Abstract
Exenatide once weekly (EQW), the first glucose-lowering agent for type 2 diabetes that is dosed one time per week, contains exenatide encapsulated in microspheres of a dissolvable matrix, which release active agent slowly and continuously into the circulation following subcutaneous injection. In two direct head-to-head comparisons, EQW resulted in better long-term glucose control, greater reductions in fasting plasma glucose, and more significant weight loss than sitagliptin. In other trials, glucose-lowering effects of EQW compared favorably with those of metformin, pioglitazone, and basal insulin. Patients on EQW exhibited a higher incidence of nausea than those on sitagliptin, although gastrointestinal adverse events occurred primarily during the first 6–8 weeks of therapy and declined thereafter. EQW was also associated with a lower incidence of nausea than two other glucagon-like peptide-1 receptor agonists, exenatide twice daily and liraglutide. Mild hypoglycemic episodes were uncommon with EQW, although risk of hypoglycemia increased in combination with sulfonylureas. When choosing between EQW and a dipeptidyl peptidase-4 (DPP-4) inhibitor, such as sitagliptin, clinicians and patients should consider the differences between the two medications in terms of glucose control (EQW superior to DPP-4 inhibitors), weight control (EQW superior to DPP-4 inhibitors), gastrointestinal tolerability during treatment initiation (EQW inferior to DPP-4 inhibitors), and mode of administration (once-weekly subcutaneous administration versus once-daily oral administration).
Introduction
Managing type 2 diabetes mellitus (T2DM) over the course of a patient’s lifetime has proven to be challenging, with less than half of patients at any given time achieving therapeutic goals.Citation1 This may be due to many factors, including the advanced state of pancreatic beta-cell dysfunction at the time of diagnosis, the relatively modest efficacy of therapeutic agents to lower blood glucose, and lack of agents that are not only glucose-lowering, but also intrinsically disease-modifying.Citation2–Citation4 In addition, numerous challenges surround patient treatment adherence, including cost and tolerability issues.Citation5 The discovery and clinical implementation of incretin-based therapies has offered health care providers the opportunity to address a number of intrinsic physiologic defects seen in diabetes and, in the case of glucagon-like peptide-1 (GLP-1) receptor agonist (GLP-1RA) therapy, offer somewhat more efficacious glucose-lowering properties than oral therapeutic alternatives. The development of extended release GLP-1RA therapy may address some of the adherence and side effect issues that have proved to be barriers to therapy in the past.
Over 40 years ago, it was discovered that the human insulin response to intravenous glucose was only 30%–40% of that observed after an oral glucose load that raised blood glucose to the same level, indicating that not only blood sugar, but also alimentary mechanisms, regulated insulin secretion.Citation6,Citation7 Subsequent studies found that this phenomenon, known as the incretin effect, was attributable in part to a 30-amino-acid hormone known as GLP-1, which is co-encoded with glucagon and is released into circulation from the distal small bowel and colon in response to nutrient ingestion.Citation8,Citation9 Upon reaching pancreatic islets, GLP-1 binds to heptahelical G protein-coupled receptors on the membrane of beta cells and stimulates insulin secretion in a glucose-dependent fashion, accounting in large part for the observed incretin effect.Citation8
Consistent with the wide expression of its receptors in diverse tissues,Citation8,Citation9 GLP-1 exhibits biologic activities beyond insulinotropism. In published studies, GLP-1 also inhibited glucagon secretion from pancreatic alpha cells at glucose levels at or above normal fasting levels, reduced gastric motility, and induced feelings of satiety via hunger centers in the hypothalamus.Citation10,Citation11 In other studies, direct infusion of GLP-1 modulated fluid intake and increased renal sodium excretion,Citation12,Citation13 whereas incretin-based therapy has been associated with blood pressure lowering, beneficial changes in lipid profiles, and improvements in hepatic, myocardial, and endothelial function.Citation14,Citation15
Two general classes of incretin therapies have been designed to capitalize on the antihyperglycemic effects of GLP-1. The first, the GLP-1RAs, act by increasing the level of systemic GLP-1 activity. Administered by subcutaneous injection, currently available GLP-1RAs include exenatide twice daily (EBID),Citation16 liraglutide once daily,Citation17 and exenatide once weekly (EQW).Citation18 The dipeptidyl peptidase 4 (DPP-4) inhibitors increase the levels of native GLP-1 by a different mechanism, ie, by inhibiting DPP-4, a widely dispersed and promiscuous protease that normally turns over native GLP-1 rapidly in vivo.Citation19 Administered orally, currently available DPP-4 inhibitors include sitagliptin,Citation20 saxagliptin,Citation21 linagliptin,Citation22 and vildagliptin.Citation23 All of the preceding incretin agents (GLP-1RAs and DPP-4 inhibitors) have been approved in the United States and the European Union for use in patients with T2DM, with the exception of vildagliptin, which has received marketing authorization in the European Union only.
The purpose of this review is to describe the properties of EQW, the most recently approved incretin therapy and the only antidiabetic agent administered one time per week. Included here will be a summary of the basic pharmacology, efficacy, and safety of this new long-acting agent, with a particular focus on its comparison to the DPP-4 inhibitor sitagliptin.
Pharmacology of EQW
Exenatide, the active ingredient in EQW, is a 39-amino-acid synthetic version of exendin-4, a peptide isolated from the lizard Heloderma suspectum that shares approximately 50% sequence identity with human GLP-1.Citation24 Exenatide binds with high affinity to GLP-1 receptors and has all of the known glucoregulatory activities of GLP-1.Citation25–Citation27 However, it is more resistant than GLP-1 to degradation by DPP-4.Citation24 In the twice-daily formulation, median peak plasma concentrations of exenatide occurred 2.1 hours after administration, and the subsequent mean terminal half-life was 2.4 hours,Citation16 pharmacokinetic parameters that permitted twice-daily administration before the two main meals of the day.
In the new once-weekly formulation, exenatide has been encapsulated in injectable microspheres that degrade in situ after administration and slowly release drug into circulation in a sustained fashion.Citation28 The structural matrix of the microsphere is composed of a medical-grade biodegradable polymer called poly-(d,l-lactide-co-glycolide) (PLG), which has been used in dissolvable surgical sutures, bone plates, and orthopedic implants for decades and in microsphere form as a long-acting drug-delivery system since 1984.Citation29–Citation31 Degradation of the PLG polymer occurs by hydrolysis of the ester linkages into lactic acid and glycolic acid, which are easily eliminated as carbon dioxide and water. It is important to note that the encapsulated exenatide in EQW, as well as the active agent released into circulation, is identical to that in EBID.
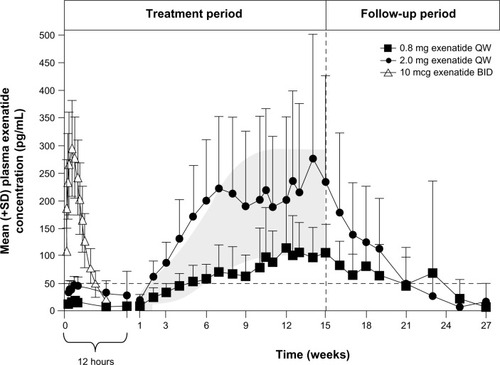
In a pharmacokinetic study on patients with T2DM receiving a single dose of EQW, exposure increased with dose (2.5, 5, 7, or 10 mg), with measurable levels of exenatide lasting for up to 10 weeks.Citation32 The same study evaluated exenatide exposure following once-weekly administrations of EQW 0.8 mg or EQW 2.0 mg in 45 patients with T2DM across a 15-week treatment period ().Citation32 In patients who received the 2.0 mg dose, the currently indicated dosage,Citation18 plasma exenatide concentrations rose over time and reached steady-state levels by approximately 6–7 weeks. At steady state, overall plasma levels were roughly comparable to the maximum concentration reached after a single injection of EBID.
Figure 1 Pharmacokinetics of EQW. Plasma exenatide concentrations following a single dose of EBID (n=39) and multiple doses of EQW (n=31).
Modified from Fineman et al. with kind permission from Springer Science+Business Media: Clin Pharmacokinet, Pharmacokinetics and pharmacodynamics of exenatide extended-release after single and multiple dosing, 2011;50(1):65–74, Fineman M, Flanagan S, Taylor K, et al., .Citation32
Abbreviations: BID, twice daily; EBID, exenatide twice daily; EQW, exenatide once weekly; QW, once weekly; SD, standard deviation.
Efficacy of EQW versus sitagliptin
Six randomized controlled trials, known by the acronym DURATION for Diabetes Therapy Utilization: Researching Changes in A1c, Weight and Other Factors Through Intervention with Exenatide Once Weekly, have been conducted to determine the efficacy and safety profiles of EQW ().Citation33–Citation38 DURATION-4Citation37 and DURATION-2Citation33 directly compared EQW with the DPP-4 inhibitor sitagliptin.
Table 1 Summary of EQW outcomes in the DURATION study program
EQW monotherapy versus sitagliptin monotherapy
DURATION-4 compared EQW monotherapy (n=248) versus sitagliptin monotherapy (n=163) in drug-naïve patients who needed to intensify their diet and exercise therapy (the trial also included a metformin arm and a pioglitazone arm, see below) ().Citation37 After 26 weeks of therapy, mean glycated hemoglobin (HbA1c) levels decreased to 6.94% with EQW (mean change, −1.53%) and 7.32% with sitagliptin (mean change, −1.15%; P<0.001). Significantly more patients treated with EQW than sitagliptin achieved HbA1c <7.0% (63% versus 43%, P<0.001) and ≤6.5% (49% versus 26%; P<0.001). Reductions in fasting plasma glucose (FPG) were also significantly greater in the EQW group (−41.4 mg/dL versus −19.8 mg/dL; P<0.001). Finally, in seven-point self-monitored blood glucose profiles, EQW was associated with greater mean reductions compared with sitagliptin (P<0.05 at all time points).
Figure 2 Effects of EQW on glycemia and weight relative to sitagliptin in the DURATION study program.
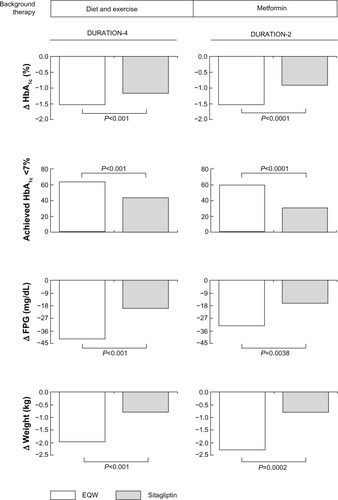
In addition to glycemic parameters, DURATION-4 evaluated other patient outcomes.Citation37 After 26 weeks of therapy, mean weight changed from baseline by −2.0 kg in the EQW group compared with −0.8 kg in the sitagliptin group (P<0.001) (). Beta-cell function, as measured by geometric mean homeostasis model assessment (HOMA)-B (C-peptide), improved more with EQW than sitagliptin (P<0.001). Changes in insulin sensitivity, as measured by HOMA-S (C-peptide), were similar in the two treatment groups.
EQW versus sitagliptin as add-on therapy to metformin
DURATION-2 compared EQW (n=160) with sitagliptin (n=166) in patients with T2DM who needed to intensify metformin monotherapy (pioglitazone was also a comparator in the trial, see below) ().Citation33 After 26 weeks of therapy, mean changes from baseline in HbA1c were −1.5% with EQW and −0.9% with sitagliptin (P<0.0001). Significantly more patients on EQW than sitagliptin achieved HbA1c targets of <7.0% and #6.5% (P<0.0001 for both comparisons), and EQW reduced FPG significantly more than sitagliptin (−32.4 mg/dL versus −16.2 mg/dL; P=0.0038). In all measurements on a six-point self-monitored blood glucose profile, reductions at week 26 were significantly greater with EQW than sitagliptin (P<0.05 at all measurements). Weight loss with exenatide (−2.3 kg) was significantly greater than with sitagliptin (−0.8 kg; P=0.0002).
A 26-week, open-label extension of DURATION-2 evaluated the safety and efficacy of continued EQW therapy, as well as the outcome of switching from sitagliptin to EQWCitation39 Patients in the EQW → EQW population maintained the significant improvements in long-term blood glucose control at 52 weeks that were observed in the original study at 26 weeks (change from baseline in HbA1c, −1.6%; change in HbA1c after the switch, +0.06%); weight remained significantly below baseline after 52 weeks of therapy (−1.8 kg; P=0.0002 versus the original baseline), but increased relative to the 26-week time point (+0.7 kg). Patients in the sitagliptin → EQW population demonstrated significant incremental improvements in both HbA1c (−0.3% after the switch; P=0.0010) and weight (−1.1 kg after the switch; P=0.0006).
EQW versus other antidiabetic agents
EQW also compared favorably with other antidiabetic agents in its glucose-lowering and weight-sparing effects (). For instance, compared with EBID in DURATION-1, patients on EQW exhibited a larger mean reduction from baseline in HbA1c (−1.9% versus −1.5%; P=0.0023), a higher proportion of patients reaching target HbA1c ≤7% (77% versus 61%; P=0.0039), and a larger mean reduction in FPG (−41.4 mg/dL versus −25.2 mg/dL; P<0.00001). Patients receiving EBID, on the other hand, had significantly greater improvements in 2-hour postprandial plasma glucose excursions (−124.2 mg/dL versus −95.4 mg/dL; P=0.00123).Citation36 Similar weight loss was observed with both agents.Citation36 DURATION-5 also compared EQW with EBID, and EQW was associated with significantly greater changes from baseline in HbA1c (−1.6% versus −0.9%; P<0.0001) and FPG (−35 mg/dL versus −12 mg/dL; P<0.0008) and similar reductions in mean bodyweight (−2.3 and −1.4 kg).Citation34
Figure 3 Effects of EQW on glycemia and weight relative to comparators in the DURATION trials.
Abbreviations: D/E, diet and exercise; EBID, exenatide twice daily; EQW, exenatide once weekly; HbA1c, glycated hemoglobin; MET, metformin; SFU, sulfonylurea; TZD, thiazolidinedione; DURATION, Diabetes Therapy Utilization: Researching Changes in A1c, Weight and Other Factors Through Intervention with Exenatide Once Weekly.
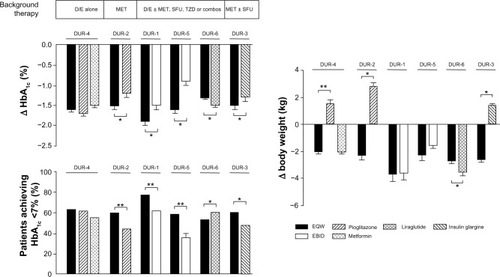
Other comparators in the DURATION trials included metformin, pioglitazone, insulin glargine, and liraglutide. In patients on diet and exercise background therapy, EQW, metformin, and pioglitzaone reduced HbA1c to similar extents, whereas in patients on metformin background therapy, EQW reduced HbA1c (−1.5%) significantly more than pioglitazone (−1.2%; P=0.017).Citation33 In patients on oral antidiabetic agent combination therapy, EQW reduced HbA1c significantly more than insulin glargine (−1.5% versus −1.3%; P<0.05),Citation35 but significantly less than liraglutide (−1.3% versus −1.5%; P<0.05).Citation38 EQW therapy was associated with moderate weight loss in all of the DURATION trials; pioglitazone and insulin glargine use were associated with weight gain ().
Safety and tolerability of EQW
Gastrointestinal side effects
Nausea was the most commonly reported gastrointestinal adverse event in patients on GLP-1RAs in the DURATION study program (). For EQW, reported events of nausea occurred predominantly in the first 6–8 weeks of therapy,Citation28 consistent with studies showing that gastrointestinal adverse events occurred more commonly during initiation of EBID than later in therapy.Citation40 Other gastrointestinal adverse events that occurred in the DURATION trials at higher rates with EQW than non-incretin therapy comparators included diarrhea, vomiting, and constipation. In direct head-to-head comparisons, EQW was associated with less nausea than either EBID or liraglutide, and more nausea than sitagliptin. The greater gastrointestinal tolerability of EQW relative to other GLP-1-RAs may reflect the more gradual rise in blood exenatide concentrations after initiating EQW therapy ().Citation41
Table 2 Rates of gastrointestinal adverse events associated with incretin therapies in the DURATION study program
Hypoglycemia
No episodes of major hypoglycemia were documented in patients on EQW in any of the DURATION trials.Citation33–Citation38 In a pooled analysis of all DURATION studies (Amylin Pharmaceuticals, data on file, 2013), the incidence of minor hypoglycemia (defined as a plasma glucose concentration <54 mg/dL) in patients who received EQW as monotherapy or in combination with metformin or thiazolidinediones was 2.0%. Sitagliptin therapy was associated with a similarly low rate of hypoglycemic episodes in the DURATION trials (1.5%). When EQW was used in combination with sulfonylureas, however, the incidence of mild hypoglycemia increased to 15.7%.
Cardiovascular risk
A randomized double-blind study found that exenatide administered at therapeutic and supratherapeutic concentrations did not produce a clinically significant change in the QT interval.Citation42 Another studyCitation43 examined 148 patients with T2DM who had been treated with EQW and found that the change in QT interval corrected for heart rate using Fridericia’s formula (QTcF) was small and clinically insignificant after 14 weeks (1.7 milliseconds) and 30 weeks (3.0 milliseconds) of therapy. No patient had a QTcF interval during treatment that exceeded 450 milliseconds or a ΔQTcF >60 milliseconds. Similar results were found after a single dose of EBID.Citation44
EQW use over periods of >6 months has shown small favorable effects on cardiovascular risk factors and biomarkers. For instance, patients treated with EQW in the 30-week DURATION-1 study who continued open-label treatment to 52 weeks had significant reductions from baseline in systolic blood pressure (SBP) (−6.2 mmHg; 95% confidence interval [CI], −8.5 to −3.9 mmHg), whereas 50% of those with baseline SBP ≥130 mmHg were observed to have lowered their SBP to normal.Citation45 The decreases in SBP were not due to changes in antihypertensive therapy, as 84% of patients who completed the study had not modified their antihypertensive therapy.Citation45 Total cholesterol and low-density lipoprotein cholesterol also decreased significantly for EQW-treated patients in DURATION-1 and DURATION-5.Citation34,Citation36 Triglycerides in EQW-treated patients decreased in both studies, as well, with significant changes observed in the 30-week study.
Pancreatic risk
A recent study examined the US Food and Drug Administration’s Adverse Event Reporting System and found an increased incidence of pancreatitis in patients on EBID or sitagliptin compared with patients on other therapies,Citation46 although potential problems associated with using spontaneous reporting as the basis for determining event rates has received comment.Citation47 Different results were obtained in an analysis of a large health insurance transaction database, which found that the absolute risk of acute pancreatitis among exenatide and sitagliptin initiators was 0.13% (37 cases among 27,996 patients) and 0.12% (19 cases among 16,267 patients), respectively; these rates were equivalent to the absolute risk in a propensity score-matched cohort of metformin/glyburide initiators.Citation48 Furthermore, in a recent integrated safety analysis of pooled data from 19 completed randomized controlled trials, composite exposure-adjusted incidence rates for pancreatitis among EBID users (n=3,261) and the pooled comparator group (n=2,333) were not statistically different.Citation40 The current package insert recommends that EQW be discontinued immediately if pancreatitis is suspected and that other antidiabetic therapies may be considered in patients with a history of pancreatitis.Citation18
The prior Adverse Event Reporting System study also found an increased rate of spontaneously reported pancreatic cancer in patients on EBID compared with patients on other therapies.Citation46 To date, though, the results of prospective or longitudinal studies that evaluated pancreatic cancer incidence rates in patients on EQW (or EBID or liraglutide) versus other antidiabetic agents have not been reported. In general terms, the American Diabetes Association recommends that cancer risk should not be a major factor in choosing between available diabetes therapies for the average patient, although the choice of therapy for patients with a very high cancer risk or recurrence of specific cancer types may require more careful consideration.Citation49
Thyroid risk
In rats and mice, sustained activation of GLP-1 receptors on thyroid C cells increased calcitonin secretion, C-cell hyperplasia, and medullary thyroid cancer.Citation50 In humans, however, GLP-1RA use did not substantially raise calcitonin levels, and an analysis of sequential changes in calcitonin levels in several thousand diabetic subjects did not reveal a relationship between liraglutide therapy and plasma calcitonin.Citation50,Citation51 In the meta-analysis described above on 3,261 EBID users and 2,333 pooled comparator users (mean exposure time, 166–171 days),Citation40 occurrences of thyroid neoplasm were benign and very rare; the exposure-adjusted incidence rate of any thyroid neoplasm was 0.3 per 100 patient-years with exenatide compared with no occurrences of thyroid neoplasm with placebo/insulin (overall risk difference, 0.27; 95% CI, 0.01–0.53). Although cancer of the thyroid is very rare (incidence of approximately 12 per 100,000 persons in the USCitation16), the use of EQW in patients with a personal or family history of medullary thyroid carcinoma or multiple endocrine neoplasia syndrome type 2 is contraindicated.Citation18
Renal side effects
There have been post-marketing reports of altered renal function with exenatide, including increased serum creatinine, renal impairment, worsened chronic renal failure, and acute renal failure, sometimes requiring hemodialysis or kidney transplantation.Citation18 Some of these events occurred in patients receiving one or more pharmacologic agents known to affect renal function or hydration status, and some occurred in patients who had been experiencing nausea, vomiting, or diarrhea, with or without dehydration. Reversibility of altered renal function has been observed in many cases with supportive treatment and discontinuation of potentially causative agents. Exenatide has not been found to be directly nephrotoxic in preclinical or clinical studies.
Other investigational approaches for reducing the frequency of exenatide administration
Given the positive outcomes of EQW studies, it is of interest to assess the clinical utility of even longer-acting preparations. Two other investigational agents have been described that allow for lower frequency of exenatide dosing. The first was a new once-monthly formulation, consisting of EQW microspheres reconstituted in a triglyceride-based diluent.Citation52 Improved patient adherence may be a potential benefit of exenatide once monthly (EQM), owing to greater convenience of administration,Citation53,Citation54 although this advantage remains unproven and will require prospective Phase III trials for confirmation. A preliminary open-label controlled study evaluated the safety and efficacy of EQM (5, 8, or 11 mg) versus EQW in 121 patients with T2DM on diet/exercise, metformin, pioglitazone, or metformin plus pioglitazone.Citation52 Across a 20-week treatment period, HbA1c decreased in the EQM 5, 8, and 11 mg groups by −1.3%, −1.3%, and −1.5%, respectively (EQW control, −1.5%), and the percentages of patients achieving HbA1c <7% were 50%, 57%, and 70%, respectively (EQW control, 48%). EQM therapy was also associated with improvements in FPG (−25, −30, and −49 mg/dL, respectively) and weight (−1.1, −0.4, and −1.1 kg, respectively). No unique safety findings were observed with EQM relative to EQW. The most frequent adverse events for EQM were headache (17%–27%) and nausea (17%–23%). No major or minor hypoglycemia was observed in any treatment group.
Another form of exenatide delivery was evaluated in a Phase II study with ITCA 650, a subcutaneous osmotic delivery system that provides for continuous delivery of exenatide at specified doses for 3 months. A 48-week study evaluated ITCA 650 at 4 doses (20, 40, 60, and 80 μg per day) in patients with T2DM.Citation55 After 48 weeks at the chronic dose selected for further Phase III studies (60 μg per day), mean HbA1c levels decreased from baseline by 1.5%, 78% of patients had HbA1c levels ≤7%, and mean weight declined by 3.5 kg. Overall, the treatment appeared to be well tolerated.
Conclusion
EQW is the first approved medication for T2DM that is administered one time per week. In direct head-to-head comparisons, EQW resulted in better glucose control (HbA1c), higher proportions of patients reaching treatment goals, greater reductions in FPG, and more significant weight loss than the DPP-4 inhibitor sitagliptin. To date, the primary limiting factor for GLP-1RA use has been gastrointestinal adverse events, notably nausea, vomiting, diarrhea, and constipation. EQW, however, was associated with less nausea than either EBID or liraglutide, but more than sitagliptin. The incidence of mild hypoglycemia in patients who received EQW as monotherapy or in combination with metformin or thiazolidinediones was low, although it was higher in patients who received concomitant treatment with a sulfonylurea.
A recent position statement from the American Diabetes Association and the European Association for the Study of Diabetes recommended maintaining HbA1c below 7% as a general treatment goal in patients with T2DM, but emphasized individualization of therapy rather than a rigid step-care approach.Citation56 The clinical utility of EQW is intriguing when looked at from the perspective of this new treatment paradigm in that it allows for earlier use of EQW in patients with diabetes, significant obesity, and issues with both dietary compliance and postprandial glycemic control.
Although many physicians may still view DPP-4 inhibitors as equivalent to injectable GLP-1RAs, both postprandial and overall glycemic control was significantly better on EQW therapy in clinical trials. Time and teaching barriers to any injectable therapy may lead many patients to favor an oral therapy, but it should be noted that administration of EQW requires only one additional step than other common injectable diabetes medications, ie, suspension of the microspheres before administration, and that a published study showed a majority of patients were capable of independently self-administering a microsphere preparation.Citation57
Regarding other therapies, early use of EQW may be hindered by the impending availability of less costly generic thiazolidinediones, as well as metformin and sulfonylureas. However, exenatide in combination with metformin or with metformin plus pioglitazone, as suggested by DeFronzo,Citation3 may possibly address physiologic defects seen in diabetes more effectively than alternative combinations. The ability to modify postprandial glycemic excursions with a once-weekly shot rather than multiple doses of insulin may also have added benefits in patient compliance and reduced hypoglycemia.
Acknowledgments
Amylin Pharmaceuticals, LLC, provided funding for this study. The authors would like to thank David Norris, PhD, of Ecosse Medical Communications (Falmouth, MA) and Steve Brunell, PhD, of Amylin Pharmaceuticals for editorial assistance provided during the preparation of the manuscript.
Disclosure
MWS is a member of an advisory board and the Speakers Bureau for Takeda Pharmaceuticals. SC and MG were employees of Amylin Pharmaceuticals, LLC, when this manuscript was drafted.
References
- Koro CE Bowlin SJ Bourgeois N Fedder DO Glycemic control from 1988 to 2000 among US adults diagnosed with type 2 diabetes: a preliminary report Diabetes Care 2004 27 1 17 20 14693960
- DeFronzo RA Lilly Lecture 1987. The triumvirate: beta-cell, muscle, liver. A collusion responsible for NIDDM Diabetes 1988 37 6 667 687 3289989
- DeFronzo RA Banting Lecture. From the triumvirate to the ominous octet: a new paradigm for the treatment of type 2 diabetes mellitus Diabetes 2009 58 4 773 795 19336687
- Kendall DM Cuddihy RM Bergenstal RM Clinical application of incretin-based therapy: therapeutic potential, patient selection and clinical use Eur J Intern Med 2009 20 Suppl 2 S329 S339 19580952
- Bergenstal RM Bailey CJ Kendall DM Type 2 diabetes: assessing the relative risks and benefits of glucose-lowering medications Am J Med 2010 123 4 374.e379 e374.e318 20362759
- Perley MJ Kipnis DM Plasma insulin responses to oral and intravenous glucose: studies in normal and diabetic subjects J Clin Invest 1967 46 12 1954 1962 6074000
- Elrick H Stimmler L Hlad CJJr Arai Y Plasma insulin response to oral and intravenous glucose administration J Clin Endocrinol Metab 1964 24 1076 1082 14228531
- Drucker DJ The biology of incretin hormones Cell Metab 2006 3 3 153 165 16517403
- Holst JJ The physiology of glucagon-like peptide 1 Physiol Rev 2007 87 4 1409 1439 17928588
- Nauck MA Niedereichholz U Ettler R Glucagon-like peptide 1 inhibition of gastric emptying outweighs its insulinotropic effects in healthy humans Am J Physiol 1997 273 5 Pt 1 E981 E988 9374685
- Tang-Christensen M Larsen PJ Göke R Central administration of GLP-1-(7–36) amide inhibits food and water intake in rats Am J Physiol 1996 271 4 Pt 2 R848 R856 8897973
- Gutzwiller JP Tschopp S Bock A Glucagon-like peptide 1 induces natriuresis in healthy subjects and in insulin-resistant obese men J Clin Endocrinol Metab 2004 89 6 3055 3061 15181098
- Gutzwiller JP Hruz P Huber AR Glucagon-like peptide-1 is involved in sodium and water homeostasis in humans Digestion 2006 73 2–3 142 150 16809911
- Mudaliar S Henry RR Incretin therapies: effects beyond glycemic control Eur J Intern Med 2009 20 Suppl 2 S319 S328 19580951
- Mudaliar S Henry RR Effects of incretin hormones on beta-cell mass and function, body weight, and hepatic and myocardial function Am J Med 2010 123 Suppl 3 S19 S27 20206728
- Byetta [package insert] San Diego, CA Amylin Pharmaceuticals, LLC 2011
- Victoza [package insert] Bagsvaerd, Denmark Novo Nordisk A/S 2011
- Bydureon [package insert] San Diego, CA Amylin Pharmaceuticals, LLC 2012
- Deacon CF Johnsen AH Holst JJ Degradation of glucagon-like peptide-1 by human plasma in vitro yields an N-terminally truncated peptide that is a major endogenous metabolite in vivo J Clin Endocrinol Metab 1995 80 3 952 957 7883856
- Januvia [package insert] Whitehouse Station, NJ Merck and Co, Inc 2007
- Onglyza [package insert] Princeton, NJ Bristol-Myers Squibb Company 2011
- Tradjenta [package insert] Ridgefield, CT Boehringer Ingelheim Pharmaceuticals, Inc 2011
- European Medicines Agency Vildagliptin: summary of product characteristics Available from: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000771/WC500020327.pdf Accessed Jun 2012
- Lovshin JA Drucker DJ Incretin-based therapies for type 2 diabetes mellitus Nat Rev Endocrinol 2009 5 5 262 269 19444259
- Cersosimo E Gastaldelli A Cervera A Effect of exenatide on splanchnic and peripheral glucose metabolism in type 2 diabetic subjects J Clin Endocrinol Metab 2011 96 6 1763 1770 21411546
- Cervera A Wajcberg E Sriwijitkamol A Mechanism of action of exenatide to reduce postprandial hyperglycemia in type 2 diabetes Am J Physiol Endocrinol Metab 2008 294 5 E846 E852 18334612
- Kolterman OG Buse JB Fineman MS Synthetic exendin-4 (exenatide) significantly reduces postprandial and fasting plasma glucose in subjects with type 2 diabetes J Clin Endocrinol Metab 2003 88 7 3082 3089 12843147
- DeYoung MB MacConell L Sarin V Trautmann M Herbert P Encapsulation of exenatide in poly-(D,L-lactide-co-glycolide) microspheres produced an investigational long-acting once-weekly formulation for type 2 diabetes Diabetes Technol Ther 2011 13 11 1145 1154 21751887
- Anderson JM Shive MS Biodegradation and biocompatibility of PLA and PLGA microspheres Adv Drug Deliv Rev 1997 28 1 5 24 10837562
- Lewis DH Controlled release of bioactive agents from lactide/glycolide polymers Chasin M Langer RS Biodegradable Polymers as Drug Delivery Systems New York Marcel Dekker 1990 1 41
- Vivitrol [package insert] Waltham, MA Alkermes, Inc 2010
- Fineman M Flanagan S Taylor K Pharmacokinetics and pharmacodynamics of exenatide extended-release after single and multiple dosing Clin Pharmacokinet 2011 50 1 65 74 21142268
- Bergenstal RM Wysham C MacConell L Efficacy and safety of exenatide once weekly versus sitagliptin or pioglitazone as an adjunct to metformin for treatment of type 2 diabetes (DURATION-2): a randomised trial Lancet 2010 376 9739 431 439 20580422
- Blevins T Pullman J Malloy J DURATION-5: exenatide once weekly resulted in greater improvements in glycemic control compared with exenatide twice daily in patients with type 2 diabetes J Clin Endocrinol Metab 2011 96 5 1301 1310 21307137
- Diamant M Van Gaal L Stranks S Once weekly exenatide compared with insulin glargine titrated to target in patients with type 2 diabetes (DURATION-3): an open-label randomised trial Lancet 2010 375 9733 2234 2243 20609969
- Drucker DJ Buse JB Taylor K Exenatide once weekly versus twice daily for the treatment of type 2 diabetes: a randomised, open-label, non-inferiority study Lancet 2008 372 9645 1240 1250 18782641
- Russell-Jones D Cuddihy RM Hanefeld M Efficacy and safety of exenatide once weekly versus metformin, pioglitazone, and sitagliptin used as monotherapy in drug-naive patients with type 2 diabetes (DURATION-4): a 26-week double-blind study Diabetes Care 2012 35 2 252 258 22210563
- Buse JB Nauck M Forst T Exenatide once weekly versus liraglutide in patients with type 2 diabetes (DURATION-6): a randomised, open-label study Lancet 2013 381 9861 117 124 23141817
- Wysham C Bergenstal R Malloy J DURATION-2: efficacy and safety of switching from maximum daily sitagliptin or pioglitazone to once-weekly exenatide Diabet Med 2011 28 6 705 714 21434995
- MacConell L Brown C Gurney K Han J Safety and tolerability of exenatide twice daily in patients with type 2 diabetes: integrated analysis of 5594 patients from 19 placebo-controlled and comparator-controlled clinical trials Diabetes Metab Syndr Obes 2012 5 29 41 22375098
- Fineman MS Shen LZ Taylor K Kim DD Baron AD Effectiveness of progressive dose-escalation of exenatide (exendin-4) in reducing dose-limiting side effects in subjects with type 2 diabetes Diabetes Metab Res Rev 2004 20 5 411 417 15343588
- Amylin Pharmaceuticals Exenatide tQT study showed no prolongation of QT interval [press release] San Diego Amylin Pharmaceuticals 7 7 2011 Available from: http://phx.corporate-ir.net/phoenix.zhtml?c=101911&p=irol-newsArticle&ID=1583328 Accessed May 2012
- Sager P Darpö B Han J Exenatide once weekly did not affect corrected QT interval in patients with type 2 diabetes Diabetes 2011 60 A294
- Linnebjerg H Seger M Kothare PA Hunt T Wolka AM Mitchell MI A thorough QT study to evaluate the effects of singledose exenatide 10 mcg on cardiac repolarization in healthy subjects Int J Clin Pharmacol Ther 2011 49 10 594 604 21961484
- Buse JB Drucker DJ Taylor KL DURATION-1: exenatide once weekly produces sustained glycemic control and weight loss over 52 weeks Diabetes Care 2010 33 6 1255 1261 20215461
- Elashoff M Matveyenko AV Gier B Elashoff R Butler PC Pancreatitis, pancreatic, and thyroid cancer with glucagon-like peptide-1-based therapies Gastroenterology 2011 141 1 150 156 21334333
- Drucker DJ Sherman SI Bergenstal RM Buse JB The safety of incretin-based therapies – review of the scientific evidence J Clin Endocrinol Metab 2011 96 7 2027 2031 21734003
- Dore DD Seeger JD Arnold Chan K Use of a claims-based active drug safety surveillance system to assess the risk of acute pancreatitis with exenatide or sitagliptin compared to metformin or glyburide Curr Med Res Opin 2009 25 4 1019 1027 19278373
- Giovannucci E Harlan DM Archer MC Diabetes and cancer: a consensus report Diabetes Care 2010 33 7 1674 1685 20587728
- Bjerre Knudsen L Madsen LW Andersen S Glucagon-like peptide-1 receptor agonists activate rodent thyroid C-cells causing calcitonin release and C-cell proliferation Endocrinology 2010 151 4 1473 1486 20203154
- Hegedus L Moses AC Zdravkovic M Le Thi T Daniels GH GLP-1 and calcitonin concentration in humans: lack of evidence of calcitonin release from sequential screening in over 5000 subjects with type 2 diabetes or nondiabetic obese subjects treated with the human GLP-1 analog, liraglutide J Clin Endocrinol Metab 2011 96 3 853 860 21209033
- MacConell L Malloy J Huang W Cirincione B Shen L Porter L Safety and efficacy of once-monthly exenatide over 20 weeks in patients with type 2 diabetes Diabetologia 2011 54 S38
- Rubin RR Adherence to pharmacologic therapy in patients with type 2 diabetes mellitus Am J Med 2005 118 Suppl 5A 27S 34S 15850551
- Saini SD Schoenfeld P Kaulback K Dubinsky MC Effect of medication dosing frequency on adherence in chronic diseases Am J Manag Care 2009 15 6 e22 e33 19514806
- Luskey L Rosenstock J Alessi T Henry RR Long-term, injection-free treatment with ITCA 650, a continuous subcutaneous delivery of exenatide via DUROS® device, leads to stable glycaemic and weight control for 48 weeks in metformin-treated type 2 diabetes Diabetologia 2011 54 Suppl 1 S39
- Inzucchi SE Bergenstal RM Buse JB Management of hyperglycemia in type 2 diabetes: a patient-centered approach: position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) Diabetes Care 2012 35 6 1364 1379 22517736
- Lorenzi G Schreiner B Osther J Boardman M Application of adult-learning principles to patient instructions: a usability study for an exenatide once-weekly injection device Clin Diabetes 2010 28 4 157 162
- Taylor K Kim D Nielsen LL Aisporna M Baron AD Fineman MS Day-long subcutaneous infusion of exenatide lowers glycemia in patients with type 2 diabetes Horm Metab Res 2005 37 10 627 632 16278786