Abstract
Background
We recently demonstrated leucine to modulate energy partitioning between adipose tissue and muscle. Further, leucine exhibits a synergy with B6, resulting in reduced adipocyte lipid storage coupled with increased muscle fat oxidation. Accordingly, a nutraceutical (NuShape™) containing 2.25 g leucine and 30 mg B6 increased fat oxidation by >30 g/day in a 28-day randomized controlled trial. The present study evaluated the long-term efficacy of this combination in modulating body weight and composition.
Methods
Two 24-week, placebo-controlled, randomized trials, one with weight maintenance (n = 20) and one hypocaloric (−500 kcal/day; n = 24), were conducted using the nutraceutical Nushape in obese subjects.
Results
The supplement resulted in fat loss in the maintenance study (−1.12 ± 0.36 and −1.82 ± 0.70 kg at 12 and 24 weeks, P < 0.01 versus placebo) while no change was found in the placebo group. In the hypocaloric study, the supplement group lost up to twice as much weight (6.18 ± 1.02 versus 3.40 ± 0.81 kg at 12 weeks and 8.15 ± 1.33 versus 5.25 ± 1.13 kg at 24 weeks, P < 0.01) and fat (4.96 ± 0.61 versus 2.31 ± 0.53 kg at 12 weeks and 7.00 ± 0.95 versus 4.22 ± 0.74 kg at 24 weeks, P < 0.01) than the placebo group.
Conclusion
This nutraceutical combination results in significant fat loss in the absence of caloric restriction and markedly enhances weight and fat loss by 50%–80% over a 24-week period.
Background
Recent data from this and other laboratories demonstrate a significant role for leucine in modulating fat oxidation and energy partitioning between adipose tissue and skeletal muscle, resulting in reductions in adipose tissue lipid storage, increased net fat oxidation, and reduced adiposity.Citation1–Citation5 These effects are mediated in part, by sirtuin (SIRT)1-dependent pathways.Citation2 We have shown leucine to activate SIRT1 signaling directly in a cell-free system,Citation6 as well as in both adipocytes and skeletal muscle cells.Citation27 Moreover, knockdown of SIRT1 attenuates leucine-induced increases in mitochondrial biogenesis, oxygen consumption, and fat oxidation.Citation2
We have also recently shown leucine to interact synergistically with vitamin B6 to attenuate adiposityCitation8 Pyridoxal phosphate inhibits adipocyte Ca2+ influx in vitro,Citation8 resulting in significant decreases in adipocyte fatty acid synthase expression and activity and corresponding reductions in adipocyte triglyceride content,Citation8 because Ca2+ signaling coordinately stimulates fatty acid synthase activity and inhibits lipolysis in adipocytes.Citation9–Citation13 Moreover, leucine and pyridoxal phosphate synergistically inhibit adipocyte triglyceride accumulation.Citation8 Consequently, the efficacy of a leucine (2.25 g/day)-B6 (30 mg pyridoxine/day) combination in stimulating fat oxidation in vivo was evaluated in a 28-day, placebo-controlled, randomized clinical trial with overweight and obese subjects and found to increase whole-body fat oxidation significantly by >30 g/day.Citation8 However, this trial was of too short a duration to determine the efficacy of this combination in modulating body weight and composition. Accordingly, we conducted the present study to determine the efficacy of a leucine/pyridoxine nutraceutical combination in augmenting body weight and fat loss during mild caloric restriction and in modulating body composition in the absence of caloric restriction.
Materials and methods
Two 24-week, placebo-controlled, parallel-group, double-blind, randomized trials were conducted, as described below. The first evaluated the effects of the supplement in subjects under mildly hypocaloric conditions and the second evaluated the effects of the supplement under weight maintenance conditions. Other than caloric restriction, procedures were identical for the two trials.
Design of both trials
Twenty obese subjects (14 females and six males aged 26.82 ± 4.24 years, body mass index 34.76 ± 2.57 [kg/m2]) for the hypocaloric trial and twenty-four obese subjects (12 females, 12 males aged 25.73 ± 4.89 years, body mass index 35.92 ± 2.85 [kg/m2]) for the maintenance trial were randomized to receive either the active supplement, termed NuShape™ (NuSirt Sciences, Inc., Nashville, TN, USA) or placebo twice daily. Subject characteristics at baseline are shown in . Each dose of the active blend provided 1.125 g leucine and 15 mg pyridoxine, for a total daily dose of 2.25 g leucine and 30 mg pyridoxine.
Table 1 Baseline subject characteristics
Subjects were studied for a 7-day lead-in period to establish their current caloric requirements, as described below (diets) and then placed on either a hypocaloric diet (500 kcal/day reduction from maintenance, study 1) or a maintenance diet (study 2) and randomized to active treatment or placebo for 24 weeks. Body weight, waist circumference, and body fat were measured at baseline and at the end of weeks 12 and 24, with subjects wearing street clothes. Body fat was measured using dual X-ray absorptiometry. Fasting levels of circulating insulin, glucose, and fasting plasma lipids (triglycerides, total cholesterol, and high-density lipoprotein cholesterol) were measured at the same time points (baseline and at weeks 12 and 24).
All subjects were weight-stable by self-report for the 4 weeks preceding study initiation and met the following exclusion criteria: significant endocrine, metabolic, or gastrointestinal disease; obesity pharmacotherapy (prescription or over-the-counter) within the preceding 4 weeks; pregnancy or lactation; initiation or change in a diet or exercise program within the preceding 4 weeks; changes in pattern of tobacco use; or use of psychotropic medications within the past 4 weeks. Both studies were approved from an ethical standpoint by the institutional review board of The University of Tennessee, Knoxville.
Diet
Baseline dietary assessments (diet records) were conducted during the lead-in period and were used to provide an initial estimate of a maintenance level of energy intake, which was then refined by calculating needs using World Health Organization equations for calculation of basal metabolic rate, adjusted for activity level to provide an estimate of total daily energy expenditure. Total daily energy expenditure was calculated as 1.3× basal metabolic rate for all subjects, because all were sedentary. Discrepancies between estimated total daily energy expenditure and baseline caloric intake were resolved, if necessary, by repeat diet records. Based on this initial estimate of caloric needs, a food exchange-based diet was developed for each subject to produce a caloric deficit of approximately 500 kcal/day for the hypocaloric study or to produce weight maintenance for the maintenance study. The diets were constructed to provide comparable levels of macronutrient and fiber, to approximate the average consumption in the US (fat, approximately 35% of total energy, carbohydrates approximately 49%, approximately protein 16%, fiber 2–3g/1,000 kJ/day). Nutritional supplements were not permitted, and caffeine intake was maintained at a constant level (individualized for each patient, according to baseline assessment). Diets were prescribed and monitored as noted above.
Subjects were provided with individual instruction regarding dietary adherence, and compliance was assessed by weekly subject interview and review of the 3-day diet diaries. There were no significant differences in energy and macronutrient intake between treatments for either trial.
Anthropometric measurements
Body weight and waist circumference were measured weekly, with subjects wearing street clothes, with no shoes, outerwear, or accessories. Body weight was measured with a calibrated scale. Height was measured with a wall-mounted stadiometer. Waist circumference was measured in the standing position with measurements obtained midway between the lateral lower rib margin and the iliac crest. Two measurements were taken mid-exhalation and the average was recorded.
Body composition
Total fat mass was assessed by dual-energy X-ray absorptiometry at baseline, 12 weeks, and 24 weeks. A Lunar Prodigy™ dual-energy X-ray absorptiometry system (GE Healthcare, Madison, WI, USA) maintained and calibrated by Lunar staff annually was used. A spine phantom was assessed every day to determine whether any drift in the machine occurred, followed by the daily calibration block; the spine phantom variation was <3% throughout the study.
Statistical analysis
Data were evaluated for significance by two-way (treatment × time) analysis of variance SAS-PC (SAS 9.2, SAS Institute, Cary, NC, USA). Criteria for parametric analysis (normality of distribution and homogeneity of variance) were confirmed prior to analysis. Data are reported as the mean ± standard deviation.
Results
There were no significant differences in energy or macronutrient intake between the placebo and supplement groups. Energy intake in the hypocaloric trial was 1429 ± 291 (placebo) and 1487 ± 310 (supplement) kcal/day, and energy intake in the maintenance trial was 2018 ± 462 (placebo) and 2073 ± 413 (supplement) kcal/day.
All subjects in the hypocaloric diet trial lost weight, fat, and waist circumference (P < 0.01). However, the supplement group lost 82% more weight and more than twice as much body fat at 12 weeks (P < 0.01, ); these differences were sustained through the conclusion of the study, with the supplement resulting in increases in body weight and fat loss of 55% and 66%, respectively (P < 0.01, ). These differences were reflected in an augmented decrease in waist circumference in the supplemented group which was evident within 4 weeks of treatment (); the supplemented group showed an approximately 3-fold greater loss of waist circumference at 4 weeks (P < 0.01, ), which was sustained as a 2-fold greater loss of waist circumference at the 24-week endpoint (P < 0.01, ).
Table 2 Effects of NuShape™ on body weight and fat in subjects on an energy-restricted diet
Table 3 Effects of NuShape™ on loss of waist circumference in subjects on an energy-restricted diet
Subjects in the weight maintenance trial did not exhibit any significant changes in body weight regardless of treatment, consistent with the experimental design. However, subjects in the supplemented group exhibit significant losses of body fat at 12 weeks, with additional fat loss at 24 weeks (P < 0.01), while body fat loss in the placebo group was not significantly different from baseline at either time point ().
Table 4 Effects of NuShape™ on body fat during weight maintenance
The supplements exerted no significant effect on plasma glucose, but plasma insulin decreased significantly during weight loss in both the placebo and NuShape groups (). However, the NuShape group exhibited a significantly greater decrease in insulin and a corresponding improvement in homeostatic assessment of insulin resistance (HOMAIR, ). In the absence of caloric restriction, the NuShape group exhibited decreases in plasma insulin at both 12 weeks and 24 weeks and an improvement in HOMAIR at 24 weeks, while no significant changes were found in the placebo group (). Treatments were without significant effect on circulating lipids.
Table 5 Effects of NuShape™ on glucose, insulin, and HOMAIR in subjects on an energy restricted diet
Table 6 Effects of NuShape™ on glucose, insulin, and HOMAIR during weight maintenance
Discussion
Results of these studies demonstrate that the NuShape nutraceutical combination results in significant fat loss in the absence of caloric restriction and markedly enhances weight and fat loss by 50%–80% over a 24-week period when compared with a placebo control. These data are consistent with our recent demonstration that NuShape stimulates a >30 g (about 300 kcal) increase in whole-body fat oxidation in obese subjects,Citation8 and demonstrates that this increase in fat oxidation results in clinically meaningful changes in body composition and weight loss.
Moderate weight reduction in the range of 5%–10% of body weight results in changes in adipose tissue characteristics, including a reduced inflammatory profile,Citation14,Citation15 and was reported to result in a 59% reduction in the odds of having metabolic syndrome after one-year assessment.Citation16 However, lifestyle changes such as caloric restriction and physical exercise are difficult to incorporate in the daily routine of most people. On the other hand, available pharmacotherapy risks systemic side effects, leading to discomfort, limited use, and/or withdrawal from the market.Citation17 Therefore, finding safe alternatives to support weight and fat loss and improve metabolic health are of great importance.
Adiposity is associated with adipocyte mitochondrial dysfunction, resulting in an increase in oxidative and inflammatory stress that contributes to insulin resistance, diabetes, and other chronic metabolic diseases.Citation18–Citation20 Visceral fat makes a greater contribution than subcutaneous fat to the secretion of proinflammatory cytokines and is therefore associated with a greater risk of metabolic dysregulation.Citation21 The NuShape treatment produced a significantly greater loss in waist circumference than did the placebo during energy restriction, suggesting a loss of visceral fat. In our previous study, we demonstrated that use of NuShape resulted in reductions of oxidative and inflammatory stress biomarkers, such as plasma malondialdehyde, 8-isoprostane F-2α, tumor necrosis factor-α, and C-reactive protein, as well as an increase in the anti-inflammatory marker adiponectin after 4 weeks, even in the absence of weight loss.Citation8 Thus, this leucine-B6 mixture appears to reduce visceral adiposity and attenuate the associated metabolic dysregulation.
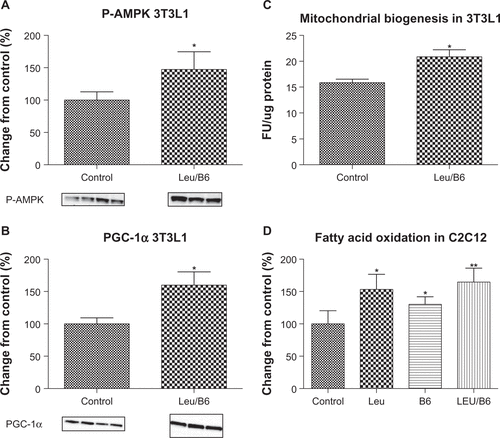
5′ AMP-activated protein kinase (AMPK) plays an important role in the development of insulin resistance because it regulates glucose and fat metabolism in response to nutrient status.Citation22 Moreover, it converges with the SIRT pathways on peroxisome proliferator-activated receptor-γ coactivator (PGC)-1 α activation, leading to increased mitochondrial function.Citation23,Citation24 Recent data suggest that decreases in AMPK and SIRT1 activity may be one of the responsible factors distinguishing insulin-resistant from insulin-responsive individuals with severe obesityCitation25,Citation26 Indeed, AMPK activity was found to be decreased in obese insulin-resistant patients compared with obese insulin-sensitive patients,Citation26 and activation of AMPK improves symptoms of impaired glucose homeostasis and insulin resistance.Citation27–Citation29 Similarly, treatment with SIRT1 activators has been shown to improve mitochondrial function and to protect mice against diet-induced obesity and insulin resistance.Citation30,Citation31 We previously found that leucine stimulates SIRT1 and SIRT1-dependent pathways, resulting in increased mitochondrial mass and fat metabolism in muscle cells and adipocytes.Citation1,Citation2 Consistent with this, our in vitro data indicate that the combination of leucine and B6 improves the oxidative capacity of cells, which results in an up to 80% increase in fatty acid oxidation in muscle and fat cells, measured as palmitate-induced oxygen consumption rate, as well as increases in adipocyte phospho-AMPK (Thr 172) and PGC-1α protein expression and mitochondrial biogenesis (), indicating an increase in oxidative capacity. Although B6 may not directly synergize with leucine to increase fatty acid oxidation in muscle cells (), it exerts synergistic effects with leucine in adipocytes, serving to both inhibit lipid storageCitation8 and synergistically increase fatty acid oxidation.
SIRT1 and AMPK are sensors of signal nutrient/energy depletion and mobilize catabolic systems to generate necessary energy. In contrast, the mTOR system senses nutrient/energy abundance and signals an anabolic response (reviewed by Lopez-Otin et alCitation32), and leucine is well known to stimulate mTOR.Citation33 Notably, the SIRT1/AMPK system suppresses mTOR signaling, raising the question of the roles of these counter-regulatory systems in our observations. Schriever et alCitation33 recently provided evidence to indicate that leucine, rather than downstream leucine catabolic products, is responsible for mTORC1 activation. In contrast, we have found a minor leucine downstream catabolic product (β-hydroxy-β-methylbutyrate) to mediate leucine stimulation of mitochondrial biogenesis and fatty acid oxidation.Citation34,Citation35 Moreover, these effects were independent of mTOR, because they were maintained during mTOR inhibition with rapamycin.Citation34
This study was designed as a follow-up of our previous trialCitation8 to assess the long-term effects of the NuShape combination in both weight maintenance and weight reduction. Despite the small number of subjects in each group, which limits interpretation of the data, the results were highly significant and in agreement with our previous in vivo and in vitro data.
Conclusion
The combination of leucine and pyridoxine found in NuShape provides a safe strategy to improve oxidative capacity and thereby significantly augments weight and fat loss.
Author contributions
MBZ designed and conducted the clinical studies. MBZ and AB were involved in data analysis and drafting the manuscript.
Supplementary figure
Figure S1 Effects of leucine-B6 combination on AMPK, PGC-1α, mitochondrial mass, and fatty acid oxidation. Differentiated adipocytes and C2C12 muscle cells were treated with the treatments indicated for 24 hours. (A) Phospho (Thr 172)-AMPK and (B) PGC-1α protein expression was detected by Western blot and quantitatively analyzed. (C) Mitochondrial mass was measured by fluorescence (485 nm excitation and 520 nm emission) using the fluorophore, nonyl acridine orange (Life Technologies, Grand Island, NY, USA). (D) Oxygen consumption rate was measured after injection of 200 μM palmitate, indicating fatty acid oxidation. Data are represented as the mean ± standard error of the mean (A–C) or mean ± standard deviation (D) (n = 6).
Abbreviations: AMPK, 5′ AMP-activated protein kinase; Leu, leucine; CTRL, control; PGC, peroxisome proliferator-activated receptor-γ coactivator; FU, fluorescent units.
Disclosure
Financial support for this study was provided by NuSirt Sciences, Inc. Both authors are employees and stockholders of NuSirt Sciences, Inc.
References
- Sun X Zemel M Leucine and calcium regulate fat metabolism and energy partitioning in murine adipocytes and muscle cells Lipids 2007 42 297 305 17406924
- Sun X Zemel MB Leucine modulation of mitochondrial mass and oxygen consumption in skeletal muscle cells and adipocytes Nutr Metab (Lond) 2009 6 26 19500359
- Macotela Y Emanuelli B Bang AM Dietary leucine – an envrionmental modifier of insulin resistance acting on multiple levels of metabolism PLoS One 2011 6 e21187 21731668
- Donato JJr Pedrosa RG Cruzat VF Pires IS Tirapegui J Effects of leucine supplementation on the body composition and protein status of rats submitted to food restriction Nutrition 2006 22 520 527 16600817
- Zhang Y Guo K LeBlanc RE Loh D Schwartz GJ Yu YH Increasing dietary leucine intake reduces diet-induced obesity and improves glucose and cholesterol metabolism in mice via multimechanisms Diabetes 2007 56 1647 1654 17360978
- Bruckbauer A Zemel MB Effects of dairy consumption on SIRT1 and mitochondrial biogenesis in adipocytes and muscle cells Nutr Metab (Lond) 2011 8 91 22185590
- Bruckbauer A Zemel MB Thorpe T Synergistic effects of leucine and resveratrol on insulin sensitivity and fat metabolism in adipocytes and mice Nutr Metab (Lond) 2012 9 77 22913271
- Zemel MB Bruckbauer A Effects of a leucine and pyridoxine-containing nutraceutical on fat oxidation, and oxidative and inflammatory stress in overweight and obese subjects Nutrients 2012 4 529 541 22822451
- Xue B Zemel M Relationship between human adipose tissue agouti and fatty acid synthase (FAS) J Nutr 2000 130 2478 2481 11015476
- Kim J Kiefer L Woychik R Agouti regulation of intracellular calcium: role of melanocortin receptors Am J Physiol 1997 272 E379 E384 9124542
- Xue B Greenberg A Kraemer F Zemel M Mechanism of intracellular calcium ([Ca2+]i) inhibition of lipolysis in human adipocytes FASEB J 2001 15 2527 2529 11641262
- Xue B The agouti gene product inhibits lipolysis in human adipocytes via a Ca2+-dependent mechanism FASEB J 1998 12 1391 1396 9761782
- Shi H Moustaid-Moussa N Wilkison W Zemel M Role of the sulfonylurea receptor in regulating human adipocyte metabolism FASEB J 1999 13 1833 1838 10506587
- Rossmeislova L Malisova L Kracmerova J Weight loss improves adipogenic capacity of human preadipocytes and modulates their secretory profile Diabetes 2013 62 1990 1995 23378611
- Olszanecka-Glinianowicz M Chudek J Szromek A Zahorska-Markiewicz B Changes of systemic microinflammation after weight loss and regain – a five-year follow up study Endokrynol Pol 2012 63 432 438 23339000
- Phelan S Wadden TA Berkowitz RI Impact of weight loss on the metabolic syndrome Int J Obes (Lond) 2007 31 1442 1448 17356528
- Colon-Gonzalez F Kim GW Lin JE Valentino MA Waldman SA Obesity pharmacotherapy: what is next? Mol Aspects Med 2013 34 71 83 23103610
- Capurso C Capurso A From excess adiposity to insulin resistance: the role of free fatty acids Vascul Pharmacol 2012 57 91 97 22609131
- Boden G Obesity and free fatty acids Endocrinol Metab Clin North Am 2008 37 635 646 viii–ix 18775356
- Medina-Gomez G Mitochondria and endocrine function of adipose tissue Best Pract Res Clin Endocrinol Metab 2012 26 791 804 23168280
- Fujihara S Mori H Kobara H Metabolic syndrome, obesity, and gastrointestinal cancer Gastroenterol Res Pract 2012 2012 483623 23304125
- Srivastava RA Pinkosky SL Filippov S Hanselman JC Cramer CT Newton RS AMP-activated protein kinase: an emerging drug target to regulate imbalances in lipid and carbohydrate metabolism to treat cardio-metabolic diseases J Lipid Res 2012 53 2490 2514 22798688
- Scarpulla RC Metabolic control of mitochondrial biogenesis through the PGC-1 family regulatory network Biochim Biophys Acta 2011 1813 1269 1278 20933024
- Canto C Gerhart-Hines Z Feige JN AMPK regulates energy expenditure by modulating NAD+ metabolism and SIRT1 activity Nature 2009 458 1056 1060 19262508
- Xu XJ Pories WJ Dohm LG Ruderman NB What distinguishes adipose tissue of severely obese humans who are insulin sensitive and resistant? Curr Opin Lipidol 2013 24 49 56 23298959
- Gauthier MS O’Brien EL Bigornia S Decreased AMP-activated protein kinase activity is associated with increased inflammation in visceral adipose tissue and with whole-body insulin resistance in morbidly obese humans Biochem Biophys Res Commun 2011 404 382 387 21130749
- Bergeron R Previs SF Cline GW Perret P Russell RR3rd Young LH Shulman GI Effect of 5-aminoimidazole-4-carboxamide-1-beta-D-ribofuranoside infusion on in vivo glucose and lipid metabolism in lean and obese Zucker rats Diabetes 2001 50 1076 1082 11334411
- Pold R Jensen LS Jessen N Long-term AICAR administration and exercise prevents diabetes in ZDF rats Diabetes 2005 54 928 934 15793229
- Cool B Zinker B Chiou W Identification and characterization of a small molecule AMPK activator that treats key components of type 2 diabetes and the metabolic syndrome Cell Metab 2006 3 403 416 16753576
- Lagouge M Argmann C Gerhart-Hines Z Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1alpha Cell 2006 127 1109 1122 17112576
- Feige JN Lagouge M Canto C Specific SIRT1 activation mimics low energy levels and protects against diet-induced metabolic disorders by enhancing fat oxidation Cell Metab 2008 8 347 358 19046567
- Lopez-Otin C Blasco MA Partridge L Serrano M Kroemer G The hallmarks of aging Cell 2013 153 1194 1217 23746838
- Schriever SC Deutsch MJ Adamski J Roscher AA Ensenauer R Cellular signaling of amino acids towards mTORC1 activation in impaired human leucine catabolism J Nutr Biochem 2013 24 824 831 22898570
- Stancliffe RA Eades M Smart K Zemel MB Role of mTOR and β-hydroxy-β-methylbutyrate (HMB) in leucine stimulation of muscle mitochondrial biogenesis and fatty acid oxidation FASEB J 2011 25 601.1
- Stancliffe RA Zemel MB Role of β-hydroxy-β-methylbutyrate (HMB) in leucine stimulation of muscle mitochondrial biogenesis FASEB J 2012 26 251.6