Abstract
Excessive loss of functional pancreatic β-cell mass, mainly due to apoptosis, is a major factor in the development of hyperglycemia in both type 1 and type 2 diabetes (T1D and T2D). In T1D, β-cells are destroyed by immunological mechanisms. In T2D, while metabolic factors are known to contribute to β-cell failure and subsequent apoptosis, mounting evidence suggests that islet inflammation also plays an important role in the loss of β-cell mass. Therefore, it is of great importance for clinical intervention to develop new therapies. γ-Aminobutyric acid (GABA), a major neurotransmitter, is also produced by islet β-cells, where it functions as an important intraislet transmitter in regulating islet-cell secretion and function. Importantly, recent studies performed in rodents, including in vivo studies of xenotransplanted human islets, reveal that GABA exerts β-cell regenerative effects. Moreover, it protects β-cells against apoptosis induced by cytokines, drugs, and other stresses, and has anti-inflammatory and immunoregulatory activities. It ameliorates the manifestations of diabetes in preclinical models, suggesting potential applications for the treatment of diabetic patients. This review outlines the actions of GABA relevant to β-cell regeneration, including its signaling mechanisms and potential interactions with other mediators. These studies increase our understanding of the regenerative processes of pancreatic β-cells, and help pave the way for the development of regenerative medicine for diabetes.
Introduction
γ-Aminobutyric acid (GABA) was initially identified as a major inhibitory neurotransmitter in the brain by Roberts and Frankel in 1950.Citation1 The biological functions of GABA are mediated by activation of the GABA receptors. There are two basic types, ie, type A and type B receptors (GABAAR and GABABR, respectively).Citation2 In the adult brain GABAAR is the most prevalent receptor, and upon binding GABA, it exerts an inhibitory effect manifested by hyperpolarization of the cell membrane.Citation2 In sharp contrast, in the developing brain GABA has a depolarizing effect, acting as the principal excitatory transmitter and exerting trophic effects, including cell proliferation and dendritic maturation.Citation2–Citation4
In addition to neurons, some nonneuronal cells, such as pancreatic islet cells, are found to produce GABA in large quantities.Citation5–Citation10 In α-cells, GABA induces membrane hyperpolarization and suppresses glucagon secretion,Citation11–Citation12 whereas in islet β-cells it induces membrane depolarization and increases insulin secretion.Citation13–Citation15 Moreover, GABA has multiple beneficial effects on β-cells, which include the stimulation of cell proliferation and antiapoptotic activities,Citation13–Citation16 making it an attractive agent for diabetes treatment. Importantly, it prevents insulitis to a remarkable degree in preclinical models,Citation13–Citation16 suggesting applications in the prevention of this disease, as well as treatment particularly in the context of clinical islet transplantation.
GABA
GABA biology
GABA is the predominant inhibitory neurotransmitter in the central nervous system (CNS). It is located in about 30% of cerebral neurons, and affects almost all neuronal activities.Citation17 In the neuron, GABA is synthesized in the cytosol from its precursor glutamate by glutamate decarboxylase (GAD), and then transported into the synaptic vesicles against a proton electrochemical gradient.Citation18 Exocytosis of synaptic vesicles is triggered when the voltage-gated calcium channels (VGCCs) open, resulting in a transient rise in cytosolic calcium. GABA is released into the synaptic space, and exerts its effect through binding to corresponding receptors.Citation18 The effect of GABA is terminated rapidly after it is removed from the synaptic space by the actions of four types of plasma membrane GABA cotransporters (GAT1–4) located in the presynaptic terminal and in the glial cells.Citation19
GABA is also present in peripheral organs, such as the testes, gastrointestinal tract, ovaries, placenta, uterus, and adrenal medulla, as well as the pancreas, where its concentration is the highest and comparable to that in the CNS.Citation8,Citation17 In accord with this, high levels of GAD have been detected in the islets of Langerhans.Citation20 Moreover, it has been reported that both pancreatic α- and β-cells express a vesicular GABA transporter, which transports GABA into the intracellular vesicles for packaging before it is released, and GAT3, which mediates cellular uptake of GABA.Citation21 The abundance of GABA and the presence of molecular machinery for GABA synthesis and release suggest an important role in pancreatic physiology.
GABA receptors
GABAARs are heteropentamers of different subunits that form fast-acting chloride channels.Citation22 There are 19 known GABAAR subunits: α1–6, β1–3, γ1–3, δ, ε, θ, π, and ρ1–3.Citation22–Citation24 GABAARs with different subunit combinations possess different pharmacological properties. In the CNS, a functional GABAAR is mostly found in a configuration containing two α-subunits, two β-subunits, and one γ-subunit.Citation25,Citation26 Many of these subunits are found in islets or β-cell lines, and it appears that the αβγ configuration also represents a functional GABAAR in the islet cells.
GABABRs are composed of two invariable subunits: B1 and B2.Citation27 This slow-acting receptor is linked to K+ channels. Activation of GABABR stimulates the opening of K+ channels, leading to hyperpolarization of the membrane potential and a reduction in the activity of adenylyl cyclase.Citation28 This prevents the opening of sodium channels and the VGCC to convey inhibitory effects.Citation29,Citation30
In the adult brain, GABA exerts inhibitory effects that are primarily due to activation of the GABAAR Cl− ion channel, which leads to Cl− influx and membrane hyperpolarization.Citation2,Citation31 However, during brain development, GABA induces depolarizing effects, acting as the principal excitatory transmitter.Citation3,Citation4,Citation32 This is because immature neurons have a higher intracellular Cl− concentration, and activation of GABAAR initiates an efflux of Cl−, leading to membrane depolarization and excitatory actions. GABA-induced depolarizing effects results in Ca2+ influx via VGCCs, and modulates a variety of Ca2+-dependent cellular processes, such as proliferation and differentiation, which are important in the formation of synapses and activity in the neuronal networks.
The shift from depolarizing to hyperpolarizing effects of GABA is associated with the onset of K+–Cl− cotransporter -2 (KCC2) expression.Citation32 The neuron-specific KCC2 is responsible for establishing the Cl− gradient in neurons through the maintenance of low intracellular Cl− concentrations.Citation32 In the islets, a functional KCC2 is found in the α-cells, but not in the β-cells,Citation33 providing a molecular mechanism underlying the opposite actions of GABA in the islet β- and α-cells.
GABA and diabetes
GABA release in the endocrine system
A screen of selected peripheral organs of rats revealed that GABA was present at the highest concentration in the pancreas.Citation8 Indeed, it has been reported that islet GABA content and release are increased in response to increasing extracellular L-glutamine concentration; when L-glutamine is at a physiological concentration (0.5 mM), the content of GABA reaches a maximum that remains stable at 5 and 10 mM. This suggests that GABA synthesis is always saturated under normal circumstances.Citation34 GABA is further metabolized in the mitochondria through the GABA-shunt pathway, which may play a role in insulin secretion.Citation20,Citation35
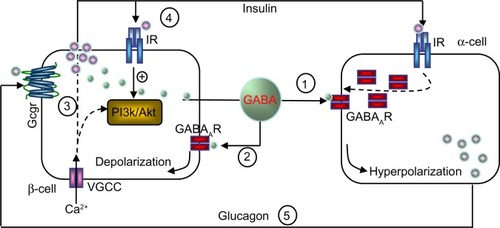
The basal release of GABA from the β-cells is relatively constant,Citation6,Citation12,Citation36 but it is modulated depending on the metabolic state of these cells.Citation37 GABA is localized in synaptic-like microvesicles,Citation6 and partially localized to large dense-core vesicles containing insulin.Citation21 Therefore, β-cells secrete GABA by both a glucose-dependent (exocytosis of insulin-containing granules) and a glucose-independent mechanism.Citation38 It appears that GABA is part of the fine-tuning machinery that is critical in maintaining islet-cell glucose competence ().
Figure 1 Hypothetical model of intraislet cell interaction.
Signaling mechanisms of GABA action
α-Cells
The signaling pathway is not fully elucidated. In neurons, GABA evokes Cl− currents that alter resting membrane potential and intracellular Ca2+ concentration.Citation39–Citation41 In this context, Ca2+ often acts as an important messenger initiating intracellular downstream signaling. During neuronal development, GABAAR-mediated depolarization and Ca2+ influx via VGCCs can activate the PI3K/PKC signaling pathway.Citation42 Activation of these protein kinases can phosphorylate GABAARs and induce receptor-trafficking events.Citation40,Citation43–Citation45
This is also the case with α-cells.Citation11 In response to increasing glucose levels, insulin released from β-cells activates the insulin receptor present on α-cells. Subsequent activation of Akt leads to phosphorylation of β-subunits of GABAAR that causes rapid translocation of the receptor to the plasma membrane. Although GABA is constantly released, the efficacy of the receptor-mediated inhibitory currents (Cl−) and membrane hyperpolarization are enhanced, due to the increased GABAAR numbers at the cell surface. In turn, membrane hyperpolarization shuts down the VGCC and inhibits α-cell exocytosis and glucagon release.Citation11 An impaired insulin/Akt/GABAAR/glucagon secretory pathway in the islet might be an underlying mechanism for unsuppressed glucagon secretion, despite hyperglycemia, in diabetic subjects.
β-Cells
Signaling differs sharply in β-cells, where GABA induces membrane depolarization.Citation14,Citation46,Citation47 In isolated rodent and human islets, GABA was shown to stimulate Akt activation, promoting β-cell proliferation and survival in a GABAAR antagonist- and/or Ca2+ channel blocker-sensitive fashion.Citation13–Citation14 This suggests that the GABAAR-mediated Ca2+-dependent PI3K/Akt pathway is a major mediator in conveying the trophic effects of GABA on β-cells.
GABABR is a G-protein-coupled receptor that initiates cyclic adenosine monophosphate signaling and Ca2+-dependent signaling. Previous studies demonstrated that in neurons GABAAR activation induced VGCC-dependent extracellular Ca2+ influx and Ca2+ release from intracellular stores, whereas GABABR evoked intracellular Ca2+ only.Citation48 This is consistent with our observation that GABA-mediated elevation of intracellular Ca2+ in human β-cells was blocked by the type A receptor antagonist (picrotoxin), while it was only partially attenuated by the type B receptor antagonist (saclofen).Citation13 Notably, our studies suggested that GABA stimulated CREB activation in a cyclic adenosine monophosphate/PKA-dependent signaling pathway mediated by GABABR.Citation13 CREB plays a key role in regulating β-cell mass homeostasis, as mice lacking CREB in their β-cells have diminished expression of IRS2Citation49 and display excessive β-cell loss.Citation50CREB is also a target gene of Akt signaling.Citation51 Interestingly, our study showed that the GABA-GABABR-induced CREB activation was independent of the PI3K/Akt pathway, because upon inhibition of PI3K/Akt, activation of CREB was not suppressed, whereas blockade of PKA-dependent CREB did not affect GABA-stimulated Akt activation.Citation13 This is of relevance, and provides a plausible mechanism by which GABA may be bypassing Akt activation in subjects with insulin resistance.
Studies examining the role of GABABR in modulating insulin secretion have shown variable results. It has been reported that in the presence of glucose at high concentration (over 10 mmol/L) GABABR displays an inhibitory effect on insulin secretion,Citation52,Citation53 whereas in the presence of lower glucose levels it has no effect.Citation53 In vivo studies suggest an important role for GABABR in regulating β-cell function. For instance, the treatment of nonobese diabetic (NOD) mice with a GABABR agonist delayed onset of type 1 diabetes (T1D),Citation54 and other studies showed GABABR-dependent improvement of β-cell survival and proliferation.Citation13,Citation16,Citation55 In apparent contradiction, GABAB1R-deficient mice (global knockout of B1 subunit) displayed improved glucose tolerance, increased pancreatic insulin content, and elevated glucose-stimulated insulin secretion, associated with enlarged islets and insulin resistance.Citation56,Citation57 The GABABR agonist baclofen inhibited glucose-stimulated insulin secretion in wild-type but not GABABR-knockout islets.Citation57 At this point, it appears that this receptor can have inhibitory or stimulatory effects on β-cell functions under various circumstances, and this merits further investigation.
It is interesting to note that in addition to GABA-receptor signaling, GABA catabolism also contributes to insulin secretion. GABA is catabolized in the GABA-shunt pathway after its transamination with 2-oxoglutarate by GABA transaminase into succinic semialdehyde and glutamate.Citation58 It is reported that both α-ketoisocaproic acidCitation58,Citation59 and glucoseCitation35 promote GABA metabolism and stimulate insulin secretion in a GABA-shunt-dependent fashion. This mechanism appears to be important, especially in rendering β-cells more competent in the face of a restriction in the metabolic flow of the citric acid cycle set by low 2-oxoglutarate dehydrogenase activity in response to high glucose stimulation.Citation35
Effects of GABA on the immune system and inflammatory pathways in diabetes
The effects of GABA on the immune system are generally inhibitory. In humans, GABAAR is expressed by T cells (CD4+ and CD8+), B cells, and some mononuclear cells, and GABAAR agonists suppress lymphocyte proliferation.Citation60–Citation62 In mice, Tian et alCitation63 identified GABAAR on T cells and showed that GABA (0.1–3 mM) partially inhibited (~50%) T-cell responses. These effects were duplicated by a GABAAR agonist (muscimol), but not by a GABABR agonist (baclofen), indicating that only GABAAR is involved. In a subsequent study, Tian et alCitation64 reported that GABA protected NOD mice against T1D, and reduced the response of their T-helper (Th)-1 cells to islet antigens.
Although T1D is a T-cell-dependent autoimmune disease, inflammatory mechanisms that are not directly T-cell-mediated also appear to play a major role in the pathogenesis.Citation65 This is particularly apparent in multiple low-dose streptozotocin-induced diabetes (MDSD).Citation66 Notably, anti-inflammatory treatment is beneficial in both MDSD and autoimmune NOD mice.Citation65,Citation67 In agreement with this, we found that GABA therapy lowered the levels of inflammatory cytokines (eg, IL-1β, TNFα, IFNγ, and IL-12) in the serum of MDSD mice.Citation14 In vitro, GABA also reduced the secretion of these cytokines. In contrast, it increased TGFβ1 production, which is a key regulatory (suppressive) cytokine.Citation14
It is clear that GABA acts on T cells of both effector and regulatory function. It suppresses the action of Th1 cells,Citation14,Citation64 as well as cytotoxic T lymphocytes, as demonstrated in a T-cell receptor (TCR) transgenic mouse model of T1D.Citation14 Importantly, we found that GABA increases regulatory T cells (Tregs) of the Foxp3+/neuropilin-1+ phenotype,Citation14 currently denoted thymus-derived Tregs. Tian et alCitation68 reported similar findings in a mouse type 2 diabetes (T2D) model. We hypothesize that this contributes to the immunosuppressive effects of GABA, and the underlying molecular mechanisms warrant further investigation. Other investigators have also shown immunosuppressive effects. For instance, Bjurstöm et alCitation69 reported that low levels of GABA (as low as 100 nM) suppressed CD4+ encephalitogenic T cells that causes experimental autoimmune encephalitis. These levels are similar to the concentration of GABA in normal plasma. Bhat et alCitation70 also reported protective effects in autoimmune encephalitis, but they concluded that GABA acted by suppressing macrophages.
We found that the immunosuppressive effects of GABA first observed in mice are also present in humans.Citation15 Indeed, it suppressed CD3-stimulated human T-cell proliferation in a GABAAR-dependent manner. Until recently, the immunosuppressive mechanisms were unclear, but we observed that GABA blocks calcium influx in human T cells. The calcium signal is a very early T-cell-activation signal, and its blockade is expected to lead to the inhibition of all subsequent activation-related events. Moreover, GABA suppressed NFκB activation in both human T cells and islet cells.Citation15 This is a key observation, because NFκB is a critically important pathway involved in both innate and adaptive immunity. Indeed, its activation appears closely linked to β-cell apoptosis initiated by inflammatory cytokines and other cell injuries.
However, it is unclear how GABA mediates these effects. Interestingly, we observed that GABA-induced effects in β-cells are associated with elevated activity of SIRT1, suggesting the involvement of this enzyme.Citation71 SIRT1 is an NAD+-dependent deacetylase that increases insulin secretion, and can block NFκB signaling. We found that the incubation of INS-1 insulinoma cells with GABA augmented SIRT1 expression, as did agonists acting on either GABAAR or GABABR. In addition, NAD+, which is essential for SIRT1 enzymatic action, was increased. GABA increased SIRT1 activity, which resulted in deacetylation of the p65 component of NFκB. This type deacetylation has previously been shown to interfere with the activation of NFκB. Of note, GABA stimulated insulin production and reduced apoptosis, and these effects were negated by SIRT1 inhibitors. We investigated whether SIRT1 and NAD+ are similarly induced by GABA in human islet cells, and found that this was indeed the case. As in INS-1 cells, the protective effect of GABA against human islet-cell apoptosis was SIRT1-dependent. Therefore, it appears that important beneficial effects of GABA on β-cells are due to increased SIRT1 and NAD+.
Of importance to islet transplantation, we found that GABA alleviates the toxic effects of immunosuppressive drugs, including rapamycin, tacrolimus (FK506), and mycophenolate mofetil.Citation15 These drugs are used to prevent rejection of transplanted organs or islets of Langerhans, and have all been implicated in impairing β-cell function and survival.Citation72–Citation74 Interestingly, while GABA reduced the toxicity of rapamycin, it collaborated with rapamycin to improve T-cell suppression. This suggests that GABA could be combined with other immunosuppressive drugs in a manner that protects islet cells and improves immunotherapy.
GABA and therapy of diabetes
GABA therapy of diabetes in preclinical models
T1D is an autoimmune disease characterized by infiltration of the pancreatic islets by T lymphocytes, macrophages, and other immune cells, and consequent loss of β-cells.Citation65,Citation75,Citation76 At the onset of T1D, the majority of β-cells are destroyed, resulting in a severe lack of insulin production.Citation77 In T2D, insulin resistance and β-cell failure remain the major pathophysiological defects; however, mounting evidence suggests that islet inflammation also plays an important role in the loss of functional β-cell mass in T2D. Therefore, therapies of T1D and T2D require both suppression of the inflammatory process and restoration of islet β-cells.
GABA induces membrane depolarization and Ca2+-dependent activation of cell-growth and survival pathways involving PI3K/Akt.Citation13–Citation14 This is in accord with our findings that GABA therapy preserves β-cell mass and prevents diabetes in three mouse models of T1D, ie, in the MDSD model, wild-type NOD mice, and transgenic TCR-8.3 NOD mice. Remarkably, in severely diabetic mice (MDSD model), GABA therapy regenerated β-cell mass and completely reversed hyperglycemia.Citation14 This was associated with anti-inflammatory and immunoregulatory events, which also appear to contribute to the success of therapy.
Our studies showed that activation of both GABAAR and GABABR are important in mediating GABA trophic effects to promote β-cell replication and survival, in both rodents and humans,Citation13 which is consistent with a study by Tian et al.Citation16 To analyze whether GABA could exert therapeutic effects on human islet cells, we used a suboptimal (marginal mass) islet-xenotransplantation model. This suboptimal mass of human islets was transplanted into immunodeficient NOD-severe combined immunodeficiency-γ mice after induction of diabetes with streptozotocin.Citation13 This in vivo approach revealed that oral GABA treatment increased graft-cell proliferation and decreased apoptosis, leading to a significantly enhanced β-cell mass. Furthermore, GABA lowered blood glucose levels and ameliorated glucose tolerance. Our results suggest that the Ca2+-dependent PI3K/Akt and CREB/IRS2 are two synergistic and independent signaling pathways that mediate the trophic effect of GABA in human islet cells.Citation13
In addition, GABA appears to be beneficial to T2D. Tian et alCitation68 demonstrated that oral treatment with GABA improves glucose tolerance and insulin sensitivity in high-fat diet-fed mice. They concluded that this was due to the inhibition of obesity-related inflammation and upregulation of Treg responses.
GAD as a target antigen in T1D
GAD is colocalized with GABACitation6 in islets of rodents and humans, and the predominant isoform (GAD65 or GAD67) varies between species.Citation78 GAD65 has long been identified as one of the major target antigens recognized by self-reactive T cells in T1D.Citation79 We hypothesize that autoimmunity against GAD in the islet depletes this enzyme and hence reduces GABA levels, and this promotes the progression of this disease. Indeed, immunomodulation with GAD65 vaccination has been extensively investigated for the prevention or treatment of T1D.Citation79 In 1994, the Swedish pharmaceutical company Diamyd Medical licensed the rights to GAD65 as the active substance in the antigen-based diabetes therapy Diamyd®. Unfortunately, despite promising results from Phase I and II trials,Citation80–Citation82 the Phase III study failed to produce a therapeutic effect.Citation83 These results are disappointing, but a recent publication suggests that the antigen therapy in combination with other agents, such as GABA, may hold promise for intervention in T1D.Citation84
Combined islet-antigen and GABA therapy
Antigen therapy of T1D by administration of insulin, GAD, peptides, or other islet antigens with various adjuvant formulations or delivery methods has proven effective in mice, but thus far clinical trials have shown only limited or no benefits.Citation79,Citation85 However, preclinical studies of combined antigen vaccination and GABA therapy suggest a possible avenue for improvement. Recently, Tian et alCitation84 reported that combined therapy with proinsulin/alum and GABA synergizes to restore normoglycemia in newly diabetic NOD mice, sometimes with permanent remission of the disease. In this study, proinsulin/alum alone failed to correct hyperglycemia, whereas GABA monotherapy restored normoglycemia for a limited period of time. Therefore, combined therapy proved necessary to induce remission, and was found to inhibit pathogenic T-cell responses and to promote β-cell proliferation.
In another study,Citation86 the combination of oral GABA treatment and immunization with a GAD/alum formulation was also found to be effective in a transplantation model. In diabetic NOD mice receiving syngeneic pancreas grafts, combined therapy was much more effective than monotherapy in prolonging the survival of β-cells.
GABA administration to humans
Studies published as far back as 1960 showed that GABA can be administered orally in large amounts to humans (several grams/day) without serious adverse effects.Citation87 To date, there are at least three reports studying the effects of GABA on endocrine pancreatic function in humans. In one study, a single oral dose of GABA (5 or 10 g) significantly increased plasma insulin and C-peptide levels in 12 healthy subjects.Citation88 In other study, 20 mg GABABR agonist baclofen was administered orally to ten healthy subjects 1 hour prior to glucose challenge and posttreatment test, which resulted in significantly increased insulin responses to glucose challenge and increased basal glucagon levels. However, glucose tolerance was not found to be changed after baclofen treatment.Citation89 In another study, it was shown that intravenous administration of GABA (2–4 mg) significantly reduced blood glucose levels in the majority of diabetic individuals, but not in the nondiabetic subjects.Citation90 These human studies were quite limited in scope, but nevertheless suggest that GABA plays a role in regulating endocrine pancreatic function. We are conducting clinical trials to study the pharmacokinetics and pharmacodynamics of GABA in healthy subjects (http://clinicaltrials.gov/show/NCT01917760), as well as its efficacy in patients with newly diagnosed T1D (http://clinicaltrials.gov/show/NCT01781884). Our Phase I GABA study in health volunteers showed that GABA orally administered was rapidly absorbed in the gastrointestinal tract and had a favorable safety profile.
Conclusion
In the past five decades, the function of GABA in the CNS has been well documented. However, the presence of a GABAergic system within the pancreas as a potential target for treating diabetes mellitus emerged only recently. In α-cells, GABA induces membrane hyperpolarization and inhibits glucagon secretion, and this involves an insulin-mediated GABAAR-trafficking mechanism. In β-cells, GABA induces membrane depolarization and enhances insulin secretion. GABA also has beneficial effects on β-cell survival and regeneration, which results in enlarged β-cell mass. Furthermore, GABA suppresses insulitis and systemic inflammatory cytokine production. All these data hold promise for GABA therapy in regulating islet cell function, glucose homeostasis, and autoimmunity. Of note, similarly to rodent studies, GABA shows trophic effects on human islets. This is important, because while a number of agents exert protective and proliferative effects on rodent islet cells, very few show similar activities on human islet cells. Orally administered GABA is safe for humans, and acts on peripheral GABA receptors but does not affect CNS functions, since it does not cross the blood–brain barrier, and thus it represents a promising new therapeutic agent for diabetes.
Acknowledgments
Our studies were supported by the Juvenile Diabetes Research Foundation (JDRF), the Canadian Institute for Health Research (CIHR), the Canadian Diabetes Association (CDA), and the National Science Foundation China (NSFC).
Disclosure
The authors report no conflicts of interest in this work.
References
- RobertsEFrankelSγ-Aminobutyric acid in brain: its formation from glutamic acidJ Biol Chem1950187155 6314794689
- OwensDFKriegsteinARIs there more to GABA than synaptic inhibition?Nat Rev Neurosci200239715 72712209120
- RepresaABen-AriYTrophic actions of GABA on neuronal developmentTrends Neurosci2005286278 28315927682
- FiszmanMLSchousboeARole of calcium and kinases on the neurotrophic effect induced by gamma-aminobutyric acidJ Neurosci Res2004764435 44115114615
- AdeghateEPoneryASGABA in the endocrine pancreas: cellular localization and function in normal and diabetic ratsTissue Cell20023411 611989965
- ReetzASolimenaMMatteoliMFolliFTakeiKDe CamilliPGABA and pancreatic beta-cells: colocalization of glutamic acid decarboxylase (GAD) and GABA with synaptic-like microvesicles suggests their role in GABA storage and secretionEMBO J19911051275 12842022191
- TaniguchiHOkadaYSeguchiHHigh concentration of gamma-aminobutyric acid in pancreatic beta cellsDiabetes1979287629 633221297
- GerberJC3rdHareTAGamma-aminobutyric acid in peripheral tissue, with emphasis on the endocrine pancreas: presence in two species and reduction by streptozotocinDiabetes197928121073 1076159847
- VincentSRHökfeltTWuJYEldeRPMorganLMKimmelJRImmunohistochemical studies of the GABA system in the pancreasNeuroendocrinology1983363197 2046339978
- OkadaYTaniguchiHSchimadaCHigh concentration of GABA and high glutamate decarboxylase activity in rat pancreatic islets and human insulinomaScience19761944265620 622185693
- XuEKumarMZhangYIntra-islet insulin suppresses glucagon release via GABA-GABAA receptor systemCell Metab20063147 5816399504
- RorsmanPBerggrenPOBokvistKGlucose-inhibition of glucagon secretion involves activation of GABAA-receptor chloride channelsNature19893416239233 2362550826
- PurwanaIZhengJLiXGABA promotes human β-cell proliferation and modulates glucose homeostasisDiabetes201463124197 420525008178
- SoltaniNQiuHAleksicMGABA exerts protective and regenerative effects on islet beta cells and reverses diabetesProc Natl Acad Sci U S A20111082811692 1169721709230
- Prud’HommeGJGlinkaYHasiloCParaskevasSLiXWangQGABA protects human islet cells against the deleterious effects of immunosuppressive drugs and exerts immunoinhibitory effects aloneTransplantation2013967616 62323851932
- TianJDangHChenZγ-Aminobutyric acid regulates both the survival and replication of human β-cellsDiabetes201362113760 376523995958
- GladkevichAKorfJHakobyanVPMelkonyanKVThe peripheral GABAergic system as a target in endocrine disordersAuton Neurosci20061241–21 816338174
- Thomas-ReetzACDe CamilliPA role for synaptic vesicles in non-neuronal cells: clues from pancreatic beta cells and from chromaffin cellsFASEB J199482209 2167907072
- GlykysJModyIActivation of GABAA receptors: views from outside the synaptic cleftNeuron2007565763 77018054854
- SorensonRLGarryDGBreljeTCStructural and functional considerations of GABA in islets of Langerhans. Beta-cells and nervesDiabetes199140111365 13741936599
- GammelsaeterRFrøylandMAragónCGlycine, GABA and their transporters in pancreatic islets of Langerhans: evidence for a paracrine transmitter interplayJ Cell Sci2004117Pt 173749 375815252115
- RudolphUKnoflachFBeyond classical benzodiazepines: novel therapeutic potential of GABAA receptor subtypesNat Rev Drug Discov2011109685 69721799515
- OlsenRWTobinAJMolecular biology of GABAA receptorsFASEB J1990451469 14802155149
- LüddensHKorpiERSeeburgPHGABAA/benzodiazepine receptor heterogeneity: neurophysiological implicationsNeuropharmacology1995343245 2547630479
- ModyIPearceRADiversity of inhibitory neurotransmission through GABA(A) receptorsTrends Neurosci2004279569 57515331240
- LüscherBKellerCARegulation of GABAA receptor trafficking, channel activity, and functional plasticity of inhibitory synapsesPharmacol Ther20041023195 22115246246
- WhiteJHWiseAMainMJHeterodimerization is required for the formation of a functional GABA(B) receptorNature19983966712679 6829872316
- HashimotoTKuriyamaKIn vivo evidence that GABA(B) receptors are negatively coupled to adenylate cyclase in rat striatumJ Neurochem1997691365 3709202330
- BettlerBKaupmannKMosbacherJGassmannMMolecular structure and physiological functions of GABA(B) receptorsPhysiol Rev2004843835 86715269338
- BoweryNGGABAB receptor pharmacologyAnnu Rev Pharmacol Toxicol199333109 1478388192
- KittlerJTMossSJModulation of GABAA receptor activity by phosphorylation and receptor trafficking: implications for the efficacy of synaptic inhibitionCurr Opin Neurobiology2003133341 347
- LudwigALiHSaarmaMKailaKRiveraCDevelopmental up-regulation of KCC2 in the absence of GABAergic and glutamatergic transmissionEur J Neurosci200318123199 320614686894
- DaviesSLRoussaELe RouzicPExpression of K+-Cl- cotransporters in the alpha-cells of rat endocrine pancreasBiochim Biophys Acta2004166717 1415533301
- Fernández-PascualSMukala-Nsengu-TshibanguAMartín Del RíoRTamarit-RodríguezJConversion into GABA (gamma-aminobutyric acid) may reduce the capacity of L-glutamine as an insulin secretagogueBiochem J2004379Pt 3721 72914763900
- Pizarro-DelgadoJBraunMHernández-FisacIMartín-Del-RíoRTamarit-RodriguezJGlucose promotion of GABA metabolism contributes to the stimulation of insulin secretion in beta-cellsBiochem J20104313381 38920695849
- SmismansASchuitFPipeleersDNutrient regulation of gamma-aminobutyric acid release from islet beta cellsDiabetologia199740121411 14159447948
- WinnockFLingZDe ProftRCorrelation between GABA release from rat islet beta-cells and their metabolic stateAm J Physiol Endocrinol Metab20022824E937 E94211882516
- BraunMWendtAKaranauskaiteJCorelease and differential exit via the fusion pore of GABA, serotonin, and ATP from LDCV in rat pancreatic beta cellsJ Gen Physiol20071293221 23117296927
- MoulderKLCormierRJShuteAAZorumskiCFMennerickSHomeostatic effects of depolarization on Ca2+ influx, synaptic signaling, and survivalJ Neurosci20032351825 183112629186
- VithlaniMMossSJThe role of GABAAR phosphorylation in the construction of inhibitory synapses and the efficacy of neuronal inhibitionBiochem Soc Trans200937Pt 61355 135819909275
- FukuraHKomiyaYIgarashiMSignaling pathway downstream of GABAA receptor in the growth coneJ Neurochem19966741426 14348858924
- PorcherCHatchettCLongbottomREPositive feedback regulation between gamma-aminobutyric acid type A (GABA(A)) receptor signaling and brain-derived neurotrophic factor (BDNF) release in developing neuronsJ Biol Chem20112862421667 2167721474450
- TerunumaMPangalosMNMossSJFunctional modulation of GABAB receptors by protein kinases and receptor traffickingAdv Pharmacol201058113 12220655480
- ChouWHWangDMcMahonTGABAA receptor trafficking is regulated by protein kinase Cε and the N-ethylmaleimide-sensitive factorJ Neurosci2010304213955 1396520962217
- BirnirBKorpiERThe impact of sub-cellular location and intracellular neuronal proteins on properties of GABA(A) receptorsCurr Pharm Des200713313169 317718045166
- BraunMRamracheyaRBengtssonMGamma-aminobutyric acid (GABA) is an autocrine excitatory transmitter in human pancreatic beta-cellsDiabetes20105971694 170120413510
- DongHKumarMZhangYGamma-aminobutyric acid up- and downregulates insulin secretion from beta cells in concert with changes in glucose concentrationDiabetologia2006494697 70516447058
- SchwirtlichMEmriZAntalKMátéZKatarovaZSzabóGGABA(A) and GABA(B) receptors of distinct properties affect oppositely the proliferation of mouse embryonic stem cells through synergistic elevation of intracellular Ca(2+)FASEB J20102441218 122819959723
- JhalaUSCanettieriGScreatonRAcAMP promotes pancreatic beta-cell survival via CREB-mediated induction of IRS2Genes Dev200317131575 158012842910
- WithersDJGutierrezJSToweryHDisruption of IRS-2 causes type 2 diabetes in miceNature19983916670900 9049495343
- DuKMontminyMCREB is a regulatory target for the protein kinase Akt/PKBJ Biol Chem19982734932377 323799829964
- GuXHKuroseTKatoSSuppressive effect of GABA on insulin secretion from the pancreatic beta-cells in the ratLife Sci1993528687 6948445998
- BriceNLVaradiAAshcroftSJMolnarEMetabotropic glutamate and GABA(B) receptors contribute to the modulation of glucose-stimulated insulin secretion in pancreatic beta cellsDiabetologia2002452242 25211935156
- BealesPEHawaMWilliamsAJAlbertiniMCGiorginiAPozzilliPBaclofen, a gamma-aminobutyric acid-b receptor agonist, delays diabetes onset in the non-obese diabetic mouseActa Diabetol199532153 567612919
- LigonBYangJMorinSBRubertiMFSteerMLRegulation of pancreatic islet cell survival and replication by gamma-aminobutyric acidDiabetologia2007504764 77317318626
- CrivelloMBonaventuraMMChamson-ReigAPostnatal development of the endocrine pancreas in mice lacking functional GABAB receptorsAm J Physiol Endocrinol Metab201330410E1064 E107623531612
- BonaventuraMMCatalanoPNChamson-ReigAGABAB receptors and glucose homeostasis: evaluation in GABAB receptor knockout miceAm J Physiol Endocrinol Metab20082941E157 E16717971510
- Pizarro-DelgadoJHernández-FisacIMartín-Del-RíoRTamarit-RodriguezJBranched-chain 2-oxoacid transamination increases GABA-shunt metabolism and insulin secretion in isolated isletsBiochem J20094192359 36819173679
- Hernández-FisacIFernández-PascualSOrtsäterHOxo-4-methylpentanoic acid directs the metabolism of GABA into the Krebs cycle in rat pancreatic isletsBiochem J2006400181 8916819942
- AlamSLaughtonDLWaldingAWolstenholmeAJHuman peripheral blood mononuclear cells express GABAA receptor subunitsMol Immunol20064391432 144216213022
- DionisioLJose De RosaMBouzatCEsandi MdelCAn intrinsic GABAergic system in human lymphocytesNeuropharmacology2011602–3513 51921093461
- MenduSKBhandageAJinZBirnirBDifferent subtypes of GABA-A receptors are expressed in human, mouse and rat T lymphocytesPloS One201278e4295922927941
- TianJChauCHalesTGKaufmanDLGABA(A) receptors mediate inhibition of T cell responsesJ Neuroimmunol199996121 2810227421
- TianJLuYZhangHChauCHDangHNKaufmanDLGamma-aminobutyric acid inhibits T cell autoimmunity and the development of inflammatory responses in a mouse type 1 diabetes modelJ Immunol200417385298 530415470076
- EizirikDLColliMLOrtisFThe role of inflammation in insulitis and beta-cell loss in type 1 diabetesNat Rev Endocrinol200954219 22619352320
- Kolb-BachofenVEpsteinSKieselUKolbHLow-dose streptozocin-induced diabetes in mice. Electron microscopy reveals single-cell insulitis before diabetes onsetDiabetes198837121 273275555
- CockfieldSMRamassarVUrmsonJHalloranPFMultiple low dose streptozotocin induces systemic MHC expression in mice by triggering T cells to release IFN-gammaJ Immunol198914241120 11282521659
- TianJDangHNYongJOral treatment with gamma-aminobutyric acid improves glucose tolerance and insulin sensitivity by inhibiting inflammation in high fat diet-fed micePloS One201169e2533821966503
- BjurstömHWangJEricssonIGABA, a natural immunomodulator of T lymphocytesJ Neuroimmunol20082051–244 5018954912
- BhatRAxtellRMitraAInhibitory role for GABA in autoimmune inflammationProc Natl Acad Sci U S A201010762580 258520133656
- Prud’hommeGJGlinkaYUdovykOHasiloCParaskevasSWangQGABA protects pancreatic beta cells against apoptosis by increasing SIRT1 expression and activityBiochem Biophys Res Commun20144523649 65425193706
- BarlowADXieJMooreCERapamycin toxicity in MIN6 cells and rat and human islets is mediated by the inhibition of mTOR complex 2 (mTORC2)Diabetologia20125551355 136522314813
- BellECaoXMoibiJARapamycin has a deleterious effect on MIN-6 cells and rat and human isletsDiabetes200352112731 273914578291
- JohnsonJDAoZAoPDifferent effects of FK506, rapamycin, and mycophenolate mofetil on glucose-stimulated insulin release and apoptosis in human isletsCell Transplant2009188833 84519500470
- AtkinsonMAEisenbarthGSType 1 diabetes: new perspectives on disease pathogenesis and treatmentLancet20013589277221 22911476858
- LehuenADianaJZacconePCookeAImmune cell crosstalk in type 1 diabetesNat Rev Immunol2010107501 51320577267
- PipeleersDChintinneMDenysBMartensGKeymeulenBGorusFRestoring a functional beta-cell mass in diabetesDiabetes Obes Metab200810Suppl 454 6218834433
- KimJRichterWAanstootHJDifferential expression of GAD65 and GAD67 in human, rat, and mouse pancreatic isletsDiabetes199342121799 18088243826
- RydenAKWesleyJDCoppietersKTVon HerrathMGNon-antigenic and antigenic interventions in type 1 diabetesHum Vaccin Immunother2014104838 84624165565
- AgardhCDCilioCMLethagenAClinical evidence for the safety of GAD65 immunomodulation in adult-onset autoimmune diabetesJ Diabetes Complications2005194238 24615993359
- LudvigssonJFaresjöMHjorthMGAD treatment and insulin secretion in recent-onset type 1 diabetesN Engl J Med2008359181909 192018843118
- LudvigssonJHjorthMChéramyMExtended evaluation of the safety and efficacy of GAD treatment of children and adolescents with recent-onset type 1 diabetes: a randomised controlled trialDiabetologia2011543634 64021116604
- LudvigssonJKriskyDCasasRGAD65 antigen therapy in recently diagnosed type 1 diabetes mellitusN Engl J Med20123665433 44222296077
- TianJDangHNguyenAVChenZKaufmanDLCombined therapy with GABA and proinsulin/alum acts synergistically to restore long-term normoglycemia by modulating T-cell autoimmunity and promoting beta-cell replication in newly diabetic NOD miceDiabetes20146393128 313425146474
- HarrisonLCWentworthJMZhangYAntigen-based vaccination and prevention of type 1 diabetesCurr Diab Rep2013135616 62323888323
- TianJDangHKaufmanDLCombining antigen-based therapy with GABA treatment synergistically prolongs survival of transplanted ss-cells in diabetic NOD micePloS One201169e2533721966502
- TowerDBThe administration of gamma-aminobutyric acid to man: systemic effects and anticonvulsant actionTowerDBRobertsEInhibition in the Nervous System and γ-Aminobutyric AcidNew YorkPergamon1960562 578
- CavagniniFPintoMDubiniAInvittiCCappellettiGPolliEEEffects of gamma aminobutyric acid (GABA) and muscimol on endocrine pancreatic function in manMetabolism198231173 777043162
- PassarielloNGiuglianoDTorellaRSgambatoSCoppolaLFrascollaNA possible role of gamma-aminobutyric acid in the control of the endocrine pancreasJ Clin Endocrinol Metab19825461145 11497042732
- KhumarianNGMamikonianRSOn the role of gamma aminobutyric acid in the regulation of the level of glycemia in diabetes mellitusZh Eksp Klin Med1967723 9 Russian5605571
- BansalPWangQInsulin as a physiological modulator of glucagon secretionAm J Physiol Endocrinol Metab20082954E751 E76118647881
- WangQJinTThe role of insulin signaling in the development of beta-cell dysfunction and diabetesIslets20091295 10121099255
- EgeaJEspinetCSolerRMNeuronal survival induced by neurotrophins requires calmodulinJ Cell Biol20011543585 59711489918
- BitoHDeisserothKTsienRWCREB phosphorylation and dephosphorylation: a Ca(2+)- and stimulus duration-dependent switch for hippocampal gene expressionCell19968771203 12148980227
- ParkSDongXFisherTLExendin-4 uses Irs2 signaling to mediate pancreatic beta cell growth and functionJ Biol Chem200628121159 116816272563
