Abstract
Anagliptin is a novel dipeptidyl peptidase-4 inhibitor that has been available in Japan since 2012. Because anagliptin is not generally used in countries other than Japan, there are only a small number of reports investigating the effects of anagliptin. In the present article, we review the safety and efficacy of anagliptin according to data obtained from preclinical trials and postmarketing studies. The usual dose of anagliptin is 200 mg daily, and increases in the dose up to 400 mg daily have been approved in cases in which the blood glucose–lowering effect is insufficient. In a Phase II trial, the reduction in the HbA1c values from baseline after 12 weeks monotherapy with 200 mg and 400 mg of daily anagliptin was 0.75%±0.50% and 0.82%±0.46%, respectively, and more than 40% of the subjects receiving anagliptin at a dose of 200 mg or 400 mg daily achieved an HbA1c level below 6.9%. Furthermore, the levels of HbA1c, fasting blood glucose, and postprandial blood glucose were significantly decreased at 52 weeks compared with the baseline values in a Phase III trial investigating the effects of anagliptin included in combination therapy with other oral antidiabetic agents. In a pooled analysis of Phase II and Phase II/III trials, the goal achievement rates for an HbA1c level below 7.0% at 12 weeks were 40.3%, 39.4%, 30.0%, and 34.8% in the patients treated with anagliptin combined with α-glucosidase inhibitors, thiazolidinediones, sulfonylureas, and biguanides, respectively. Meanwhile, the serum lipid concentrations significantly improved after the administration of anagliptin in a pooled analysis of Phase III trials, and no serious adverse effects have been reported in preclinical trials. Therefore, the use of anagliptin in patients with type 2 diabetes is considered to be safe and effective for both monotherapy and combination therapy.
Introduction
Treatment with dipeptidyl peptidase-4 (DPP-4) inhibitors, novel oral antidiabetic agents (OADs), results in improvements in the blood glucose levels in patients with type 2 diabetes mellitus following the stimulation of endogenous insulin secretion, inhibition of glucagon release, and reduction of gastric emptying via the enhanced production of incretin hormones (glucagon-like peptide-1 [GLP-1] and gastric inhibitory polypeptide). Sitagliptin, the first DPP-4 inhibitor, was approved for use by the US Food and Drug Administration in 2006 and has been available in Japan since 2009. Seven drugs belonging to this class are currently available for prescription in the clinical setting under the medical insurance law at the time of December 2014 in Japan ().
Table 1 Pharmacological characteristics and use in the clinical setting in Japan and efficacy of DPP-4 inhibitors in patients with type 2 diabetes based on the data for Phase III clinical trials
Recent trends in the rates of antidiabetic agent prescriptions in our facility are shown in . While the number of patients prescribed α-glucosidase inhibitors, sulfonylureas, thiazolidinediones, and glinides has been on the decline, the rate of prescription of DPP-4 inhibitors has increased markedly in recent years, reaching 50%–60%. In contrast, the rate of prescription of insulin and biguanides has remained stable at approximately 30%. This trend is similar to that observed for data obtained from databases in JapanCitation1 and the United States.Citation2 Therefore, it is not an exaggeration to state that the introduction of DPP-4 inhibitors has changed the treatment strategies for patients with type 2 diabetes.
Figure 1 Prescription rates of antidiabetic agents.
Abbreviations: DPP-4, dipeptidyl peptidase-4; GLP-1, glucagon-like peptide-1.
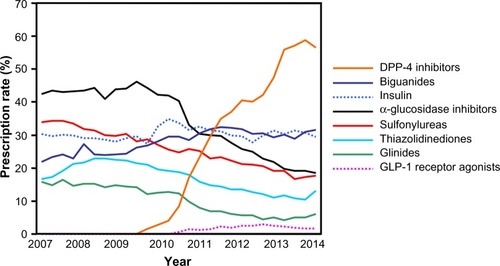
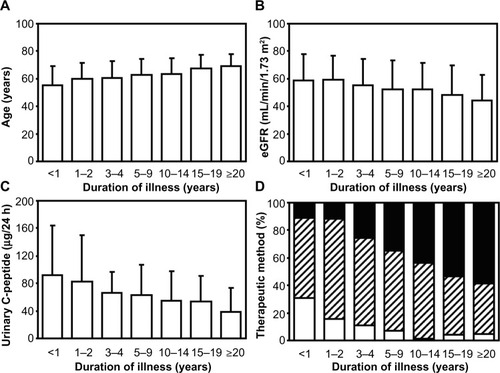
Metformin, an OAD in the family of biguanides, is recognized to be the first-line drug among antidiabetic agents.Citation3 Metformin administration is superior in the glucose-lowering effect for patients with type 2 diabetes, and regardless of the presence or absence of obesity,Citation4 the prescription rate has remained unchanged at approximately 30% (). This observation is considered to account for the relatively lower prescription rates among patients with type 2 diabetes who received consecutive treatments in our department, including many elderly subjects and individuals with renal impairmentCitation5,Citation6 who are contraindicated for biguanide administration.Citation7–Citation9 Patients with type 2 diabetes tend to demonstrate a decreased renal function, as indicated by the estimated glomerular filtration rate and reduced endogenous insulin secretion along with the long-term duration of diabetes and aging (). When selecting antidiabetic agents for patients with type 2 diabetes and renal dysfunction, it is necessary to use various OADs, including biguanides, sulfonylureas, and thiazolidinediones, carefully due to their side effects. On the other hand, it is a major advantage of DPP-4 inhibitors that agents in this class may be administered continuously, even in elderly subjects or patients with renal dysfunction, including those receiving maintenance dialysis.Citation10
Figure 2 Data for (A) patient age, (B) estimated glomerular filtration rate (eGFR), (C) urinary C-peptide, and (D) therapeutic methods for type 2 diabetes among the groups divided according to the duration of illness.
Whereas linagliptin and teneligliptin do not require dose adjustment, it is recommended that the dose of other DPP-4 inhibitors is reduced depending on the degree of renal dysfunction (). The lowering effect on the HbA1c level obtained in Phase III clinical trialsCitation11–Citation17 does not differ significantly based on the drug. Anagliptin (Suiny®), a novel DPP-4 inhibitor, was developed by Sanwa Kagaku Kenkyusho Co, Ltd (Nagoya, Japan) and Kowa Pharmaceutical Co, Ltd (Tokyo, Japan) and has been available in Japan since November 2012. Because anagliptin was released relatively recently and this drug is not generally used in countries other than Japan (at the time of December 2014), there are only a small number of reports investigating the effects of anagliptin. However, it has been reported that anagliptin demonstrates serum lipid–lowering and antiatherogenic effects, which have not yet been observed in other DPP-4 inhibitors. We herein describe the effects of anagliptin primarily in Japanese patients with type 2 diabetes.
Efficacy of anagliptin in patients with type 2 diabetes
Efficacy of monotherapy after 12 weeks of treatment
The recommended dose of anagliptin is 200 mg daily (100 mg, BID) in Japan.Citation18 Because treatment with anagliptin exhibits dose-dependent improvements in the HbA1c and blood glucose levels according to the results of a Phase II trial including a dose-ranging study (50–400 mg daily),Citation19 increases in the dose up to 400 mg daily (200 mg, BID) have been approved.Citation18 In one study, the reduction in the HbA1c values from baseline after 12 weeks monotherapy with 200 mg (n=69) and 400 mg (n=68) of daily anagliptin was 0.75%±0.50% and 0.82%±0.46%, respectively, and more than 40% of the subjects receiving anagliptin at a dose of 200 mg or 400 mg daily achieved an HbA1c level below 6.9%.Citation19 In another Phase II/III trial, the administration of monotherapy for 12 weeks demonstrated a reduction of 0.66%±0.50% and 0.75%±0.55% in the HbA1c levels with 200 mg (n=63) and 400 mg (n=58) of daily anagliptin, respectively.Citation20 The differences in the HbA1c levels versus the placebo group among the subjects receiving 200 mg and 400 mg of anagliptin daily were −0.72% and −0.82%, respectively.Citation20
According to a pooled analysis of the data obtained from Phase II and Phase II/III trials, the goal achievement rate of an HbA1c level less than 7.0% was 51.1% in the group treated with anagliptin at a dose of 200 mg (100 mg, BID) at 12 weeks, which was significantly superior to that attained with voglibose, an α-glucosidase inhibitor.Citation21
Long-term efficacy of monotherapy
KakuCitation17 reported that the use of monotherapy with anagliptin at a daily dose of 200 mg (with dose increases up to 400 mg permitted if the glucose-lowering effect was insufficient) showed continuous improvements in the HbA1c levels over 52 weeks in 150 patients with type 2 diabetes whose blood glucose levels were poorly controlled (6.9%≤ HbA1c<10.5%) even after receiving nonpharmacological therapy. The changes in the HbA1c, fasting blood glucose, and postprandial 2-h blood glucose levels were −0.63%±0.85% (baseline value: 8.22%±1.06%), −12.5±32.2 mg/dL (baseline value: 166.1±38.4 mg/dL), and −31.0±47.8 mg/dL (baseline value: 255.1±60.3 mg/dL), respectively. Furthermore, improvements were noted in the levels of 1,5-AG, glycoalbumin, serum insulin, proinsulin, homeostatic model assessment-β, total cholesterol, and low-density lipoprotein (LDL)–cholesterol.
Combination therapy
The changes in the HbA1c levels observed in a Phase III trialCitation22 of anagliptin as an add-on therapy to α-glucosidase inhibitors (acarbose 20.2%, voglibose 43.6%, and miglitol 36.2%), biguanides (metformin), sulfonylureas (glimepiride 73.5%, gliclazide 10.3%, and glibenclamide 16.2%), and thiazolidinediones (pioglitazone) are shown in . In this trial, treatment with anagliptin at a dose of 200 mg daily (100 mg, BID) or a placebo was administered for an initial 12 weeks in patients with type 2 diabetes whose HbA1c levels were between 6.9% and 10.4%, even after treatment with other OADs. Anagliptin was subsequently administered at a dose of 200 mg daily for 40 weeks in both groups. Increases in the dose of anagliptin up to 400 mg daily (200 mg, BID) were allowed if the HbA1c level was >6.9% at 28 weeks. Consequently, the improvements in parameters indicating glycemic control at 12 weeks were significantly greater in the anagliptin/anagliptin group than in the placebo/anagliptin group, regardless of whether OADs were administered before the trial. Furthermore, the levels of HbA1c, fasting blood glucose, and postprandial blood glucose were significantly decreased at 52 weeks compared with the baseline values.
Table 2 Changes in the levels of HbA1c, fasting blood glucose, and postprandial blood glucose in the groups treated with combination therapy with anagliptin and other oral antidiabetic agents in a Phase III clinical trial
As it has been reported that metformin increases the plasma-active GLP-1 in humansCitation23 via the inhibitory effects of DPP-4,Citation24 combination therapy with anagliptin and metformin is considered to be suitable for the treatment of type 2 diabetes based on the enhanced endogenous GLP-1 activity. Furthermore, combination therapy with anagliptin and α-glucosidase inhibitors also has advantages, as it has been reported that GLP-1 secretion is prolonged by acarbose.Citation25 On the other hand, the maximum drug concentration (C max) and area under the blood concentration–time curve (AUC) from time zero to 24 h after dosing (AUC0–24h) for anagliptin are reduced with the concomitant administration with miglitol,Citation26 whereas both the C max and AUC0–24h values for anagliptin are elevated in subjects under treatment with the combination therapy consisting of metformin and anagliptin.Citation27 The degree of improvement in glycemic control achieved with combination therapy using anagliptin and sulfonylureas appears to be lower than that attained with α-glucosidase inhibitors, biguanides, or thiazolidinediones. Nevertheless, there is concern regarding the potential for hypoglycemia in patients with type 2 diabetes treated with DPP-4 inhibitors and sulfonyulureas.Citation28 This issue should be investigated in detail in a real clinical setting.
According to the results of a pooled analysis of the data obtained from Phase II and Phase II/III trials,Citation21 the goal achievement rates of an HbA1c level less than 7.0% at 12 weeks in patients receiving combination therapy with anagliptin and other OADs are 40.3%, 39.4%, 30.0%, and 34.8% for α-glucosidase inhibitors, thiazolidinediones, sulfonylureas, and biguanides, respectively.
Daily blood glucose profile
Uchino and KakuCitation29 investigated the daily blood glucose profiles using continuous glucose monitoring in 20 patients with type 2 diabetes under treatment with 200 mg of anagliptin (100 mg, BID) or 50 mg of sitagliptin (QD) in an open-label, two-period crossover study. The area under the curve for the blood glucose level (4,421.9±1,187.0 mg h/dL), average 24-h blood glucose level (184.10±49.39 mg/dL), M-value (29.4±32.7 mg/dL), and mean amplitude of glycemic excursion (104.97±32.71 mg/dL) during the anagliptin administration period were significantly lower than the corresponding values obtained in the control period (4,906.0±924.9 mg h/dL, 204.25±38.53 mg/dL, 39.54±26.27 mg/dL, and 126.50±19.03 mg/dL, respectively), whereas these parameters were not significantly different from the corresponding values noted during the sitagliptin administration period (4,469.2±1,125.7 mg h/dL, 186.06±46.83 mg/dL, 30.35±12.42 mg/dL, and 110.35±30.95 mg/dL, respectively).
Lipid-lowering effect
Managing the serum lipid concentrations is important in patients with type 2 diabetes as dyslipidemia is an important risk factor for diabetic macroangiopathy in Japanese subjects.Citation30 According to a pooled analysis of data obtained from Phase III trials, the serum LDL-cholesterol, triglyceride, total cholesterol and non–high-density lipoprotein (HDL)-cholesterol levels significantly improved after the administration of anagliptin at a dose of 200 mg (100 mg, BID) or 400 mg (200 mg, BID) ().Citation31 Meanwhile, the serum concentrations of HDL-cholesterol increased significantly in the subjects with an HDL-cholesterol level less than 40 mg/dL and decreased in the entire patient population. These observations suggest that there is an additional mechanism independent of the lipid-lowering effect of these drugs that occurs secondary to improvements in the blood glucose level, as the correlation coefficient between the change in the HbA1c level and the LDL-cholesterol (r=0.112, P=0.003) or triglyceride (r=0.075, P=0.07) levels was rather small. Therefore, treatment with anagliptin is expected to prevent atherosclerotic diseases in addition to diabetic microangiopathy by improving glycemic control in patients with type 2 diabetes.
Table 3 Changes in the serum lipid concentrations in a Phase III trial
Antiatherogenic effect
DPP-4 inhibitors are thought to reduce the risk of cardiovascular events, particularly myocardial infarction, in patients with type 2 diabetes.Citation32 Recently, Ervinna et alCitation33 reported that the administration of anagliptin attenuates atherosclerosis secondary to suppression of the proliferation of vascular smooth muscles and monocyte inflammatory reactions in apo-E–deficient mice, an animal model of progressive atherosclerosis.
Kakuda et alCitation34 showed that oxidative stress markers, such as urinary 8-OHdG and serum high-sensitivity-CRP, are ameliorated and the plasma level of high–molecular weight adiponectin, which has an antiatherogenic effect, increases after the initiation of therapy with 200 mg of anagliptin daily, in addition to observed improvements in blood glucose control and the serum LDL-cholesterol, triglyceride, non–HDL-cholesterol and remnant-like particle-cholesterol levels. Imai et alCitation35 also reported that treatment with anagliptin or miglitol reduces the oral sucrose load–inducible gene expression of inflammatory cytokines, such as interleukin-1β and interleukin-18, and tumor necrosis factor-α, in the peripheral leukocytes of Otsuka Long-Evans Tokushima fatty rats. However, it remains unclear which of these anti-inflammatory effects is specifically caused by anagliptin and which occurs secondary to improvements in postprandial hyperglycemia, which is commonly seen in patients with glucose impairment.
Mimura et alCitation36 demonstrated that treatment with anagliptin facilitates the restoration of mucosal damage in mice exhibiting experimental colitis induced by dextran sulfate sodium. The authors suggested the possibility of applying anagliptin administration as a novel therapeutic approach for the treatment of inflammatory bowel disease. Further investigations are required to confirm this hypothesis in both basic and clinical research.
Safety and patient acceptability
Safety in preclinical trials
The frequency of adverse effects (AEs) is not significantly different between subjects administered anagliptin and those administered a placebo according to the results of a Phase II trial performed over 12 weeks.Citation19 Most AEs were mild and no events resulting from increases in the dose of anagliptin were observed.
AEs were noted in 23.5% (19 of 81 patients) and 15.7% (11 of 70 patients) of subjects receiving preprandial and postprandial administration, respectively, during treatment with long-term monotherapy consisting of 200–400 mg of daily anagliptin (100–200 mg, BID) for 52 weeks.Citation17 In that study, the complications observed in more than 2% of the subjects included constipation (4.9%) and gastritis (4.9%), whereas only one patient (0.7%) showed mild hypoglycemia. Although statistically significant changes were detected in some laboratory data and vital signs compared with the baseline values, they were not considered to be meaningful due to the small variation within the normal ranges. Furthermore, no serious AEs were reported in another Phase III trial investigating the effects of combination therapy with anagliptin and other OADs.Citation22 Side effects observed in more than 2% of the subjects in that trial are shown in . Because the frequency of hypoglycemia was relatively high in the sulfonylurea group, it is important to pay attention to the potential for hypoglycemia in patients receiving combination therapy with anagliptin and sulfonylureas, as described earlier. Other than hypoglycemia, no side effects of concern have been documented. Therefore, treatment with anagliptin is considered to be superior in terms of safety and tolerability for both monotherapy and combination therapy.
Table 4 Side effects observed in more than 2% of subjects treated with anagliptin and other oral antidiabetic agents in a Phase III trial in Japan
Safety in the real clinical setting
One case of hypokalemia and muscle weakness was reported as serious side effects of anagliptin during postmarketing surveillance by Sanwa Kagaku Kenkyusho Co, Ltd.Citation37 In addition, a small number of nonserious events, including gastrointestinal symptoms (n=9) and somnolence (n=3), were observed among approximately 20,000 diabetic patients treated with anagliptin for 6 months after the drug’s release. The case of a 60-year-old Japanese male with type 2 diabetes complicated by the onset of angioedema 4 days after the initiation of anagliptin was reported by Hamasaki and Yanai.Citation38 Although an increased risk of angioedema has been described in patients using both DPP-4 inhibitors and angiotensin-converting enzyme inhibitors,Citation39 no medications other than anagliptin were given in this case. Because DPP-4 influences the rate of degradation of bradykinin, the authors suggested that DPP-4 inhibitors may increase the half-life of bradykinin.
Currently, the biggest clinical concern with DPP-4 inhibitors is the increased incidence of new heart failure with these medications.Citation40,Citation41 Although alogliptin, another DPP-4 inhibitor, did not demonstrate an increased risk for cardiovascular disease and heart failure,Citation42,Citation43 there have not yet been any studies on the association between anagliptin treatment and heart failure.
Safety in patients with renal impairment
The ratio of AUC from time zero to infinity after dosing (AUC0–∞) in subjects with a normal kidney function following the single administration of anagliptin at a dose of 400 mg is 1.65 ng h/mL, 1.76 ng h/mL, 2.70 ng h/mL, and 3.22 ng h/mL for those with mild renal impairment (n=6, 60 mL/min/1.73 m2 ≤ creatinine clearance <90 mL/min/1.73 m2), moderate renal impairment (n=6, 30 mL/min/1.73 m2 ≤ creatinine clearance <60 mL/min/1.73 m2), severe renal impairment (n=6, 15 mL/min/1.73 m2≤ creatinine clearance <30 mL/min/1.73 m2), and end-stage kidney disease under maintenance hemodialysis (n=6), respectively.Citation18 Because AUC values are greater in the progressive stages of renal dysfunction, the dose of anagliptin is usually reduced to 100 mg once daily in patients with severe renal impairment (). There are currently no studies investigating safety or patient acceptability in subjects with type 2 diabetes and renal impairment. However, treatment with anagliptin may have a glucose-lowering effect at half-doses in patients with renal dysfunction, similarly to linagliptin,Citation44 which does not require dose reduction.
Conclusion
Anagliptin is thought to be a potent DPP-4 inhibitor demonstrating safety and tolerability in patients with type 2 diabetes. Treatment with anagliptin improves glycemic control for both monotherapy and combination therapy, and it is possible that anagliptin prevents atherosclerotic disease as well as diabetic microangiopathy. However, there are too few reports of the effects of anagliptin. There is currently no data on the use of this drug for more than 1 year or the application of combination therapy with anagliptin and insulin. Additionally, the antiatherogenic effect of anagliptin was not supported by any comprehensive clinical data. Further investigation is therefore necessary to clarify the safety, efficacy, and patient acceptability of anagliptin in subjects with type 2 diabetes.
Acknowledgments
The authors thank Tomoko Koyanagi in the secretarial section of Edogawa Hospital for her valuable help with data collection.
Disclosure
The authors report no conflicts of interest in this work.
References
- KohroTYamazakiTSatoHTrends in antidiabetic prescription patterns in Japan from 2005 to 2011Int Heart J201354293 9723676369
- TurnerLWNarteyDStaffordRSSinghSAlexanderGCAmbulatory treatment of type 2 diabetes in the US, 1997–2012Diabetes Care2014374985 99224198301
- American Diabetes AssociationStandards of medical care in diabetes – 2014Diabetes Care201437Suppl 1S14 S8024357209
- ItoHIshidaHTakeuchiYLong-term effect of metformin on blood glucose control in non-obese patients with type 2 diabetes mellitusNutr Metab2010201783
- ItoHTakeuchiYIshidaHHigh frequencies of diabetic micro-and macroangiopathies in patients with type 2 diabetes mellitus with decreased estimated glomerular filtration rate and normoalbuminuriaNephrol Dial Transplant20102541161 116719892756
- ItoHOshikiriKMifuneMThe usefulness of the revised classification for chronic kidney disease by the KDIGO for determining the frequency of diabetic micro- and macroangiopathies in Japanese patients with type 2 diabetes mellitusJ Diabetes Complications2012264286 29022621778
- National Kidney FoundationKDOQI clinical practice guideline for diabetes and CKD: 2012 updateAm J Kidney Dis2012605850 88623067652
- TuttleKRBakrisGLBilousRWDiabetic kidney disease: a report from an ADA consensus conferenceDiabetes Care201437102864 288325249672
- Japan Diabetes SocietyTreatment Guide for Diabetes 2012–2013TokyoBunkodo2012
- ItoHMifuneMMatsuyamaEVildagliptin is effective for glycemic control in diabetic patients undergoing either hemodialysis or peritoneal dialysisDiabetes Ther201342321 32923801219
- AschnerPKipnesMSLuncefordJKSitagliptin Study 021 GroupEffect of the dipeptidyl peptidase-4 inhibitor sitagliptin as monotherapy on glycemic control in patients with type 2 diabetesDiabetes Care200629122632 263717130196
- Pi-SunyerFXSchweizerAMillsDDejagerSEfficacy and tolerability of vildagliptin monotherapy in drug-naïve patients with type 2 diabetesDiabetes Res Clin Pract2007761132 13817223217
- DeFronzoRAFleckPRWilsonCAMekkiQAlogliptin Study 010 GroupEfficacy and safety of the dipeptidyl peptidase-4 inhibitor alogliptin in patients with type 2 diabetes and inadequate glycemic control: a randomized, double-blind, placebo-controlled studyDiabetes Care200831122315 231718809631
- Del PratoSBarnettAHHuismanHNeubacherDWoerleHJDugiKAEffect of linagliptin monotherapy on glycaemic control and markers of β-cell function in patients with inadequately controlled type 2 diabetes: a randomized controlled trialDiabetes Obes Metab2011133258 26721205122
- KadowakiTKondoKEfficacy, safety and dose-response relationship of teneligliptin, a dipeptidyl peptidase-4 inhibitor, in Japanese patients with type 2 diabetes mellitusDiabetes Obes Metab2013159810 81823464664
- RosenstockJAguilar-SalinasCKleinECV181-011 Study InvestigatorsEffect of saxagliptin monotherapy in treatment-naïve patients with type 2 diabetesCurr Med Res Opin200925102401 241119650754
- KakuKEfficacy and safety of long-term monotherapy with anagliptin in Japanese patients with type 2 diabetes – a multi-centre. Randomised, open-label, parallel-group study (administered before meals vs after meals)Jpn Pharmacol Ther20124010733 744 Japanese [Abstract in English]
- Sanwa Kagaku Kenkyusho Co, Ltd 2015 Available from: http://med.skk-net.com/resources/show?colum=refference_files&field=doc_mng&num=1&thread_id=d2a5fafb-796e-4409-9986-2d4ba2f5b2adAccessed January 25, 2015 Japanese
- KakuKDose-ranging study of anagliptin in Japanese patients with type 2 diabetes – a multi-centre, randomized, placebo-controlled, double-blind, parallel-group studyJpn Pharmacol Ther20124011944 973 Japanese [Abstract in English]
- KakuKEfficacy and safety of anagliptin in Japanese patients with type 2 diabetes – a multi-centre, randomized, placebo-controlled, double-blind, parallel-group studyJpn Pharmacol Ther20124011985 995 Japanese [Abstract in English]
- KakuKEffects of anagliptin on the new goal achievement rates for glycemic control in Japanese patients with type 2 diabetesJpn Pharmacol Ther20124110965 974 Japanese [Abstract in English]
- KakuKEfficacy and safety of anagliptin add-on therapy in Japanese patients with type 2 diabetesJpn Pharmacol Ther2012409745 770 Japanese [Abstract in English]
- MannucciEOgnibeneACremascoFEffect of metformin on glucagon-like peptide 1 (GLP-1) and leptin levels in obese nondiabetic subjectsDiabetes Care2001243489 49411289473
- LindsayJRDuffyNAMcKillopAMInhibition of dipeptidyl peptidase IV activity by oral metformin in type 2 diabetesDiabet Med2005225654 65715842525
- QualmannCNauckMAHolstJJOrskovCCreutzfeldtWGlucagon-like peptide 1 (7–36 amide) secretion in response to luminal sucrose from the upper and lower gut. A study using alpha-glucosidase inhibition (acarbose)Scand J Gastroenterol1995309892 8968578189
- KimKKakuKDrug interaction between anagliptin, a novel dipeptidyl peptidase-4 inhibitor, and miglitol, an α-glucosidase inhibitor, in Japanese patients with type 2 diabetesJpn Pharmacol Ther20124010871 881 Japanese [Abstract in English]
- KimKKakuKDrug interaction between anagliptin, a novel α-glucosidase inhibitor, and metformin in Japanese patients with type 2 diabetesJpn Pharmacol Ther20124010883 894 Japanese [Abstract in English]
- HermansenKKipnesMLuoESitagliptin Study 035 GroupEfficacy and safety of the dipeptidyl peptidase-4 inhibitor, sitagliptin, in patients with type 2 diabetes mellitus inadequately controlled on glimepiride alone or on glimepiride and metforminDiabetes Obes Metab200795733 74517593236
- UchinoHKakuKA novel dipeptidyl peptidase-4 inhibitor, anagliptin, improved the daily blood glucose profileJpn Pharmacol Ther20124010859 869 Japanese [Abstract in English]
- SoneHTanakaSTanakaSJapan Diabetes Complications Study GroupSerum level of triglycerides is a potent risk factor comparable to LDL cholesterol for coronary heart disease in Japanese patients with type 2 diabetes: subanalysis of the Japan Diabetes Complications Study (JDCS)J Clin Endocrinol Metab201196113448 345621865372
- KakuKEffects of anagliptin on serum lipids in Japanese patients with type 2 diabetes – a pooled analysis of long-term therapy with anagliptinJpn Pharmacol Ther2012409771 784 Japanese [Abstract in English]
- MonamiMAhrénBDicembriniIMannucciEDipeptidyl peptidase-4 inhibitors and cardiovascular risk: a meta-analysis of randomized clinical trialsDiabetes Obes Metab2013152112 12022925682
- ErvinnaNMitaTYasunariEAnagliptin, a DPP-4 inhibitor, suppresses proliferation of vascular smooth muscles and monocyte inflammatory reaction and attenuates atherosclerosis in male apo E-deficient miceEndocrinology201315431260 127023337530
- KakudaHKobayashiJKakudaMYamakawaJTakekoshiNThe effect of anagliptin treatment on glucose metabolism and lipid metabolism, and oxidative stress in fasting and postprandial states using a test meal in Japanese men with type 2 diabetesEndocrine2014
- ImaiCHarazakiTInoueSMochizukiKGodaTInhibition of postprandial hyperglycemia by either an insulin-dependent or - independent drug reduces the expression of genes related to inflammation in peripheral leukocytes of OLETF ratsBiosci Biotechnol Biochem201377112305 230824200798
- MimuraSAndoTIshiguroKDipeptidyl peptidase-4 inhibitor anagliptin facilitates restoration of dextran sulfate sodium-induced colitisScand J Gastroenterol201348101152 115924047394
- Sanwa Kagaku Kenkyusho Co, Ltd 2015 Available from: http://med.skk-net.com/information/download/578/file1Accessed January 25, 2015 Japanese
- HamasakiHYanaiHThe development of angioedema in a patient with type 2 diabetes due to a novel dipeptidyl peptidase-IV inhibitor, anagliptinInt J Cardiol20131683e10623972359
- BrownNJByiersSCarrDMaldonadoMWarnerBADipeptidyl peptidase-IV inhibitor use associated with increased risk of ACE inhibitor-associated angioedemaHypertension2009543516 52319581505
- SciricaBMBhattDLBraunwaldESAVOR-TIMI 53 Steering Committee and InvestigatorsSaxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitusN Engl J Med2013369141317 132623992601
- WuSHopperISkibaMKrumHDipeptidyl peptidase-4 inhibitors and cardiovascular outcomes: meta-analysis of randomized clinical trials with 55,141 participantsCardiovasc Ther2014324147 15824750644
- WhiteWBCannonCPHellerSREXAMINE InvestigatorsAlogliptin after acute coronary syndrome in patients with type 2 diabetesN Engl J Med2013369141327 133523992602
- WhiteWBPratleyRFleckPCardiovascular safety of the dipeptidyl peptidase-4 inhibitor alogliptin in type 2 diabetes mellitusDiabetes Obes Metab2013157668 67323489301
- ItoHAbeMAntokuSComparison of the antidiabetic effects of linagliptin among groups with a normal renal function and a mild or severe renal impairment – retrospective observation study of Japanese patients with type 2 diabetes mellitusExpert Opin Pharmacother2015163289 29625529857
- AndoSTsugamiESuzukiSRecent trend in the prescription rates of antidiabetic agents in our facilityJournal of Japan Diabetes Society201356Suppl 1S164
