filtration rate (eGFR), total cholesterol (TC), triglycerides (TG), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C). Formula calculation: HALP score = HGB×ALB×LYM/PLT;BMI (Kg/m2) = weight/height/height.
Statistical analysis: used Prism 8.0.1 and R version 4.3.1 software. Normally distributed measurement data were expressed as mean ± standard deviation (SD) and compared using two independent samples t-tests; Non-normally distributed measurement data were presented as median and quartile spacing (M [P25%, P75%]) and compared using the Mann–Whitney U-test. Count data were compared using the chi-square test. Mantel-Haenszel is used for Chi-square trend test. Restricted cubic splinear analysis was used to analyze the nonlinear relationship between the risk of DR and HALP score. Binary logistic regression was used to analyze the independent risk factors for DR. The receiver operating characteristic curve (ROC) and the area under the curve (AUC) were used to determine the diagnostic value of HALP scores for DR occurrence in T2DM patients. P<0.05 was considered statistically significant.
Results
Comparison of General Data and Laboratory-Related Indicators in T2DM Patients
Comparison of General Data and Laboratory-Related Indicators in All Patients
In all T2DM patients, the HALP score of DR patients was significantly lower than that of NDR patients (P<0.001). The age, diabetes course, SBP, BUN and HDL-C of patients in the DR group were significantly higher than those with NDR (P<0.05). The smoking history, HGB, LYM, ALT, ALB, UA, eGFR and TG in DR patients were significantly lower than that of NDR patients (P<0.05). There were no significant difference between the two in gender, drinking history, DBP, BMI, PLT, HbA1c, FBG, AST, Cr, TC, LDL-C (, ).
Table 1 Comparison of General Data and Laboratory-Related Indicators in All Patients
Comparison of General Data and Laboratory-Related Indicators in Male Patients
In all male patients with T2DM, the HALP score of patients with DR was significantly lower than that of patients with NDR (P<0.001). The age, diabetes course, SBP, BUN, HDL-C of DR patients were significantly higher than that of NDR patients (P<0.05). The smoking history, BMI, HGB, LYM, ALT, ALB, UA, eGFR and TG of DR patients were significantly lower than those of NDR patients (P<0.05). There were no obvious difference between the two in terms of drinking history, DBP, PLT, HbA1c, FBG, AST, Cr, TC, LDL-C (, ).
Table 2 Comparison of General Data and Laboratory-Related Indicators in Male Patients
Comparison of General Data and Laboratory-Related Indicators in Female Patients
Among all female T2DM patients, the HALP score of DR patients was significantly lower than that of NDR patients (P=0.003). Diabetes course, SBP and BUN in DR patients were significantly higher than those with NDR (P<0.05). DR patients with LYM and TG were significantly lower than those with NDR (P<0.05). There were no significant difference between the two in age, smoking history, drinking history, DBP, BMI, HGB, PLT, HbA1c, FBG, AST, ALT, ALB, UA, eGFR, Cr, TC, LDL-C, HDL-C (, ).
Table 3 Comparison of General Data and Laboratory-Related Indicators in Female Patients
Trend Test of HALP Score and DR in T2DM Patients
In all patients, according to the HALP score quartile spacing level, the HALP score was converted into an ordered multi-classified variable, namely, group A (HALP score <38.0729), group B (38.0729 <HALP score <48.2878), group C (48.2878 <HALP score<62.3251), and group D (HALP score>62.3251). Four groups of patients were tested for Mantel-Haenszel Chi-square Trend Test.
In all male patients, according to the HALP score quartile spacing level, the HALP score was converted into an ordered multi-classified variable, namely, group A (HALP score <39.2414), group B (39.2414<HALP score < 49.1894), Group C (49.1894<HALP score<63.2877), Group D (HALP score>63.2877). Mantel-Haenszel Chi-square Trend Test was carried out on 4 groups of patients.
In all female patients, according to the HALP score quartile spacing level, the HALP score was converted into an ordered multi-classified variable, that is, group A (HALP score <36.2487), group B (36.2487 <HALP score < 46.5918), Group C (46.5918<HALP score<60.3351), Group D (HALP score>60.3351). Mantel-Haenszel Chi-square Trend Test was carried out on 4 groups of patients.
The Results indicated that there was a linear trend between HALP score and DR incidence, and DR incidence was negatively related to HALP score in all patients. The above results were also shown in male patients. However, in female patients, although the incidence of DR was still negatively associated with HALP score, there was no obvious linear trend between the two ().
Table 4 Trend Test of HALP Score and DR in T2DM Patients
Multi-Factor Regression Analysis of DR in T2DM Patients
Multi-Factor Regression Analysis of DR in All T2DM Patients
In all patients, taking DR as the dependent variable, the variables of P<0.05 are screened in turn: HALP score, age, course of disease, history of smoking, SBP, BUN, HDL-C, ALT, UA, eGFR, TG were included in the binary logistic regression equation. HL test indicated the model has a good fit (X2=7.460, P=0.488). Regression analysis showed that HALP score, disease course, SBP and BUN were independent risk factors for DR. For each unit added to the HALP score, the DR risk of T2DM patients was reduced by 1.7% ().
Table 5 Multi-Factor Regression Analysis of DR in All T2DM Patients
Multi-Factor Regression Analysis of DR in All Male T2DM Patients
In all male patients, taking DR as the dependent variable, the variables of P<0.05 are screened in turn: HALP score, age, course of disease, smoking history, BMI, SBP, BUN, HDL-C, ALT, UA, eGFR, TG were included in the binary logistic regression equation. HL test indicated the model has a good fit (X2=7.787, P=0.455). Regression analysis showed that HALP score, disease course, SBP and BUN were independent risk factors for DR. For each additional unit of HALP score, the risk of DR in T2DM patients was reduced by 1.4% ().
Table 6 Multi-Factor Regression Analysis of DR in Male Patients
Multi-Factor Regression Analysis of DR in All Female T2DM Patients
In all female patients, taking DR as the dependent variable, the variables of P<0.05 are screened in turn: HALP score, disease course, SBP, BUN, TG were included in the binary logistic regression equation. HL test indicated the model has a good fit (X2=5.608, P=0.691). Regression analysis showed that HALP score, disease course, SBP and BUN were independent risk factors for DR. For every additional unit of HALP score, the DR risk of T2DM patients was reduced by 2.1% ().
Table 7 Multi-Factor Regression Analysis of DR in Female Patients
Nonlinear Relationship Analysis of HALP Score and DR in T2DM Patients
Nonlinear Relationship Analysis of HALP Score and DR in All Patients
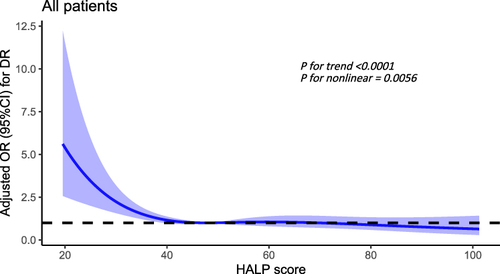
In all T2DM patients, after adjusting the disease course, SBP and BUN, there was a significant nonlinear relationship between HALP score and DR (P for trend < 0.0001, P for nonlinear = 0.0056), and the risk of DR increases significantly when the HALP score was below 47.87 ().
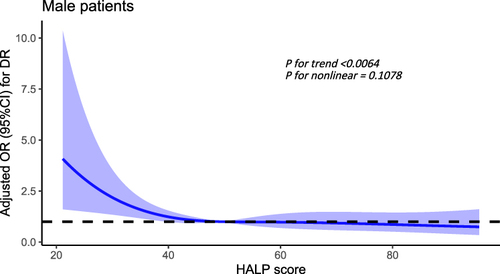
Nonlinear Relationship Analysis of HALP Score and DR in Male Patients
In all male patients, after adjusting the disease course, SBP and BUN, there was a significant linear relationship between HALP score and DR (P for trend <0.0064, P for nonlinear = 0.1078), when the HALP score was below 49.52, the risk of DR increases significantly ().
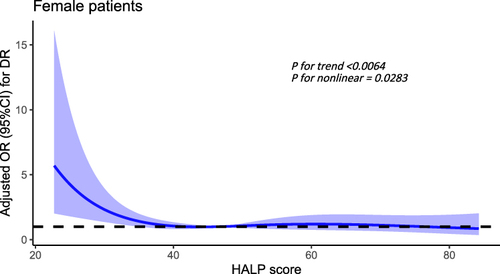
Nonlinear Relationship Analysis of HALP Score and DR in Female Patients
In all female patients, after adjusting the disease course, SBP and BUN, there was a significant nonlinear relationship between HALP score and DR (P for trend<0.0064, P for nonlinear = 0.0283), when the HALP score was below 41.89, the risk of DR increases significantly. ().
Diagnostic Value of HALP Score for DR in T2DM Patients
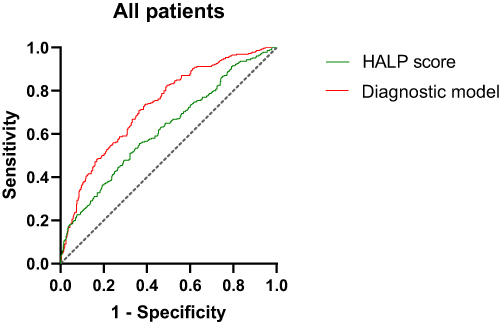
Diagnostic Value of HALP Score for DR in All Patients
ROC curve analysis showed that in all T2DM patients, the area under the curve (AUC) of HALP score diagnostic DR was 0.623, the sensitivity was 55.6%, and the specificity was 63.4%. The model formula of HALP score combined with disease course, SBP and BUN diagnosis DR was: Y=−2.888–0.019HALP score+0.014SBP+0.076Diabetes course+0.165BUN. The AUC of DR diagnosed by the combined diagnostic model was 0.730, the sensitivity was 73.4%, and the specificity was 61.1% (, ).
Table 8 Diagnostic Value of HALP Score and Combined Diagnostic Mode for DR in T2DM Patients
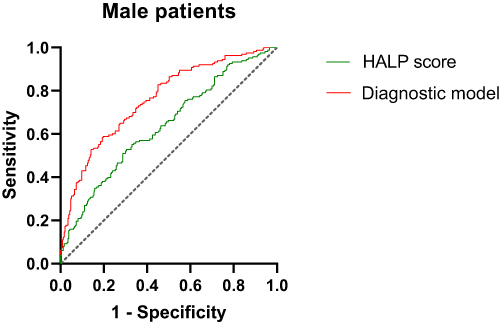
Diagnostic Value of HALP Score for DR in Male Patients
ROC curve analysis showed that in male patients, the AUC of HALP score diagnostic DR was 0.637, the sensitivity was 52.8%, and the specificity was 69.5%. The model formula of HALP score combined with disease course, SBP and BUN diagnosis DR was:Y=−3.167–0.018HALP score+0.014SBP+0.109Diabetes course+0.156BUN. The AUC of DR diagnosed by the combined diagnostic model was 0.761, the sensitivity was 58.3%, and the specificity was 80.3% (, ).
Diagnostic Value of HALP Score for DR in Female Patients
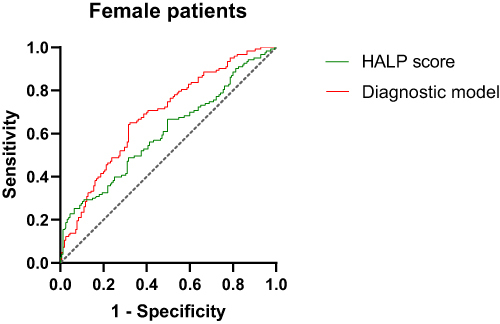
ROC curve analysis showed that in female patients, the AUC of HALP score diagnostic DR was 0.603, the sensitivity was 48.8%, and the specificity was 68.4%. The model formula of HALP score combined with disease course, SBP and BUN diagnosis DR was: Y=−2.623–0.020HALP score+0.014SBP+0.043Diabetes course+0.197BUN. The AUC of DR diagnosed by the combined diagnostic model was 0.684, the sensitivity was 65%, and the specificity was 67.7% (, ).
Discussion
With the increase in the incidence of diabetes, the prevalence of global DR has also increased significantly. DR is divided into two stages: non-proliferative diabetic retinopathy (NPDR) and proliferative diabetic retinopathy (PDR). When patients progress to the PDR stage, serious visual impairment may occur.Citation11 At present, antivascular endothelial growth factor (VEGF) therapy has become the first-line treatment of PDR.Citation12 However, in clinical practice, the use of anti-VEGF drugs is still limited due to poor patient compliance and economic burden. In addition, anti-VEGF therapy requires frequent injections of vitreous body, so there are strong requirements for the professionalism of ophthalmologists. But in some areas where medical resources are scarce, they do not even have fundus examination equipment to screen DR. The shortage of ophthalmology equipment and ophthalmologists still makes the diagnosis and treatment of DR a huge challenge.Citation13 What’s more worrying is that DR is not just a simple eye disease. In recent years, many studies have confirmed the impact of DR on stroke, heart failure and all-cause mortality, and no matter what stage DR is in, it increases the risk of all-cause mortality compared with diabetics without DR.Citation2,Citation14 DR seriously affects the quality of life of patients. In order to help more patients detect DR at an early stage, we are constantly looking for risk factors affecting DR, hoping to assist in the early diagnosis of DR with more easily available biological indicators.
There is growing evidence that inflammation is a key driver of DR. The high glucose circulation in diabetic patients activates the cascade reaction of inflammation. The chronic accumulation of inflammation and the continuous infiltration of inflammatory cells continue to act on retinal blood vessels, resulting in impaired vascular permeability and abnormal proliferation of blood vessels, and gradually develop to DR.Citation15 When inflammation occurs, the body’s vascular reaction and leukocyte reaction are activated. Platelets actively participate in the regulation of vascular endothelial permeability. And the platelet P-selectin exposed after platelet activation mediates and white blood cells to form platelet-leukocyte aggregation, induces the recruitment of neutrophils and macrophages, thus regulating inflammatory expression.Citation16–18 Platelets have become an important player in the process of inflammation. It has been reported that in Crohn’s disease model mice, serum phenylacetyl glutamine aggravates colitis in mice by mediating platelet activation, and anti-platelet therapy provides a new treatment for inflammatory bowel disease.Citation19 Lymphocytes are important constituent cells of the body’s immune response, which play the role of immune defense and immune monitoring, and reflect the inflammatory state and immune ability in the body. Previous studies have found that the reduction of lymphocyte mitochondrial DNA content is independently associated with IL-6, IL-10 and TNF-α, indicating that chronic inflammation is closely related to apoptosis of lymphocytes.Citation20 In diabetics, lymphocyte apoptosis is significantly higher than that of normal patients. Under electron microscope, it can be observed that the lymphocyte is smaller and morphologically damaged, the number of mitochondria is reduced, and the mitochondrial structure changes, resulting in a low immune status of patients and increasing the occurrence of infection.Citation21 In our study, it was found that the lymphocyte count in the DR group was lower than that in the NDR group, indicating that lymphocyte apoptosis continued to play a role in the progression of diabetes complications. Albumin is a common nutritional indicator that helps to evaluate the nutritional status of patients. There is also a close relationship between albumin and inflammation. Albumin can selectively inhibit the production of mitochondrial ROS and prevent the release of extraneutrophil traps, thus inhibiting inflammation.Citation22 However, in the inflammatory state, the half-life of albumin is shortened and the exudation increases, leading to the occurrence of hypoproteinemia, and the interaction between inflammation and hypoproteinemia causes a malignant cycle.Citation23 Wang et al found in the health and nutrition survey that albumin levels can be used as an indicator for long-term follow-up of diabetic patients and can be used to monitor the prognosis of diabetes.Citation6 Our study found that the albumin level in patients with DR was significantly lower than that of patients with NDR, which was also consistent with the results of Wang et al. Hemoglobin is another common nutritional index, which is often used to evaluate kidney injury in patients with diabetic nephropathy.Citation24 Adele et al analyzed the correlation between hemoglobin and DR and found that hemoglobin levels were low and the incidence of anemia was high in DR patients, and similar results were found in our study.Citation25
To sum up, platelets, lymphocytes, albumin and hemoglobin all play an important role in the complications of diabetes and diabetes. The HALP score integrates these four indicators, comprehensively considers the patient’s inflammation and nutrition, evaluates the patient’s health, and responds more steadily to the patient’s general state. The HALP score is calculated by multiplying the lymphocyte count by albumin multiplied by hemoglobin and then divided by platelet count. The lower the HALP score, the higher the patient’s inflammatory level and the worse the nutritional state.Citation26 HALP scores have been widely used in the evaluation of cancer and vascular diseases.Citation26–28 Our study linked the HALP score with DR for the first time, and explored the diagnostic value of HALP score for DR in T2DM patients. Considering the difference in hemoglobin in male and female patients, we classified male and female patients. It was found that in all patients, male patients and female patients, the HALP score of the DR group was significantly lower than that of the NDR group, and the HALP score was negatively correlated with the incidence of DR, which further confirmed that inflammation and nutrition played a role in DR lesions. Regression analysis showed that HALP score was an independent risk factor for DR. In addition, regression analysis results showed that SBP, course of disease and BUN were also risk factors for DR, which was also consistent with previous research results.Citation29,Citation30 Then we used the restrictive cubic spline to find the critical value of HALP score of DR risk in T2DM patients, and found that the critical value of HALP score in male patients was higher than that of female patients, and it may be related to the higher hemoglobin of male patients than that of female patients. Finally, we used HALP score to evaluate DR. We found that even if HALP score was an independent risk factor of DR, when using HALP score alone to diagnose DR, neither specificity nor sensitivity were ideal. Therefore, we combine the DR independent risk factors found in the study to establish a combined diagnostic model of HALP score, SBP, course of disease and BUN, which is better than using HALP score to diagnose DR alone. We established combined diagnostic model among all patients, male patients and female patients, and found that it had higher diagnostic value among male patients, so more attention should be paid to the monitoring of HALP score in male patients clinical practice. Compared with fundoscopy, HALP score does not require the active participation of patients. At the same time, it is simpler and more economical, and may become a new biological indicator for diagnostic DR.
Of course, there are still many shortcomings in this study. First of all, this study was a retrospective study, and the causal relationship between DR and HALP score could not be determined. Secondly, this study had not taken into account the patient’s diet and drug application history, resulting in possible bias in the results. In addition, although the combined diagnostic model of HALP score, SBP, course and BUN was more accurate than the diagnosis of HALP score alone, its sensitivity and specificity were still not high, and could not replace the fundus examination. Finally, the sample size of this study was relatively small, and there was a lack of multi-center joint research. A large number of subsequent clinical trials are needed to verify.
Conclusion
Our results discussed for the first time that HALP score was closely related to the occurrence of DR, especially in male T2DM patients. The accuracy of HALP score, course of disease, SBP and BUN combined model diagnosis DR was high, which provides a basis for early detection, intervention and treatment of DR.
Ethics and Consent Statements
This study was conducted in strict adherence to the Helsinki Declaration of Principles and received approval from the Ethics Committee of Hebei General Hospital. In addition, this study was a retrospective non-interventional study, and the patient’s information was anonymous and confidential, so the signed informed consent was exempted.
Disclosure
The authors report no conflicts of interest in this work.
Acknowledgments
This paper has been uploaded to Research Gate as a preprint: https://www.researchgate.net/publication/378810421_Relationship_between_the_Hemoglobin_Albumin_Lymphocyte_count_Platelet_count_HALP_score_and_type_2_diabetes_retinopathy.
Additional information
Funding
References
- Fung TH, Patel B, Wilmot EG, Amoaku WM. Diabetic retinopathy for the non-ophthalmologist. Clin Med. 2022;22(2):112–116. PMID: 35304370; PMCID: PMC8966825. doi:10.7861/clinmed.2021-0792
- Modjtahedi BS, Wu J, Luong TQ et al. Severity of diabetic retinopathy and the risk of future cerebrovascular disease, cardiovascular disease, and all-cause mortality. OPHTHALMOLOGY.2021;128(8):1169–1179. doi:10.1016/j.ophtha.2020.12.019
- Lin KY, Hsih WH, Lin YB, Wen CY, Chang TJ. Update in the epidemiology, risk factors, screening, and treatment of diabetic retinopathy. J Diabetes Investig. 2021;12(8):1322–1325. PMID: 33316144; PMCID: PMC8354492. doi:10.1111/jdi.13480
- Amorim M, Martins B, Fernandes R. Immune fingerprint in diabetes: ocular surface and retinal inflammation. Int J Mol Sci. 2023;24(12):9821. PMID: 37372968; PMCID: PMC10298084. doi:10.3390/ijms24129821
- Wang S, Pan X, Jia B, et al. Exploring the correlation between the Systemic Immune Inflammation Index (SII), Systemic Inflammatory Response Index (SIRI), and Type 2 diabetic retinopathy. Diabetes Metab Syndr Obes. 2023;16:3827–3836. doi:10.2147/DMSO.S437580
- Wang GX, Fang ZB, Li JT, et al. The correlation between serum albumin and diabetic retinopathy among people with type 2 diabetes mellitus: NHANES 2011-2020. PLoS One. 2022;17(6):e0270019. PMID: 35709212; PMCID: PMC9202838. doi:10.1371/journal.pone.0270019
- Zhou J, Yang D Prognostic significance of Hemoglobin, Albumin, Lymphocyte and Platelet (HALP) score in hepatocellular carcinoma. J Hepatocell Carcinoma. 2023; 10: 821–31. doi:10.2147/JHC.S411521
- Alshuweishi Y, Basudan AM, Alfaifi M, et al. Association of the HALP score with dyslipidemia: a large, nationwide retrospective study. MEDICINA-LITHUANIA. 2023;59. doi:10.3390/medicina59112002
- Kocaoglu S, Alatli T. The efficiency of the HALP score and the modified halp score in predicting mortality in patients with acute heart failure presenting to the emergency department. JCPSP-J COLL PHYSICI. 2023;32:2022. doi:10.29271/jcpsp.2022.06.706.
- Tian M, Li Y, Wang X, et al. The Hemoglobin, Albumin, Lymphocyte, and Platelet (HALP) score is associated with poor outcome of acute ischemic stroke. Front Neurol. 2020;11: 610318. doi:10.3389/fneur.2020.610318
- Tan TE, Wong TY. Diabetic retinopathy: looking forward to 2030. Front Endocrinol. 2023;13:1077669. PMID: 36699020; PMCID: PMC9868457. doi:10.3389/fendo.2022.1077669
- Wang W, Lo ACY. Diabetic retinopathy: pathophysiology and Treatments. Int J Mol Sci. 2018;19(6):1816. PMID: 29925789; PMCID: PMC6032159. doi:10.3390/ijms19061816
- Teo ZL, Tham YC, Yu M, Cheng CY, Wong TY, Sabanayagam C. Do we have enough ophthalmologists to manage vision-threatening diabetic retinopathy? A global perspective. Eye. 2020;34(7):1255–1261. PMID: 31992863; PMCID: PMC7314752. doi:10.1038/s41433-020-0776-5
- Zhu XR, Zhang YP, Bai L, et al. Prediction of risk of diabetic retinopathy for all-cause mortality, stroke and heart failure: evidence from epidemiological observational studies. MEDICINE. 2017;96: e5894. doi:10.1097/MD.0000000000005894
- Al-Kharashi AS. Role of oxidative stress, inflammation, hypoxia and angiogenesis in the development of diabetic retinopathy. Saudi J Ophthalmol. 2018;32(4):318–323. PMID: 30581303; PMCID: PMC6300752. doi:10.1016/j.sjopt.2018.05.002
- Rossaint J, Margraf A, Zarbock A. Role of platelets in leukocyte recruitment and resolution of inflammation. Front Immunol. 2018;9: 2712. doi:10.3389/fimmu.2018.02712.
- Gros A, Ollivier V, Ho-Tin-Noé B. Platelets in inflammation: regulation of leukocyte activities and vascular repair. Front Immunol. 2014;5: 678. doi:10.3389/fimmu.2014.00678
- Thomas MR, Storey RF. The role of platelets in inflammation. Thromb Haemost. 2015;114(3):449–458. PMID: 26293514. doi:10.1160/TH14-12-1067
- Feng R, Tian Z, Mao R, et al. Gut microbiome-generated phenylacetylglutamine from dietary protein is associated with crohn’s disease and exacerbates colitis in mouse model possibly via platelet activation. J Crohns Colitis. 2023;17(11):1833–1846. doi:10.1093/ecco-jcc/jjad098
- Abu Bakar MH, Hairunisa N, Zaman Huri H. Reduced mitochondrial DNA content in lymphocytes is associated with insulin resistance and inflammation in patients with impaired fasting glucose. Clin Exp Med. 2018;18(3): 373–82. doi:10.1007/s10238-018-0495-4
- Xu H, Chen Y, Li Y, et al. Mitochondrial apoptosis of lymphocyte is induced in type 2 diabetes. Chin Med J. 2014;127(2):213–217. PMID: 24438606. doi:10.3760/cma.j.issn.0366-6999.20131906
- Zheng Y, Zhu Y, Liu X, et al. The screening of albumin as a key serum component in preventing release of neutrophil extracellular traps by selectively inhibiting mitochondrial ROS generation. Can J Physiol Pharmacol. 2021;99(4):427–438. PMID: 32799676. doi:10.1139/cjpp-2019-0670
- Soeters PB, Wolfe RR, Shenkin A. Hypoalbuminemia: pathogenesis and clinical significance. JPEN J Parenter Enteral Nutr. 2019;43(2):181–193. PMID: 30288759; PMCID: PMC7379941. doi:10.1002/jpen.1451
- Song HY, Wei CM, Zhou WX, et al. Association between admission hemoglobin level and prognosis in patients with type 2 diabetes mellitus. World J Diabetes. 2021;12(11):1917–1927. doi:10.4239/wjd.v12.i11.1917
- Bahar A, Kashi Z, Ahmadzadeh Amiri A, et al. Association between diabetic retinopathy and hemoglobin level. CASP J Intern Med. 2013;4: 759. PMID: 24294469.
- Farag CM, Antar R, Akosman S, et al. What is hemoglobin, albumin, lymphocyte, platelet (HALP) score? A comprehensive literature review of HALP’s prognostic ability in different cancer types. Oncotarget. 2023;14: 153. doi:10.18632/oncotarget.28367
- Xu H, Zheng X, Ai J, et al. Hemoglobin, albumin, lymphocyte, and platelet (HALP) score and cancer prognosis: a systematic review and meta-analysis of 13,110 patients. Int Immunopharmacol. 2023:114. doi:10.1016/j.intimp.2022.109496.
- Liu L, Gong B, Wang W, et al. Association between haemoglobin, albumin, lymphocytes, and platelets and mortality in patients with heart failure. ESC Heart Fail. 2024; 1051–60. doi:10.1002/ehf2.14662
- Wong TY, Cheung CM, Larsen M, Sharma S, Simó R. Diabetic retinopathy. Nat Rev Dis Primers. 2016;2(1):16012. PMID: 27159554. doi:10.1038/nrdp.2016.12
- Okada S, Ichiki K, Tanokuchi S, et al. Factors related to the development and progression of diabetic retinopathy in patients with type 2 diabetes. J Int Med Res. 1996;24(2):214–220. doi:10.1177/030006059602400206