Abstract
Obesity is a major predisposing factor for the development of type 2 diabetes (T2D) and is an escalating public health issue around the world. The transition from obesity to T2D is preceded by the induction of a state of insulin resistance, which occurs in response to genetic factors and environmental influences, such as diet. Recent advances have implicated inflammatory immune cells and cytokines as critical pathogenic mediators of insulin resistance and T2D. In particular proinflammatory T helper (Th)1 cells and M1 macrophages are recruited to adipose tissue in response to high fat diet and directly promote the development of insulin resistance. The function of these two cell types is linked by the actions of the cytokine interferon (IFN)γ and one of its major transcriptional regulators T-bet. Recent studies in animal models of T2D demonstrate that T-bet is critical for the development of insulin resistance in response to high fat diet as T-bet-deficient animals are protected from the development of insulin resistance. These data indicate that T-bet and type 1 immunity may constitute novel sites of therapeutic intervention for the treatment of insulin resistance and T2D, in obese human patients.
Introduction
The prevalence of obesity has steadily increased in recent decades and currently, more than 10% of the world’s adult population is obese, as determined by a body mass index (BMI) equal to or greater than 30 kg/m2.Citation1 In the USA, the prevalence of obesity is 34%, and this is forecast to increase to 42% by 2030.Citation2 Obesity is caused by an imbalance between intake and expenditure of energy and is particularly associated with increased adiposity. It is an important risk factor for the development of type 2 diabetes (T2D), cardiovascular disorders, musculoskeletal problems, such as osteoarthritis, and increased risk of certain cancers, including colon and breast cancer.Citation1 According to US statistics, more than 80% of people with T2D are obese or overweight.Citation3 Thus, obesity predisposes to a range of debilitating health problems and will represent a significant burden of the public health system for the foreseeable future.
Study of the pathogenesis of obesity and T2D has identified important roles for the immune system in the induction of insulin resistance and development of T2D. As we more fully elucidate the underlying immunological mechanism, it is possible that we will identify new therapeutic strategies for the treatment of T2D based on modulation of immune responses. Our aim is to review the current literature elucidating mechanisms of immune regulation of insulin resistance and T2D, with particular focus on the last decade of work defining the role for the transcription factor T-bet for control of type 1 immunity, and its relevance in T2D.
Progression from obesity to T2D
Obesity can lead to the impairment of insulin sensitivity in sites of glucose disposal, such as skeletal muscle, liver, and adipose tissue – a condition known as “insulin resistance”. The normal actions of insulin include stimulation of glucose uptake and glycogen synthesis in the skeletal muscle and liver, and inhibition of gluconeogenesis in the liver. Insulin also causes inhibition of lipolysis and increases lipogenesis in adipose tissue. Insulin resistance is characterized by reduced glucose uptake by skeletal muscle, increased release of glucose from the liver, and increased release of lipids from adipose tissue into the blood.Citation4 Overall, this results in glucose intolerance and relative hyperglycemia in obese insulin-resistant individuals.
β-cells in the pancreatic islets adapt to hyperglycemia with compensatory responses, such as expansion of the total β-cell mass and increased insulin secretion.Citation5 The resultant hyperinsulinemia is able to maintain normoglycemia to some extent; however, chronic progressive insulin resistance and compensatory hypersecretion of insulin can induce β-cell stress and eventually, β-cell failure, resulting in progression to frank hyperglycemia and T2D.Citation6 Poor glycemic control in individuals with T2D can lead to various downstream complications that include renal failure, blindness, neuropathy, and cardiovascular disorders, such as myocardial infarction and stroke.Citation7
Animal models of obesity
Understanding the mechanisms of obesity-induced insulin resistance has been an area of intensive research in recent years. These mechanisms have been elucidated primarily using animal models, including the high fat diet (HFD) model, ob/ob mice, and Zucker fatty rats.Citation8 In the HFD model, male mice or rats are made obese, insulin resistant, and glucose intolerant by feeding them a lipid- and calorie-rich diet for several weeks. Most studies use either a 45% or 60% HFD (denoting the percentage of total digestible calories obtained from lipid sources).Citation8 The ob/ob mouse is homozygous for a recessive inactivating mutation in leptin, a satiety hormone, and this mutation causes hyperphagia, obesity, and hyperglycemia.Citation8 Zucker fatty rats are homozygous for a recessive mutation in the leptin receptor, which causes leptin resistance secondary to a hypofunctional leptin receptor. Similar to ob/ob mice, these rats are hyperphagic and obese and become insulin resistant and glucose intolerant.Citation8 The intake of an HFD or hyperphagia in these animal models causes increased accumulation of white adipose tissue, which is classified either subcutaneous or visceral. Increased deposition of visceral adipose tissue is generally more deleterious for glucose homeostasis and insulin sensitivity in these animal models.Citation9 Ectopic lipid accumulation is also observed in the liver (steatosis) and muscle of these rodents, and this is a strong predictor of hepatic and skeletal muscle insulin resistance.Citation10
Insulin signaling and insulin resistance
Insulin signaling is a complex process involving several key factors (these have been reviewed more extensively elsewhere).Citation11 Important steps in the insulin signaling cascade include the binding of insulin to its receptor, followed by its autophosphorylation and tyrosine phosphorylation of insulin receptor substrate (IRS)-1 and IRS-2. IRS-1 and IRS-2 then bind to phosphatidylinositol 3-kinase (PI3K), and this induces a series of downstream events resulting in activation of Akt. Activation of Akt in insulin-sensitive cells has various effects, including the translocation of the insulin-sensitive glucose transporter (GLUT-4) to the cell membrane, activation of glycogen synthesis, and inhibition of gluconeogenic genes in the liver.Citation4,Citation6 Impairments of this signaling network can result in insulin resistance, including modulation of the activity of the key signaling molecules, such as IRS-1, IRS-2, and Akt. The kinases IKK (IkB kinase) and JNK-1 (c-Jun N-terminal kinase 1) negatively regulate IRS-1 and IRS-2 by serine phosphorylation, inducing degradation of IRS proteins and inhibition of insulin receptor signaling.Citation11,Citation12 Increased IRS-1 serine phosphorylation is observed in insulin-resistant states.Citation13,Citation14 SOCS (suppressor of cytokine signaling) proteins compete with IRS proteins for binding sites on the insulin receptor, inhibit IRS tyrosine phosphorylation, and induce IRS-1 and IRS-2 degradation.Citation15,Citation16 The protein phosphatases PP2A (protein phosphatase 2A) and PHLPP (PH domain and leucine rich repeat protein phosphatase) inhibit Akt activity via dephosphorylation of the activating sites.Citation17,Citation18 Further, the factor TRB3 (tribbles homolog 3) inhibits Akt activity by sequestering the unphosphorylated form, thereby blocking its phosphorylation and activation.Citation19 These mechanisms are regulated by metabolic and inflammatory factors that induce insulin resistance and predispose to the development of T2D.
Lipid-derived molecules are mediators of insulin resistance
Increased plasma concentration of saturated fatty acids such as palmitate is an important cause of insulin resistance in obesity.Citation20 Palmitate stimulation of cultured skeletal muscle myotubes has been shown to inhibit insulin signaling and glucose uptake in rat soleus muscle preparations.Citation21,Citation22 These inhibitory effects are mediated, in part, by the sphingolipid ceramide, which is synthesized as a consequence of palmitate-induced impairments of mitochondrial lipid oxidationCitation20 and inhibits insulin signaling by blocking Akt activation.Citation21,Citation23 Palmitate also induces insulin resistance via engagement of toll-like receptor 2 (TLR2) and subsequent activation of JNK and IKKβ kinases.Citation24 Myriocin, a ceramide synthesis inhibitor, prevents palmitate-induced impairment of insulin signaling in skeletal muscle cells and diabetes, in rodent models.Citation23,Citation25 Conversely, inhibition of acid ceramidase, an enzyme that reduces ceramide levels by converting to sphingosine, restores the palmitate-induced suppression of insulin signaling in skeletal muscle.Citation26
Unsaturated fatty acids also induce insulin resistance in key metabolic tissues. Linoleate induces increased cellular accumulation of diacylglycerols, and linoleate-rich infusions and diacylglycerols induce insulin resistance in rodent and human skeletal muscles, independently of ceramides.Citation25,Citation27,Citation28 Diacylglycerols induce the activation of PKC (protein kinase C) isoforms, and these can inhibit the activity of IRS-1, leading to insulin resistance.Citation20,Citation29 Thus, lipid-derived molecules have direct effects on insulin-sensitive tissues and can induce insulin resistance.
Mechanisms of inflammation-induced insulin resistance
Appreciation of the role of inflammatory processes in the development of insulin resistance has increased over the last 15 years. It is now recognized that obesity induces an inflammatory state that promotes the development of insulin resistance.Citation9,Citation30 Adipose tissue has high levels of resident macrophages, and these cells are critical players that mediate the development of obesity-associated insulin resistance.Citation31 Adipose tissue macrophages (ATMs) are classified into classically or alternatively activated macrophages, based on their pro- or anti-inflammatory functions respectively and can be identified by their differential expression of cell surface markers and cytokines.
M1 or classically activated macrophages express CD11c, secrete proinflammatory cytokines, such as tumor necrosis factor (TNF)α, interleukin (IL)-6, and IL-1β, and their differentiation is promoted by interferon (IFN)γ and lipopolysaccharide.Citation31,Citation32 M2 or alternatively activated macrophages express MGL-1 (macrophage galactose lectin 1), secrete anti-inflammatory molecules, such as IL-10, transforming growth factor (TGF)β, and IL-1 receptor antagonist, and differentiate in response to IL-4 and IL-13.Citation31,Citation32 Under normal homeostatic conditions, ATMs have a predominantly M2 phenotype; however, obesity induces a shift in the ATM population, from the M2 to M1 phenotype. This occurs via two main mechanisms: phenotypic conversion of M2 to M1 and recruitment of M1 macrophages into adipose tissue.Citation6,Citation31–Citation34 This phenotypic conversion can be mediated by multiple stimuli, such as lipotoxicity and adipocyte dysfunction, including hypertrophy, hypoxia, and necrosis. Necrotic adipocytes also stimulate recruitment and the accumulation of M1 macrophages and secretion of proinflammatory cytokines and chemokines, including monocyte chemoattractant protein (MCP)-1.Citation6,Citation33,Citation35,Citation36
M1 ATMs produce proinflammatory cytokines, such as TNFα, IL-6, and IL-1β, in response to obesity, and circulating levels of these cytokines are increased in human patients and mouse models of T2D.Citation30 These cytokines have several effects that could link obesity to insulin resistance, most notably, the induction of local inflammatory pathways. Engagement of TNFα receptors activates the NF-κB (nuclear factor kappa-light-chain-enhancer of activated B cells) and MAP (mitogen-activated protein) signaling pathways via IKK and JNK1 kinases.Citation10 Liver-specific deletion of IKK protects from hepatic insulin resistance in both the HFD and ob/ob mouse models.Citation37 Similarly, JNK1-deficient mice are protected from obesity-induced insulin resistance. Compared with wild type controls, these mice gain less weight and have a relatively higher body temperature, indicating increased energy expenditure.Citation14 IL-6 suppresses insulin receptor signal transduction by inhibiting IRS-1, IRS-2, and Akt in a SOCS3-dependent manner.Citation15,Citation38 These cytokines also cause increased lipolysis in adipose tissue, by reducing the expression of stabilizing lipid droplet proteins, including perilipin and FSP27 (fat specific protein 27).Citation39,Citation40 This could promote ectopic lipid deposition in other insulin-sensitive tissues.
NLRP (Nucleotide-binding oligomerization domain Leucine-rich Repeat and Pyrin domain-containing family) inflammasome-dependent IL-1β, secretion from macrophages and adipocytes plays an important role in the pathogenesis of insulin resistance.Citation41 The NLRP3 inflammasome mediates the activation of caspase 1, which is required for the processing of pro-IL-1β to the active IL-1β form.Citation42 IL-1β is directly toxic to pancreatic β-cells and induces insulin resistance via inflammatory pathways.Citation41 The saturated fatty acid palmitate and ceramide activate the inflammasome complex in macrophages, while the unsaturated fatty acid oleate does not.Citation43,Citation44 Increased inflammasome activity has been observed in monocyte-derived macrophages from T2D patients.Citation45 Inflammasome activation promotes insulin resistance via inhibitory serine phosphorylation of IRS-1 in liver and adipocytes, and inhibition of Akt activity.Citation44 Mice deficient in NLRP3 or caspase 1 are protected from HFD-induced insulin resistance and glucose intolerance.Citation44,Citation46 Thus, inflammasome activation and the production of inflammatory cytokines by adipose tissue macrophages can inhibit insulin signaling and cause insulin resistance.
The adaptive immune system regulates insulin sensitivity in obesity
The adaptive immune system is composed of a vast array of differing lymphocyte subsets. The predominant lymphocytes include CD4+ T cells, regulatory T cells (Tregs), CD8+ T cells, and B cells, and these cells perform numerous critical functions, such as secretion of cytokines, the limiting of self-reactivity, killing of infected cells, and production of antibodies. CD4+ T cells are central regulators of the adaptive immune system, and these can diverge along multiple lineages, such as T helper (Th)1, Th2, Th17, and Tregs, in response to environmental cues.Citation47 These lineages exhibit unique cytokine signatures and functional properties.Citation47
Lymphocytes play a critical role in the control of adiposity and glucose homeostasis in response to a HFD, in mouse models.Citation31,Citation48 When fed a normal diet, adipose tissue resident Tregs and Th2 T cells maintain an anti-inflammatory environment via the production of anti-inflammatory cytokines and induction of alternative macrophage activation.Citation31,Citation49 In response to a HFD, Th1 type T cells are recruited to subcutaneous and visceral adipose tissue, in animal models. This recruitment increases the ratio of Th1 cells to Tregs and leads to a proinflammatory state in visceral adipose tissue. This is temporally correlated with an increased number of M1-type macrophages in adipose tissue, induction of insulin resistance, and impaired glucose homeostasis, in mice.Citation34,Citation50,Citation51 There is some evidence that this mechanism may be relevant to humans as analysis of a small cohort of obese patients revealed that the Th1/Treg ratio in visceral adipose tissue was positively correlated with BMI.Citation52
Lymphocyte-deficient RAG2−/− mice have exacerbated weight gain and insulin resistance in response to a HFD, which can be partially corrected by the transfer of CD4+ T cells.Citation52 These results highlight the function of anti-inflammatory CD4+ T cells, and loss of these cells in HFD mice contributes to insulin resistance.Citation50,Citation53 Treatment of obese mice with immune therapies, such as immunosuppressive anti-CD3 monoclonal antibodies, was shown to result in the induction of anti-inflammatory responses in adipose tissue, including increased FoxP3+ Tregs and alternatively activated macrophages, increased IL-10, and reduced TNFα. This was associated with reduced insulin resistance and improved glucose tolerance in the anti-CD3 treated animals.Citation52 Thus, distinct CD4+ T cell subsets have opposing proinflammatory and anti-inflammatory functions in obesity. Th1 responses are central to the recruitment of M1 macrophages and the induction of insulin resistance in models of obesity-induced diabetes. These effects are counterbalanced by the function of Tregs and Th2 cells, which maintain an anti-inflammatory state and enhance insulin sensitivity.
Other lymphocyte subsets, including CD8+ T cells and B cells, also regulate glucose homeostasis in models of obesity. CD8+ T cells are recruited to adipose tissue after an HFD, and activation of CD8+ T cells was shown to be enhanced in the epididymal adipose tissue from animals on an HFD compared with a normal diet.Citation48 These activated CD8+ T cells support the recruitment and expansion of macrophages expressing high levels of TNFα. Similarly, B cells, particularly class-switched immunoglobulin (Ig) G+ B cells, are recruited to adipose tissue after an HFD, accompanied by a specific enhancement in the production of IgG2c isotype antibodies.Citation54 These antibodies were shown to be pathogenic as transfer from obese mice into B cell-deficient recipients caused increased numbers of M1 macrophages in visceral adipose tissue, increased TNFα production, increased fasting insulin levels, and exacerbated glucose intolerance.Citation52 The pathogenic roles of CD8+ T cells and B cells were further illustrated by improved glucose tolerance in CD8+ T cell- and B cell-deficient mice on an HFD.Citation48,Citation52 Thus several lymphocyte lineages contribute to the development of insulin resistance in response to obesity.
IFNγ promotes insulin resistance
IFNγ is the signature Th1 cytokine, and its expression in adipose tissue increases in obesity.Citation55 IFNγ receptor engagement and activation of the downstream JAK (Janus kinase) and STAT1 (signal transducer and activator of transcription 1) pathway causes decreased insulin-stimulated glucose uptake, inhibited differentiation, and reduced triglyceride content in adipocytes.Citation56 IFNγ stimulates the expression of macrophage and T cell chemoattractants and secretion of TNFα from cultured adipocytes and visceral adipose tissue preparations.Citation55 Moreover, IFNγ acts on adipose tissue macrophages to facilitate M2 to M1 phenotypic conversion in obesity.Citation57 In addition, IFNγ directly impairs insulin signaling by reducing insulin receptor IRS-1 and GLUT-4 expression and Akt-1 phosphorylation.Citation56 IFNγ-deficient mice had improved insulin sensitivity and glucose tolerance after an HFD, consistent with the role of IFNγ as a proinflammatory factor in adipose tissue.Citation55,Citation58 This favorable metabolic phenotype was accompanied by increased physical activity and lower food intakeCitation58 and was likely due, in part, to the reduced recruitment of M1 macrophages and other inflammatory cells to adipose tissue, in IFNγ−/− mice.Citation55,Citation57
T-bet is a central regulator of IFNγ production and immune function
T-bet is a Tbox family transcription factor that was first identified as a transcriptional inducer of IFNγ in CD4+ T cells.Citation59 Its expression is induced in naïve CD4+ T cells by T cell receptor (TCR) and cytokine stimulation, particularly STAT1-inducing cytokines, such as IFNγ. Expression of T-bet leads to the expression of a transcriptional program that stabilizes Th1 cell differentiation, including IFNγ, IL-12Rβ2 and Hlx-1,Citation60 which promote further T-bet expression in a positive feedback loop, and other molecules that are required for Th1 function, such as CXCR3 (C-X-C motif chemokine receptor type 3).Citation61 In addition T-bet directly antagonizes the development of the Th2 and Th17 lineages, by suppressing the function of the lineage defining transcription factors GATA3 (GATA binding protein 3) and RORγt (retinoid acid receptor-related orphan receptor gamma t) respectively.Citation62,Citation63 Overexpression of T-bet is sufficient to reprogram Th2 cells to express IFNγ, and T-bet-deficient CD4+ T cells fail to differentiate into Th1 cells under polarizing conditions.Citation59 Thus T-bet regulates the expression of a Th1 transcriptional program in CD4+ T cells and is necessary and sufficient to promote Th1 polarization.
T-bet is expressed in a variety of other immune cell types and regulates type 1 immunity more generally. This is achieved by promoting the differentiation of multiple immune cell lineages that contribute to cell-mediated immunity and by suppressing the function of counterregulatory lineages.Citation64,Citation65 T-bet is required for the development of optimal CD8+ T cell responses, where it transactivates the expression of IFNγ and cytotoxic molecules, such as perforin and granyzme B.Citation66 T-bet-deficient CD8+ T cells fail to upregulate activation markers, express lower levels of IFNγ and increased levels of Tc2 cytokines, including IL-4 and IL-10, and fail to develop into functional cytotoxic T lymphocytes (CTLs) in vitro and in vivo.Citation67
B cell antibody responses are regulated by cytokines, particularly differentiation and isotype class switching. The production of the IgG2a/c isotype is associated with activation of cell-mediated immunity and is induced in response to IFNγ stimulation of B cells. T-bet-deficient B cells have impaired production of class-switched IgG2b and IgG3, and almost complete absence of IgG2a downstream of IFNγ signaling.Citation68,Citation69
T-bet also controls adaptive immune responses by modulating the function of innate immune cells, including dendritic cells. T-bet is induced, in response to IFNγ, in dendritic cells via a STAT1-dependent mechanismCitation70 and is required for IFNγ production in response to IL-12 and IL-18 stimulation. Immunization of mice with T-bet-deficient dendritic cells resulted in reduced IFNγ in responding CD4+ T cells, most likely the result of reduced IFNγ production from the dendritic cells. Furthermore the adjuvant properties of CpG (unmethylated DNA of microbial origin) in the clearance of Listeria monocytogenes are dependent on the IFNγ-induced expression of T-bet in dendritic cells.Citation71 T-bet-deficient mice also show profound defects in the frequencies of natural killer (NK) and natural killer T (NKT) cell lineagesCitation72 and functional impairments in NK cells, in the context of metastatic cancer.Citation73
The importance of T-bet for the development of optimal immune responses has been demonstrated in multiple in vivo models of infection and autoimmunity. T-bet-deficient mice are more susceptible to a range of intracellular pathogens, including Mycobacterium tuberculosis, Leishmania major, Staphylococcus aureus, and Salmonella typhimurium. In keeping with generalized deficiencies in type 1 immunity, T-bet-deficient mice are less susceptible to autoimmune disorders, including inflammatory bowel disease, experimental autoimmune encephalomyelitis, collagen-induced arthritis, systemic lupus erythematosus, and type 1 diabetes.Citation64 Strikingly, T-bet-deficient mice also develop spontaneous allergic airway inflammation that is reminiscent of human asthma,Citation74 in keeping with their inability to suppress the expression of Th2 cytokines during Th1 polarization.Citation75 Thus T-bet is a central transcriptional regulator for type 1 immunity and is required for optimal function of multiple innate and adaptive immune cell lineages.
Role of T-bet in obesity and T2D
A recent study has demonstrated that T-bet plays a critical role in the development of insulin resistance in animal models of obesity (). T-bet knockout mice fed an HFD showed increased weight gain and adiposity; however, they were refractory to the induction of insulin resistance.Citation76 The authors argue that the uncoupling of weight gain and insulin resistance in the absence of T-bet was mediated by the adaptive immune system and impaired IFNγ production.
Figure 1 T-bet/IFNγ effects in obesity and insulin resistance.
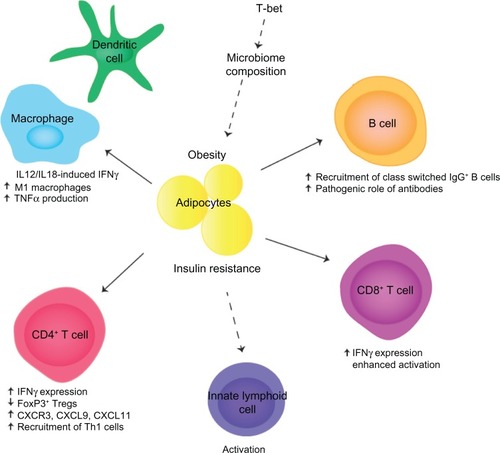
A detailed analysis revealed that T-bet-deficient mice had increased body weight and perigonadal and mesenteric fat deposits on both normal diet and HFD.Citation76 This was correlated with improved insulin sensitivity and glucose tolerance on both diets, suggesting that T-bet regulates insulin sensitivity in the basal state as well as in response to an HFD. Quantification of lymphocytes from adipose tissue demonstrated reduced numbers of CD4+ T cells, CD8+ T cells, and NK cells, and a reduced production of inflammatory cytokines, including IFNγ, TNFα, IL-1β, and IL-6. Generation of doubly deficient T-bet−/− × RAG2−/− mice implicated the adaptive immune system as these lymphocyte-deficient mice were no longer protected from the development of insulin resistance.Citation76 These data indicate that the T-bet-dependent effects on insulin sensitivity reside within the adaptive immune system.
The T-bet-deficient mice displayed increased percentages of FoxP3+ Tregs in perigonadal fat and reduced expression of CXCR3 and its ligands CXCL9 and CXCL11, which are required for the recruitment of Th1 T cells into inflammatory sites. It is likely that reduced levels of inflammation and insulin resistance are due to reduced recruitment of Th1 T cells into visceral fat, although the numbers of Th1 or Th2 cells were not directly quantified. The predicted downstream consequences of decreased Th1 recruitment are reduced activation of M1 inflammatory macrophages. This is consistent with the observed reduction in inflammatory cytokine production, although the authors did not quantify the frequencies of M1 and M2 macrophages.Citation76
The generation of doubly deficient T-bet−/− × IFNγ−/− mice indicates that IFNγ is also a key mediator of these processes. The phenotypes of IFNγ−/− mice and T-bet−/− × IFNγ−/− mice were found to be almost identical,Citation76 indicating that IFNγ deficiency is likely to be a primary cause of the observed protection from insulin resistance in T-bet-deficient mice. Additionally T-bet directly antagonizes the function of the Th2 lineage defining transcription factor GATA3.Citation62 Thus, increases in Th2 cells, in combination with reduced Th1 cells, may also contribute to the observed protection. T-bet also suppresses the production of IL-2 in activated T cells,Citation77 and thus, T-bet-deficient T cells may produce elevated levels of IL-2 in adipose tissue. Since IL-2 supports the survival of Tregs in vivo, this may sustain increased levels of Tregs in adipose tissue, maintain an anti-inflammatory environment, and prevent induction of insulin resistance.
While the transfer of wild type CD4+ T cells into T-bet−/− × RAG2−/− doubly deficient mice was found to partially restore insulin resistance, indicating an important role for CD4+ T cells, complete restoration was not observed.Citation76 These data indicate that other T-bet-expressing lymphocyte lineages may also contribute to the development of insulin resistance. Other potential candidates include CD8+ T cells and B cells, which are involved in the pathogenic processes leading to insulin resistance and express T-bet. T-bet is required for optimal activation, cytokine production, and CTL effector function in CD8+ T cellsCitation66,Citation67 and thus may make an important contribution to the development of insulin resistance in obesity. Similarly, T-bet is required for the optimal function of B cells, particularly class switching to IgG2a/c. As there is a specific enhancement of pathogenic IgG2a/c antibodies in response to an HFD and IgG2a/c production is controlled by T-bet,Citation68,Citation69 the T-bet deficiency in B cells may also contribute to the protection from insulin resistance in obese T-bet-deficient mice. Thus, it is likely that T-bet regulates insulin sensitivity by controlling the function of multiple lymphocyte lineages, including CD4+ and CD8+ T cells and B cells.
Recently, the role of the microbiome in the pathogenesis of obesity and T2D has been further elucidated. Obese and T2D patients have been found to have alterations in the composition of their microbiome,Citation78–Citation80 and gut microflora regulates the development of obesity in animal models.Citation81,Citation82 The microbiome composition changes in mouse models during the development of obesity, and fecal transplantation transfers an obese phenotype to wild type recipients.Citation83,Citation84 These data indicate that microflora play a causative role in the development of obesity. T-bet regulates mucosal T cell activation, and T-bet deficiency alters the composition of microflora, in mice, via T-bet actions in dendritic cells and innate lymphoid cells.Citation85–Citation87 Thus, it is tempting to speculate that T-bet deficiency may also alter the microbiome in the context of an HFD and obesity and that this may also contribute to the inflammatory and metabolic processes that regulate the T2D.
Whilst the data from mouse models demonstrating a role for the immune system and T-bet in the regulation of insulin sensitivity during obesity is compelling, much less convincing data exists from human patients. Small clinical studies in T2D patients have identified increased ratios of inflammatory Th1 to Th2 or Th1 to Tregs in adipose tissue and increased expression of major histocompatibility complex (MHC) molecules in adipocytes.Citation50,Citation52,Citation88 These data are suggestive of ongoing CD4+ T cell-mediated inflammation within adipose tissue in T2D patients.
Data from genome-wide association studies indicate that the majority of genetic linkages to T2D are associated with genes that regulate β-cell function.Citation89 Strikingly, there is little evidence of linkage to immune or inflammatory pathways, such as MHC or cytokine signaling, as is observed for classical autoimmune diseases, such as type 1 diabetes.Citation90 Peroxisome proliferator-activated receptor (PPAR)γ polymorphisms are associated with T2D,Citation89,Citation91 and PPARγ agonists improve insulin sensitivity in T2D patients.Citation92 Whilst the therapeutic effects of these agonists were assumed to be due to a direct action on adipocytes, the recent discovery that PPARγ is preferentially expressed by adipose tissue resident Tregs indicates that the primary mechanism of PPARγ agonists may instead be immune regulation.Citation53,Citation93 Similarly, other genes identified by genome-wide association studies in T2D may have hitherto unappreciated roles in the immune system.
There have been promising studies demonstrating that blockade of inflammatory cytokines and pathways can be used to reduce insulin resistance in T2D.Citation9,Citation30 The discovery that T-bet is required for the development of insulin resistance in preclinical models indicates that immune therapies based on antagonism of Th1 immunity or induction of regulatory T cells may also be of therapeutic utility in T2D. Suppression of type 1 immunity could potentially be achieved by direct pharmacological antagonism of T-bet function or alternatively, reduction of T-bet expression using gene silencing technologies. Alternatively, blockade of cytokines that induce T-bet expression, such as IFNγ and/or IL-12, may also provide a means to suppress T-bet and type 1 immunity in the context of T2D. Such therapeutic strategies must be weighed against the potential side effects of T-bet inhibition, such as reduced immunity to intracellular pathogens and tumors and/or increased susceptibility to airway inflammation, as suggested by the phenotype of T-bet-deficient mice.Citation74 Finally, expansion of Tregs via low-dose IL-2 treatment may also serve to dampen inflammation generally, which may prove an effective means of increasing insulin sensitivity in T2D patients.
It is now appreciated that inflammation and the immune system play a critical role in the development of T2D, in the context of obesity. Immune cells, including M1 macrophages and Th1 T cells, induce insulin resistance in response to environmental factors, such as dietary lipids, doing so via the action of immune mediators, including cytokines, such as IFNγ. With the demonstration that T-bet-deficient mice are protected from the development of insulin resistance in the context of obesity, it is now clear that T-bet is a central regulator of insulin resistance via its ability to promote IFNγ production, Th1 T cell function, and type 1 immunity. These effects are likely to be involved in the development of insulin resistance and T2D in human patients, and thus, a new generation of therapeutics that antagonize the function of T-bet, its upstream regulators, or downstream mediators may prove efficacious for the alleviation of insulin resistance and T2D, in obese patients.
Review criteria
The articles in this Review were identified by a literature search in PubMed for articles published from 2000 to 2013. The keywords used were “type 2 diabetes”, “obesity”, “insulin resistance”, “immune system”, “T-bet”, “Th1”, “IFNγ”. Additional relevant articles selected from the authors’ database were also included.
Disclosure
The authors report no conflicts of interest in this work.
References
- who.int [homepage on the Internet] Obesity and overweight World Health Organization 2013 [updated May 2014; cited January 20, 2014]. Available from: http://www.who.int/mediacentre/factsheets/fs311/en/ Accessed May 21, 2014
- Finkelstein EA Khavjou OA Thompson H Obesity and severe obesity forecasts through 2030 Am J Prev Med 2012 42 6 563 570 22608371
- diabetes.niddk.nih.gov [homepage on the Internet] Diabetes overview National Diabetes Information Clearinghouse 2008 [updated April 2, 2014; cited January 20, 2014]. Available from: http://diabetes.niddk.nih.gov/dm/pubs/overview/ Accessed May 21, 2014
- Schenk S Saberi M Olefsky JM Insulin sensitivity: modulation by nutrients and inflammation J Clin Invest 2008 118 9 2992 3002 18769626
- Weir GC Bonner-Weir S Five stages of evolving beta-cell dysfunction during progression to diabetes Diabetes 2004 53 Suppl 3 S16 S21 15561905
- Kalupahana NS Moustaid-Moussa N Claycombe KJ Immunity as a link between obesity and insulin resistance Mol Aspects Med 2012 33 1 26 34 22040698
- Brownlee M Biochemistry and molecular cell biology of diabetic complications Nature 2001 414 6865 813 820 11742414
- Lutz TA Woods SC Overview of animal models of obesity Curr Protoc Pharmacol 2012 Chapter 5:Unit5.61
- Shoelson SE Lee J Goldfine AB Inflammation and insulin resistance J Clin Invest 2006 116 7 1793 1801 16823477
- Samuel VT Shulman GI Mechanisms for insulin resistance: common threads and missing links Cell 2012 148 5 852 871 22385956
- Taniguchi CM Emanuelli B Kahn CR Critical nodes in signalling pathways: insights into insulin action Nat Rev Mol Cell Biol 2006 7 2 85 96 16493415
- Paz K Hemi R LeRoith D A molecular basis for insulin resistance. Elevated serine/threonine phosphorylation of IRS-1 and IRS-2 inhibits their binding to the juxtamembrane region of the insulin receptor and impairs their ability to undergo insulin-induced tyrosine phosphorylation J Biol Chem 1997 272 47 29911 29918 9368067
- Cai D Yuan M Frantz DF Local and systemic insulin resistance resulting from hepatic activation of IKK-beta and NF-kappaB Nat Med 2005 11 2 183 190 15685173
- Hirosumi J Tuncman G Chang L A central role for JNK in obesity and insulin resistance Nature 2002 420 6913 333 336 12447443
- Rui L Yuan M Frantz D Shoelson S White MF SOCS-1 and SOCS-3 block insulin signaling by ubiquitin-mediated degradation of IRS1 and IRS2 J Biol Chem 2002 277 44 42394 42398 12228220
- Hirashima Y Tsuruzoe K Kodama S Insulin down-regulates insulin receptor substrate-2 expression through the phosphatidylinositol 3-kinase/Akt pathway J Endocrinol 2003 179 2 253 266 14596677
- Siddle K Signalling by insulin and IGF receptors: supporting acts and new players J Mol Endocrinol 2011 47 1 R1 R10 21498522
- Gao T Furnari F Newton AC PHLPP: a phosphatase that directly dephosphorylates Akt, promotes apoptosis, and suppresses tumor growth Mol Cell 2005 18 1 13 24 15808505
- Du K Herzig S Kulkarni RN Montminy M TRB3: a tribbles homolog that inhibits Akt/PKB activation by insulin in liver Science 2003 300 5625 1574 1577 12791994
- Chavez JA Summers SA A ceramide-centric view of insulin resistance Cell Metab 2012 15 5 585 594 22560211
- Schmitz-Peiffer C Craig DL Biden TJ Ceramide generation is sufficient to account for the inhibition of the insulin-stimulated PKB pathway in C2C12 skeletal muscle cells pretreated with palmitate J Biol Chem 1999 274 34 24202 24210 10446195
- Thrush AB Harasim E Chabowski A Gulli R Stefanyk L Dyck DJ A single prior bout of exercise protects against palmitate-induced insulin resistance despite an increase in total ceramide content Am J Physiol Regul Integr Comp Physiol 2011 300 5 R1200 R1208 21325642
- Chavez JA Knotts TA Wang LP A role for ceramide, but not diacylglycerol, in the antagonism of insulin signal transduction by saturated fatty acids J Biol Chem 2003 278 12 10297 10303 12525490
- Senn JJ Toll-like receptor-2 is essential for the development of palmitate-induced insulin resistance in myotubes J Biol Chem 2006 281 37 26865 26875 16798732
- Holland WL Brozinick JT Wang LP Inhibition of ceramide synthesis ameliorates glucocorticoid-, saturated-fat-, and obesity-induced insulin resistance Cell Metab 2007 5 3 167 179 17339025
- Chavez JA Holland WL Bär J Sandhoff K Summers SA Acid ceramidase overexpression prevents the inhibitory effects of saturated fatty acids on insulin signaling J Biol Chem 2005 280 20 20148 20153 15774472
- Itani SI Ruderman NB Schmieder F Boden G Lipid-induced insulin resistance in human muscle is associated with changes in diacylglycerol, protein kinase C, and IkappaB-alpha Diabetes 2002 51 7 2005 2011 12086926
- Qu X Seale JP Donnelly R Tissue and isoform-selective activation of protein kinase C in insulin-resistant obese Zucker rats – effects of feeding J Endocrinol 1999 162 2 207 214 10425458
- Kim JK Fillmore JJ Sunshine MJ PKC-theta knockout mice are protected from fat-induced insulin resistance J Clin Invest 2004 114 6 823 827 15372106
- Donath MY Shoelson SE Type 2 diabetes as an inflammatory disease Nat Rev Immunol 2011 11 2 98 107 21233852
- Mathis D Immunological goings-on in visceral adipose tissue Cell Metab 2013 17 6 851 859 23747244
- Osborn O Olefsky JM The cellular and signaling networks linking the immune system and metabolism in disease Nat Med 2012 18 3 363 374 22395709
- Lumeng CN DelProposto JB Westcott DJ Saltiel AR Phenotypic switching of adipose tissue macrophages with obesity is generated by spatiotemporal differences in macrophage subtypes Diabetes 2008 57 12 3239 3246 18829989
- Lumeng CN Bodzin JL Saltiel AR Obesity induces a phenotypic switch in adipose tissue macrophage polarization J Clin Invest 2007 117 1 175 184 17200717
- Kitade H Sawamoto K Nagashimada M CCR5 plays a critical role in obesity-induced adipose tissue inflammation and insulin resistance by regulating both macrophage recruitment and M1/M2 status Diabetes 2012 61 7 1680 1690 22474027
- Prieur X Mok CY Velagapudi VR Differential lipid partitioning between adipocytes and tissue macrophages modulates macrophage lipotoxicity and M2/M1 polarization in obese mice Diabetes 2011 60 3 797 809 21266330
- Arkan MC Hevener AL Greten FR IKK-beta links inflammation to obesity-induced insulin resistance Nat Med 2005 11 2 191 198 15685170
- Senn JJ Klover PJ Nowak IA Mooney RA Interleukin-6 induces cellular insulin resistance in hepatocytes Diabetes 2002 51 12 3391 3399 12453891
- Bézaire V Mairal A Anesia R Lefort C Langin D Chronic TNFalpha and cAMP pre-treatment of human adipocytes alter HSL, ATGL and perilipin to regulate basal and stimulated lipolysis FEBS Lett 2009 583 18 3045 3049 19695247
- Ranjit S Boutet E Gandhi P Regulation of fat specific protein 27 by isoproterenol and TNF-α to control lipolysis in murine adipocytes J Lipid Res 2011 52 2 221 236 21097823
- Wen H Ting JP O’Neill LA A role for the NLRP3 inflammasome in metabolic diseases – did Warburg miss inflammation? Nat Immunol 2012 13 4 352 357 22430788
- Martinon F Burns K Tschopp J The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta Mol Cell 2002 10 2 417 426 12191486
- Wen H Gris D Lei Y Fatty acid-induced NLRP3-ASC inflammasome activation interferes with insulin signaling Nat Immunol 2011 12 5 408 415 21478880
- Vandanmagsar B Youm YH Ravussin A The NLRP3 inflammasome instigates obesity-induced inflammation and insulin resistance Nat Med 2011 17 2 179 188 21217695
- Lee HM Kim JJ Kim HJ Shong M Ku BJ Jo EK Upregulated NLRP3 inflammasome activation in patients with type 2 diabetes Diabetes 2013 62 1 194 204 23086037
- Stienstra R van Diepen JA Tack CJ Inflammasome is a central player in the induction of obesity and insulin resistance Proc Natl Acad Sci U S A 2011 108 37 15324 15329 21876127
- Zhu J Yamane H Paul WE Differentiation of effector CD4 T cell populations (*) Annu Rev Immunol 2010 28 445 489 20192806
- Nishimura S Manabe I Nagasaki M CD8+ effector T cells contribute to macrophage recruitment and adipose tissue inflammation in obesity Nat Med 2009 15 8 914 920 19633658
- Shu CJ Benoist C Mathis D The immune system’s involvement in obesity-driven type 2 diabetes Semin Immunol 2012 24 6 436 442 23333525
- Feuerer M Herrero L Cipolletta D Lean, but not obese, fat is enriched for a unique population of regulatory T cells that affect metabolic parameters Nat Med 2009 15 8 930 939 19633656
- Lumeng CN Deyoung SM Bodzin JL Saltiel AR Increased inflammatory properties of adipose tissue macrophages recruited during diet-induced obesity Diabetes 2007 56 1 16 23 17192460
- Winer S Chan Y Paltser G Normalization of obesity-associated insulin resistance through immunotherapy Nat Med 2009 15 8 921 929 19633657
- Cipolletta D Feuerer M Li A PPAR-γ is a major driver of the accumulation and phenotype of adipose tissue Treg cells Nature 2012 486 7404 549 553 22722857
- Winer DA Winer S Shen L B cells promote insulin resistance through modulation of T cells and production of pathogenic IgG antibodies Nat Med 2011 17 5 610 617 21499269
- Rocha VZ Folco EJ Sukhova G Interferon-gamma, a Th1 cytokine, regulates fat inflammation: a role for adaptive immunity in obesity Circ Res 2008 103 5 467 476 18658050
- McGillicuddy FC Chiquoine EH Hinkle CC Interferon gamma attenuates insulin signaling, lipid storage, and differentiation in human adipocytes via activation of the JAK/STAT pathway J Biol Chem 2009 284 46 31936 31944 19776010
- O’Rourke RW White AE Metcalf MD Systemic inflammation and insulin sensitivity in obese IFN-γ knockout mice Metabolism 2012 61 8 1152 1161 22386937
- Wong N Fam BC Cempako GR Deficiency in interferon-gamma results in reduced body weight and better glucose tolerance in mice Endocrinology 2011 152 10 3690 3699 21791564
- Szabo SJ Kim ST Costa GL Zhang X Fathman CG Glimcher LH A novel transcription factor, T-bet, directs Th1 lineage commitment Cell 2000 100 6 655 669 10761931
- Szabo SJ Sullivan BM Peng SL Glimcher LH Molecular mechanisms regulating Th1 immune responses Annu Rev Immunol 2003 21 713 758 12500979
- Lord GM Rao RM Choe H T-bet is required for optimal proinflammatory CD4+ T-cell trafficking Blood 2005 106 10 3432 3439 16014561
- Hwang ES Szabo SJ Schwartzberg PL Glimcher LH T helper cell fate specified by kinase-mediated interaction of T-bet with GATA-3 Science 2005 307 5708 430 433 15662016
- Lazarevic V Chen X Shim JH T-bet represses T(H)17 differentiation by preventing Runx1-mediated activation of the gene encoding RORγt Nat Immunol 2011 12 1 96 104 21151104
- Lazarevic V Glimcher LH T-bet in disease Nat Immunol 2011 12 7 597 606 21685955
- Lazarevic V Glimcher LH Lord GM T-bet: a bridge between innate and adaptive immunity Nat Rev Immunol 2013 13 11 777 789 24113868
- Glimcher LH Townsend MJ Sullivan BM Lord GM Recent developments in the transcriptional regulation of cytolytic effector cells Nat Rev Immunol 2004 4 11 900 911 15516969
- Sullivan BM Juedes A Szabo SJ von Herrath M Glimcher LH Antigen-driven effector CD8 T cell function regulated by T-bet Proc Natl Acad Sci U S A 2003 100 26 15818 15823 14673093
- Peng SL Szabo SJ Glimcher LH T-bet regulates IgG class switching and pathogenic autoantibody production Proc Natl Acad Sci U S A 2002 99 8 5545 5550 11960012
- Rubtsova K Rubtsov AV van Dyk LF Kappler JW Marrack P T-box transcription factor T-bet, a key player in a unique type of B-cell activation essential for effective viral clearance Proc Natl Acad Sci U S A 2013 110 34 E3216 E3224 23922396
- Lugo-Villarino G Maldonado-Lopez R Possemato R Penaranda C Glimcher LH T-bet is required for optimal production of IFN-gamma and antigen-specific T cell activation by dendritic cells Proc Natl Acad Sci U S A 2003 100 13 7749 7754 12802010
- Lugo-Villarino G Ito S Klinman DM Glimcher LH The adjuvant activity of CpG DNA requires T-bet expression in dendritic cells Proc Natl Acad Sci U S A 2005 102 37 13248 13253 16135562
- Townsend MJ Weinmann AS Matsuda JL T-bet regulates the terminal maturation and homeostasis of NK and Valpha14i NKT cells Immunity 2004 20 4 477 494 15084276
- Werneck MB Lugo-Villarino G Hwang ES Cantor H Glimcher LH T-bet plays a key role in NK-mediated control of melanoma metastatic disease J Immunol 2008 180 12 8004 8010 18523263
- Finotto S Neurath MF Glickman JN Development of spontaneous airway changes consistent with human asthma in mice lacking T-bet Science 2002 295 5553 336 338 11786643
- Szabo SJ Sullivan BM Stemmann C Satoskar AR Sleckman BP Glimcher LH Distinct effects of T-bet in TH1 lineage commitment and IFN-gamma production in CD4 and CD8 T cells Science 2002 295 5553 338 342 11786644
- Stolarczyk E Vong CT Perucha E Improved insulin sensitivity despite increased visceral adiposity in mice deficient for the immune cell transcription factor T-bet Cell Metab 2013 17 4 520 533 23562076
- Hwang ES Hong JH Glimcher LH IL-2 production in developing Th1 cells is regulated by heterodimerization of RelA and T-bet and requires T-bet serine residue 508 J Exp Med 2005 202 9 1289 1300 16275766
- Ley RE Turnbaugh PJ Klein S Gordon JI Microbial ecology: human gut microbes associated with obesity Nature 2006 444 7122 1022 1023 17183309
- Karlsson FH Tremaroli V Nookaew I Gut metagenome in European women with normal, impaired and diabetic glucose control Nature 2013 498 7452 99 103 23719380
- Zhang X Shen D Fang Z Human gut microbiota changes reveal the progression of glucose intolerance PLoS One 2013 8 8 e71108 24013136
- Bäckhed F Ding H Wang T The gut microbiota as an environmental factor that regulates fat storage Proc Natl Acad Sci U S A 2004 101 44 15718 15723 15505215
- Bäckhed F Manchester JK Semenkovich CF Gordon JI Mechanisms underlying the resistance to diet-induced obesity in germ-free mice Proc Natl Acad Sci U S A 2007 104 3 979 984 17210919
- Ley RE Bäckhed F Turnbaugh P Lozupone CA Knight RD Gordon JI Obesity alters gut microbial ecology Proc Natl Acad Sci U S A 2005 102 31 11070 11075 16033867
- Turnbaugh PJ Ley RE Mahowald MA Magrini V Mardis ER Gordon JI An obesity-associated gut microbiome with increased capacity for energy harvest Nature 2006 444 7122 1027 1031 17183312
- Neurath MF Weigmann B Finotto S The transcription factor T-bet regulates mucosal T cell activation in experimental colitis and Crohn’s disease J Exp Med 2002 195 9 1129 1143 11994418
- Garrett WS Lord GM Punit S Communicable ulcerative colitis induced by T-bet deficiency in the innate immune system Cell 2007 131 1 33 45 17923086
- Powell N Walker AW Stolarczyk E The transcription factor T-bet regulates intestinal inflammation mediated by interleukin-7 receptor+ innate lymphoid cells Immunity 2012 37 4 674 684 23063332
- Deng T Lyon CJ Minze LJ Class II major histocompatibility complex plays an essential role in obesity-induced adipose inflammation Cell Metab 2013 17 3 411 422 23473035
- Billings LK Florez JC The genetics of type 2 diabetes: what have we learned from GWAS? Ann N Y Acad Sci 2010 1212 59 77 21091714
- Barrett JC Clayton DG Concannon P Type 1 Diabetes Genetics Consortium Genome-wide association study and meta-analysis find that over 40 loci affect risk of type 1 diabetes Nat Genet 2009 41 6 703 707 19430480
- Sanghera DK Blackett PR Type 2 diabetes genetics: beyond GWAS J Diabetes Metab 2012 3 198 6948 23243555
- Olefsky JM Treatment of insulin resistance with peroxisome proliferator–activated receptor γ agonists J Clin Invest 2000 106 4 467 472 10953021
- Cipolletta D Kolodin D Benoist C Mathis D Tissular T(regs): a unique population of adipose-tissue-resident Foxp3+CD4+ T cells that impacts organismal metabolism Semin Immunol 2011 23 6 431 437 21724410
