Abstract
Advanced glycation end products (AGEs) constitute a complex group of compounds produced endogenously during the aging process and under conditions of hyperglycemia and oxidative stress. AGEs also have an emerging exogenous origin. Cigarette smoke and diet are the two main exogenous sources of AGEs (glycotoxins). Modern Western diets are rich in AGEs which have been implicated in the pathogenesis of several metabolic and degenerative disorders. Accumulating evidence underlies the beneficial effect of the dietary restriction of AGEs not only in animal studies but also in patients with diabetic complications and metabolic diseases. This article reviews the evidence linking dietary glycotoxins to several disorders from diabetic complications and renal failure to liver dysfunction, female reproduction, eye and cognitive disorders as well as cancer. Furthermore, strategies for AGE reduction are discussed with a focus on dietary modification.
Introduction
Advanced glycation end products (AGEs) represent a complex group of compounds derived from the nonenzymatic glycation of proteins, lipids, and nucleic acids formed endogenously but also from exogenous supplies (also referred as glycotoxins).Citation1 Tobacco smokeCitation2 and certain foods rich in glycotoxins that are formed by specific cooking methodsCitation3 represent the two major exogenous sources of glycotoxins. The pool of body AGEs reflects the balance between endogenous production and exogenous intake, with the effective mechanisms of AGE detoxification systems as well as their excretion from the kidneys.Citation4,Citation5
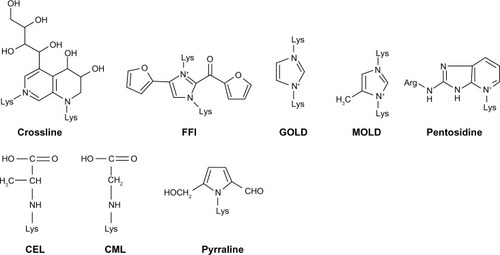
AGEs are heterogeneous molecules () that share some common characteristics including the formation of covalent cross-links between proteins, the effect of turning some foodstuffs a yellow-brown color (the ‘browning’ effect), and the ability to generate fluorescence. They have diverse chemical structures but have a common characteristic lysine residue in their molecule. Based on their properties, AGEs can be categorized as: (a) fluorescent and cross-linking AGEs, including pentosidine, crossline, 2-(2-furoyl)-4(5)-(2-furanyl)-1H-imidazole, glyoxal-lysine dimer, and methylglyoxal-lysine dimer (MOLD); and (b) nonfluorescent and non-cross-linking agents such as N-carboxymethyl lysine (CML), N3-(carboxyethyl)lysine, and pyrraline.Citation6,Citation7
Figure 1 Chemical structure of some advanced glycation end products (AGEs): crossline, 2-(2-furoyl)-4(5)-(2-furanyl)-1H-imidazole (FFI), glyoxal-lysine dimer (GOLD), methylglyoxal-lysine dimer (MOLD), pentosidine, N3-(carboxyethyl)lysine (CEL), N-carboxymethyl lysine (CML), and pyrraline.
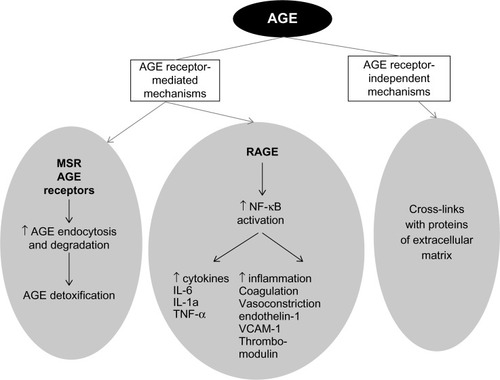
AGEs’ actions are mainly mediated through two classes of AGE receptors with different biological effects (). The receptors responsible for their clearance are macrophage scavenger receptor-1 (MSR-1) and the AGE-specific receptors AGE-R1, AGE-R2, and AGE-R3. These receptors are responsible for maintaining AGE homeostasis through regulation of their degradation and removal. The multi-ligand receptor for advanced glycation end products (RAGE) mediates pro-atherogenic, inflammatory, and immune responses via activation of nuclear factor-kappa B (NF-κB), resulting in the increased expression/synthesis of proinflammatory cytokines such as interleukin (IL)-6, IL-1a, and tumor necrosis factor-alpha.Citation6 Furthermore, activation of NF-κB leads to increased reactive oxygen species (ROS) productionCitation1,Citation8,Citation9 through molecules mediating inflammation, vasoconstriction, and coagulation such as vascular cell adhesion molecule-1, endothelin-1, and thrombomodulin ().Citation6 Apart from the AGE receptor-mediated mechanisms, AGEs can form cross-links with basic components in the basement membrane of the extracellular matrix, modifying its structural characteristics ().Citation10
Figure 2 Schematic representation of advanced glycation end product (AGE) mechanisms of action.
Abbreviations: IL-1a, interleukin-1a; IL-6, interleukin-6, MSR, macrophage scavenger receptor; NF-κB, nuclear factor-kappa B, RAGE, receptor for advanced glycation end products; TNF-α, Tumor necrosis factor-alpha; VCAM-1, vascular cell adhesion molecule-1.
When defense mechanisms are damaged, as in patients with diabetes mellitus, or when inflammatory mechanisms are upregulated, as in tissues in which AGEs are accumulated,Citation11 the deleterious impact of AGEs prevails. This involves pro-oxidant and inflammatory effects that increase intracellular oxidative stress and ROS production; therefore, chronic exposure to AGEs may be implicated in a broad spectrum of diseases through inflammation and propagation of tissue damage.
This review focuses on the impact of dietary glycotoxins in the pathogenesis of several diseases. We also review therapeutic management, focusing mainly on food restriction, which appears to have beneficial effects on the adverse effects of these harmful molecules.
Dietary glycotoxins
Approximately 10% of consumed AGEs are absorbed though the intestine. One-third of the absorbed AGEs are excreted within 48 hours in the urine, whereas the other two-thirds of those absorbed remain in the tissues.Citation12 The exogenous formation of AGEs takes place spontaneously in food under specific cooking methods – predominantly, cooking at high temperature of short duration. Nutrient composition (high lipid and protein content), presence or absence of moisture, and pH are also parameters that affect AGE content. For instance, in dry-heat cooking (grilling, frying, roasting, baking, and barbecuing) over short periods of time, more AGEs are formed than in high-moisture cooking (boiling, steaming, poaching, stewing, and slow cooking) over longer periods of time. presents the AGE contents of selected food items from a large database provided by Goldberg et al.Citation3
Table 1 Advanced glycation end product (AGE) content in commonly consumed foodsTable Footnotea
It seems obvious that the modification of cooking methods is a sufficient way of reducing a meal’s AGE content. For instance, cooking foods at low temperatures for long time periods in the presence of higher water content – boiling, steaming, poaching, stewing – minimizes the AGE content, compared with broiling or frying. This is of great significance given that dietary AGEs have been directly associated with serum and tissue AGE levels in healthy individuals as well as in patients suffering from diabetes and chronic kidney disease.Citation12–Citation14 This was even more profound in a study of 26 patients with renal failure undergoing peritoneal dialysis who followed a diet with high versus low AGE content for a short period of time (4 weeks).Citation15 A significant reduction in serum AGE levels was observed in the group following a low AGE-content diet, whereas in the group on a high AGE diet, a significant increase in serum AGE levels was reported.
Nowadays, conventional diets are considered to provide 25 to 75 mg of AGEs, estimated mainly with pyrraline and N-carboxymethyl lysine.Citation16 Vlassara et alCitation13 and Uribarri et alCitation15 reported a significant reduction in serum AGE levels of diabetics as well as renal failure patients due to diets low in dietary glycotoxins. In a human study analyzing the metabolism of Amadori products, only approximately 5% was recovered from the urine and feces.Citation17
These data underlie the contribution of nutritional AGEs to total body burden and, furthermore, imply a possible mechanism of reducing circulating AGE levels through dietary interventions.
AGE metabolism and excretion
The kidney is the organ predominantly responsible for AGE removal. Here the filtered AGEs are partially degraded in the tubule system, while those remaining are excreted in the urine.Citation18 Renal impairment leads to decreased clearance of AGEs and their accumulation in tissues,Citation19,Citation20 while their plasma levels progressively rise. AGEs are shown to affect almost all renal components,Citation21,Citation22 with the proximal tubule responsible for AGE catabolism.Citation23 Data from experimental animal and human studies have confirmed the crucial role of the kidneys in the metabolic regulation and clearance of glycotoxins.Citation12,Citation15,Citation24
It has been shown that AGEs in the kidney originate either from the circulation, are generated from intrarenal proteins, or are due to impaired AGE-removal mechanisms. The role of the kidney in trapping AGEs was shown after chronic intravenous infusion of AGE (CML)-modified rat albumin in normal rats. The AGE content of the kidneys increased by 50% in the treated animals compared with in the control ones.Citation25 Interestingly, this process resulted in widening of the basement membrane, increased glomerular tuft volume, mesangial extracellular matrix expansion, glomerulosclerosis, and albuminuria.Citation25
In addition to renal tissues, the liver is also a site of metabolism and degradation of AGEs, predominantly through a scavenger receptor mechanism expressed in hepatic sinusoidal cells and to lesser extent Kupffer cells.Citation26,Citation27 After the intravenous administration of AGE-modified bovine serum albumin in a rat experimental model, Smedsrød et al demonstrated a rapid and almost exclusive accumulation in liver endothelial cells (60% uptake) and to lesser extent Kupffer cells (25% uptake), while parenchymal cells accounted only for approximately 10%–15% of hepatic deposition.Citation26 This endocytic uptake is mediated by a scavenger receptorCitation27 whose function has been shown to be negatively modulated by AGEs.Citation28 Besides its pivotal role in AGE degradation, the liver has the potency to generate low levels of inflammatory molecules, causing oxidative stress and, in the case of AGEs, is presumed to contribute to inflammatory processes through the activation of RAGE after interaction with its ligands (AGEs) in hepatocytes and hepatic stellate cells.Citation29
Implications for health
Nowadays, AGEs are implicated in the pathogenesis of several disorders from diabetic complications and renal failure to female reproduction, liver disorders, neurodegenerative, and eye diseases as well as cancer. The pathological role of food-derived AGEs in various types of disorders is analyzed following.
AGEs and diabetes
It is now well-documented that AGEs are related to the progression of diabetic complications including diabetic nephropathy, diabetic peripheral neuropathy, diabetic cardiomyopathy and peripheral arterial disease, diabetic ocular disease, and atherosclerotic disease.Citation30 Dietary glycotoxins have been shown experimentally to contribute to the development of type 2 (T2D) diabetes,Citation5 and animal studies with rodents following a high AGE diet for a long period of time document the induction of insulin resistance and T2D.Citation31,Citation32
In diabetes patients, restriction of dietary-glycotoxin intake has been shown to decrease inflammatory markers,Citation13 improve insulin resistance, and lower plasma insulin levels.Citation33 Furthermore, it has been associated with lower leptin and increased adiponectin levels, as well as altered AGE-receptor expression (decreased RAGE and increased AGE-R1 expression),Citation5 while impaired vascular and endothelial function has been reported following a high AGE meal.Citation34,Citation35
AGEs and kidney disease
Chronic kidney disease and AGEs participate in what has been termed a “vicious cycle”, as this condition is associated with increased oxidative and carbonyl stress – that is, the production of reactive carbonyl compounds and AGEs. AGEs and other carbonyl-modified proteins, in turn, contribute to the further decline of renal function.Citation36 This vicious cycle is implied, as in patients with end-stage renal disease, serum AGE levels are elevated,Citation37,Citation38 and, in turn, AGEs contribute to further decrease of the glomerular filtration rate. Several studies have demonstrated the mechanisms via which AGEs can damage the kidneys, either through AGE–RAGE interaction, deposition of AGEs, or by in situ glycation.Citation39,Citation40
Kidney disease has been linked to the decreased excretion of an oral load of AGEs, resulting in an inverse correlation between renal function and serum levels of AGEs.Citation41 AGE-induced renal injury is due to renal cellular-signaling alterations, mainly through the interaction of AGEs with their receptor, RAGE. Protein kinase C, mitogen-activated protein kinase, and NF-κB are some of the signaling molecules involved in the tissue injury related to AGE pathways.Citation39 In addition, tubular expression of connective tissue growth factor via the tissue growth factor-beta-independent RAGE-ERK/p38-mitogen-activated protein kinase-Smad3 cross-talk pathway is another way through which AGEs are involved in renal injury.Citation40 Moreover, AGEs directly affect the structural integrity of the renal tissue through the cross-linking of matrix proteins (collagen).Citation42,Citation43
In podocytes, the AGE–RAGE interaction induces the upregulation of monocyte chemoattractant protein-1 expression, while MCP-1 gene transcription has been shown to be regulated by NF-κB and SP1 protein, and this interaction results in the generation of intracellular ROS.Citation44
Dietary AGEs have also been shown to contribute to kidney disease, as demonstrated in studies of patients with renal failure with dietary restriction of glycotoxin intake. In patients with renal failure, dietary glycotoxins were positively associated with high AGE serum levels.Citation14 Thus, dietary restriction of AGEs may contribute to the reduction of AGE-related renal injury and associated mortality, through several mechanisms including the reduction of oxidative stress and inflammation.
Moreover, a study which included a female rat model that exerted the metabolic and hormonal characteristics of women with hyperandrogenemia, suggested that dietary glycotoxins, in combination with increased androgen exposure, exert a more profound negative impact on the kidney.Citation45 In addition, the study demonstrated that dietary glycotoxins and androgen excess induce an inflammatory environment for the kidney, which could further aggravate its structure and function.Citation45
AGEs and liver disease
Several human and experimental studies have shown an association between AGEs with several hepatic disorders from simple steatosis, and biochemical aberrations to hepatic cirrhosis.Citation29,Citation46–Citation48 In patients with nonalcoholic steatohepatitis, AGEs were histochemically documented in the liver, and serum levels of AGEs were related to the severity of liver dysfunction.Citation48
Experimental studies of AGE administration in rodents confirm their increased liver tissue deposition parallel to an increase in the hepatic expression of RAGE and vascular endothelial growth factor (VEGF), which is implicated in hepatic fibrosis.Citation49 In another study of male mice, the long-term administration of a low AGE-content diet was related to enhanced expression of AGE-R1 and decreased RAGE expression in the liver tissue parallel to a beneficial effect on oxidative stress and extended lifespan, compared to rodents following an isocaloric diet of standard AGE content.Citation50 Furthermore, in a mouse model of high-versus-regular AGE-content diet, signs of liver inflammation were observed in the high AGE content diet subgroup.Citation51
AGEs and polycystic ovary syndrome (PCOS)
The elevated levels of serum AGEs observed in PCOS women associated positively with insulin-resistance indices, testosterone, and anti-Müllerian hormone levels when compared with healthy age- and body mass index-matched women.Citation52,Citation53 This is a unique characteristic in women with PCOS as opposed to those who experience only some of the clinical features of the syndrome.Citation54 These observations, combined with the immunohistochemical evidence of increased AGE deposition in human polycystic ovaries,Citation55 imply a possible direct impact of AGEs in the ovarian physiology of women with PCOS.
Dietary AGEs are also considered to have an impact on PCOS pathophysiology, since evidence has been provided from animal studies with AGE-enriched diets. In those experiments, animals fed with enriched AGE diets had an increased immunochemically documented accumulation of AGEs in ovarian tissue and elevated serum levels and this was associated with an impaired hormonal and metabolic profile expressed as elevated insulin and androgen levels, compared to animals fed a low AGE-content diet.Citation56,Citation57 The potential involvement of dietary AGEs to PCOS pathophysiology is implied from a recent study of women with the syndrome who followed specific, consecutive, nutritional interventions for short period of time based on diet AGE content (high and subsequently low AGE content).Citation58 As shown by researchers, a disturbed metabolic and hormonal profile expressed as elevated insulin and testosterone and increased markers of insulin resistance and oxidative stress was observed in women with PCOS following the diet high in AGE content compared to preceding levels during a regular hypocaloric diet.Citation58 On the other hand, the low AGE diet seemed to have a beneficial effect on oxidative stress.Citation58 Along these lines, in vivo studies of PCOS-like animal models, PCOS-like models, fed with high AGE diets showed that androgens and dietary AGEs have a synergistic effect on the intra-ovarian detoxifying system operated by glyoxalase-1, resulting in the increased deposition of AGEs in ovarian tissues.Citation57 In addition, a high AGE diet compromises the expression of the scavenger receptor in peripheral blood monocytes and therefore interferes with the clearance mechanisms and subsequently contributes to loading the tissues with AGEs.Citation59
A recent study of human granulosa KGN cells cultured with variable concentrations of human glycated albumin or insulin revealed a novel interference between AGEs with insulin intracellular signaling and glucose transport in the ovarian microenvironment.Citation60 The AGEs showed a direct inhibitory effect on insulin signaling in the human granulosa cells, resulting in the prevention of the membrane translocation of GLUT4. Therefore, intra-ovarian AGE accumulation, from endogenous or exogenous sources, may contribute to the pathophysiology of states characterized by anovulation and insulin resistance such as PCOS.Citation60
These in vitro observations may prove to be even more important on clinical grounds, since AGEs have been shown to be present in the umbilical cord blood. In pregnant women with PCOS and with gestational diabetes, the mother’s AGE serum levels have been shown to correlate with the infant’s levels as well as with other oxidative molecules, findings which suggest that the mother’s oxidative stress affects the infant’s environment.Citation61
AGEs and neurological disorders
AGEs have been related to the pathogenesis of several neurological degenerative diseases, including Alzheimer’s disease, Parkinson’s disease, and amyotrophic lateral sclerosis as well as peripheral diabetic polyneuropathies.Citation62 The pathophysiological mechanism involved is believed to be related to pathological amyloid glycation and the induction of oxidative stress leading to potential neurotoxic effects.Citation62
A probable involvement of oral AGEs in the initiation and progression of cognitive aberrations is proposed by animal data showing systemic and brain changes in old mice following chronic exposure to a neurotoxic methyl-glyoxal diet.Citation63 Moreover, these effects are probably mediated through an inhibitory effect on Sirtuin 1 (SIRT1) gene expression that has been shown to be related to cognitive decline and Alzheimer’s disease. In humans, a reduced intake of food-derived AGEs has been considered an effective strategy to prevent neurodegenerative diseases given that in subjects over 60 years old an association has been shown between high serum AGEs and cognitive decline.Citation63
AGEs and eye diseases
AGEs are also involved in vision loss, and have been linked with macula degeneration, cataract formation, diabetic retinopathy, and glaucoma.Citation64
The pathogenetic mechanisms involved include AGE accumulation in ocular tissues where they mediate the aberrant cross-linking of extracellular matrix proteins and the disruption of endothelial junctional complexes that affects cell permeability and mediates angiogenesis. Moreover, AGEs severely affect cellular metabolism by disrupting adenosine triphosphate production, enhancing oxidative stress, and modulating the gene expression of anti-angiogenic and anti-inflammatory genes.Citation64 A recent study demonstrated the direct impact of a high AGE diet on the ocular tissues of normal rats of different ages.Citation65 In that study, it was demonstrated that increased peripheral AGE levels were positively correlated with significant increased tissue immunoreactivity of AGEs and RAGE in retinal and uveal tissues associated with increased retinal VEGF-A expression.Citation65 Furthermore the upregulation of RAGE and VEGF-A expression was observed in the ocular tissue of both baby and adult animals fed with a high AGE content diet, suggesting that dietary AGEs can have negative effects on eye tissues regardless of age.Citation65
AGEs and cancer
The potential involvement of AGEs in cancer risk, development, and disease progression is largely undetermined and remains to be elucidated. In vitro studies of breast, prostate, and lung cancer cell lines imply a possible contribution of AGEs to cancer proliferation, migration, invasion, and survival.Citation66–Citation68 Human studies are yet inconclusive but significant observations have been reported. Detection of AGEs has been reported in various human tumorsCitation69 and, interestingly, a higher accumulation of AGEs has been observed in malignant tissues (such as prostate cancer) compared to benign ones.Citation70 Some epidemiologic studies suggest an inverse association between soluble AGEs and the risk of pancreaticCitation71 and colorectal cancer,Citation72 while other studies have failed to report similar effects.Citation73 A suggested molecular link between glycotoxins and cancer biology involves the activation of an inflammatory cycle of persistent oxidative stress that favors cancer development.Citation74
Strategies for AGE reduction
As previously described, accumulating evidence from animal studies as well as clinical studies in diabetic patients and healthy individuals converge on the observation that the dietary intake of foods with a high AGE content predisposes to inflammation and deteriorating biological effects, whereas AGE restriction seems to have an opposite, beneficial impact. In line with this, several researches have been conducted over the last decades to investigate possible strategies to influence the AGE pathway (). However, despite the wide range of promising pharmacologic agents for AGE reduction, like blockage of AGE formation, cross-link breakers, RAGE blockade, and others, human studies are scanty and are in an experimental state, including clinical trial studies, therefore their clinical application has not been established. Most experimental data – both in vitro and in vivo – as well as clinical studies, converge to suggest the significance of dietary AGE restriction. Therefore, the current strategy to avoid the body accumulation of AGEs is mainly to decrease their intake (via diet restriction and tobacco avoidance or cessation).
Table 2 Interventions targeting the advanced glycation end product (AGE) pathway
Dietary restriction of AGEs
Dietary restriction of the consumption of AGEs from exogenous sources through modifications in eating habits may reflect an initial, effective therapeutic intervention in the management of several AGE-related disorders as indicated, so far, by many animal and human studies of dietary AGE restriction.Citation15,Citation75–Citation77
In experimental studies, a reduction in the intake of exogenous, food-derived glycotoxins has been linked to a beneficial effect on insulin sensitivity, abdominal obesity, and body weight regulation, and, furthermore, has been shown to have a protective effect against T2D.Citation76,Citation77 Moreover, in diabetic rodents, dietary AGE restriction was shown to improve diabetic complications (macroangiopathy vascular disease, diabetic nephropathy, recover healing ability)Citation31,Citation50,Citation75–Citation77 and has been related to prolonged life expectancy – probably through reduced oxidant stress and organ dysfunction.Citation50
AGE restriction in individuals with diabetes and kidney failure for a short period of time decreases inflammation and has a beneficial effect on the endothelium and insulin sensitivity.Citation13,Citation15,Citation34 In healthy individuals, lower AGE intake has been related to lower serum AGE levelsCitation78 and improvement in basal oxidative status, inflammation,Citation79 and insulin-resistance markers.Citation80 It should be highlighted that, as far as we are aware, AGE restriction studies in humans so far have been performed for relatively short periods of time (approximately 4 weeks),Citation78–Citation80 and clinical studies exploring long-term effects are missing.
In conclusion, accumulating evidence suggests the modification of dietary habits as an effective strategy for the alleviation of many AGE-related disorders; however, the evidence, though highly suggestive, is still insufficient to prompt precautions.Citation81 The restriction of glycotoxin consumption and the recognition of foods with high AGE contents require constant communication between the scientific community, authorities, and public in order to inform consumers about the potential hazardous effects of AGE consumption and reinforce the importance of reducing dietary AGEs.
General practices for lowering dietary AGE intake () include the consumption of foods with low AGE contents (such as fruits and vegetables, seafood, whole grains, bread, low-fat milk) while avoiding or reducing the consumption of high AGE content food (including sugary items such as candy, cookies, and beverages; highly processed foods, such as packaged meats, cheese, and snack-type foods; and fats, including butter, full-fat cheeses, and fried foods), adaptation of specific cooking methods (such as cooking foods at low temperatures with high moisture – boiling, steaming, poaching, stewing – for a relatively long period of time) and adoption of healthy lifestyle habits (such as exercise, maintaining normal body weight, and cessation of tobacco consumption).
Table 3 Recommendations for lowering advanced glycation end product (AGE) consumption
Blockage of AGEformation
Blockage of AGE formation has been considered as a potential anti-AGE therapeutic strategy targeting the initial steps of AGE formation. Aminoguanidine was first studied as a compound with potential AGE-inhibiting propertiesCitation82 and has been shown to be effective in preventing diabetic complications in various animal studies, such as those on retinopathyCitation83 and nephropathy.Citation84 Further, it has been shown to ameliorate neuropathy even in normal rodents.Citation85
N-(2-Acetamidoethyl) hydrozinecarboximidamide hydrochloride (ALT-946) is another compound that has been studied with reference to its renal protective effects,Citation86,Citation87 while 3-benzyloxycarbonylmethyl-4-methyl-thiazol-3-ium bromide (C36) was shown to improve markers of cardiovascular system function in a group of streptozotocin-induced diabetic rats.Citation88 Hydroxymethylglutaryl-coenzyme A reductase inhibitors (statins) and pyridoxamine, the natural form of vitamin B6, are among the substances shown to be effective at inhibiting AGEs in diverse ways.Citation89–Citation91
Putative cross-link breakers
Breaking collagen cross-links has been thought a possible therapeutic approach against AGE-provoked unfavorable biological outcomes. The first study on AGE breakers with a rational design was published in 1996 by Vasan et al.Citation92 The strategy was based on the design of compounds able to covalently react and then cleave the alpha-dicarbonyl derivatives which were proposed as intermediates of AGE formation. The prototype was identified as N-phenacylthiazolium and was shown to cleave cross-links.Citation92 A consecutive derivative of N-phenacylthiazolium (alagebrium [3-phenyacyl-4,5-dimethyl-thiazolium chloride] or ALT-711) has been examined in view of its ability to attenuate diabetes-associated myocardial structural abnormalities in rats.Citation93 In a series of studies with diabetic animal models, alagebrium was demonstrated to have a beneficial effect on diabetic complications – renal disease, diabetic cardiomyopathy, and atherosclerosis.Citation94 AGE breakers have been shown to improve arterial complianceCitation95 and several parameters of cardiac function in patients with stable diastolic heart failure.Citation96
RAGE blockade
Blockage of the ligand–RAGE axis is another promising therapeutic approach based on the fact that a significant part of oxidative stress in cells is provoked by exposure to AGEs via RAGE. Blockage of this axis can be achieved either by inactivating RAGE using high-molecular-weight substrate analogs, low-molecular-weight inhibitors, or anti-RAGE antibodies, or by inhibiting the signal transduction pathway that follows the ligand–RAGE interaction.Citation97 Substances believed to have a suppressive effect on RAGE expression are several categories of antihypertensive drugs (calcium-channel blockers, angiotensin converting enzyme inhibitors, angiotensin II receptor blockers), antidiabetic agents (thiazolidinediones), and cholesterol-lowering drugs (statins).Citation98 Theoretically, the group of agents that could potentially suppress the RAGE axis consists of the soluble form of RAGE (soluble receptor for advanced glycation end products [sRAGE]); antihypertensive drugs – telmisartan, ramipril, olmesartan, candesartan, losartan, valsartan, nifedipine; pigment epithelium-derived factor; statins, inhibitors of cholesterol synthesis; and nitrogen-containing bisphosphonates used as anti-osteoporotic drugs.Citation99 The beneficial effects of sRAGE on the development and progression of diabetic atherosclerosis have been documented in animal models of diabetic apolipoprotein E-null mice.Citation100,Citation101 Furthermore, a favorable effect of sRAGE administration on diabetic retinopathy has also been described.Citation102,Citation103
Other agents
Poly(ADP-ribose) polymerase (PARP) inhibitors have been shown to ameliorate early peripheral diabetic neuropathy as well as endothelial and myocardial function in experimental studies of laboratory animals.Citation104,Citation105
Another possible therapeutic approach against the AGE pathway is through agents inhibiting glycotoxin absorption. An example of such is provided by AST-120, an oral adsorbent, which has been shown to exert a beneficial effect on chronic kidney disease by eliminating uremic toxins.Citation106 Furthermore, its ability to lower serum AGE levels by binding to CML has been reported in patients with chronic renal failure.Citation106
Orlistat, an inhibitor of intestinal lipases, has been shown to have a beneficial effect on serum AGE levels and metabolic profile in obese women with PCOS and in obese control women.Citation107 Finally, inhibitors of advanced glycation acting as potent endoplasmic reticulum stress modulators have been reported to have beneficial effects on metabolic dysfunction.Citation108
Conclusion
Dietary AGEs represent a class of proinflammatory and oxidative stress-promoting agents implicated not only in normal aging and diabetes, but also in a wide spectrum of diseases. A common pathogenetic mechanism in many AGE-related disorders is the induction or deterioration of oxidative stress that is mainly mediated through the activation of the AGE–RAGE axis and/or through the suppression of internal defensive systems. Furthermore, chronic exposure to dietary AGEs is speculated to act as an additional, aggravating factor for tissue injury and deleterious biological effects. In the modern society, AGE consumption – as component of modern Westernized fast and high-temperature cooked diets – is excessive. Therefore, AGE restriction is now recognized as a necessary intervention. Prospective studies are needed to clarify whether dietary AGEs significantly contribute to oxidant stress. Furthermore, it is of great significance to determine whether the modulation of dietary AGE content has beneficial effects on certain clinical conditions. Restriction of food-derived AGEs through diet and tobacco consumption reflects an efficient approach to avoid body accumulation of AGEs. It remains to be proven whether therapeutic agents targeting AGE formation, AGE cross-linking, and AGEs–RAGE interaction will be approved for use in clinical practice.
Disclosure
The authors declare no conflicts of interest in this work.
References
- VlassaraHPalaceMRDiabetes and advanced glycation endproductsJ Intern Med2002251287 10111905595
- CeramiCFoundsHNichollITobacco smoke is a source of toxic reactive glycation productsProc Natl Acad Sci U S A1997942513915 139209391127
- GoldbergTCaiWPeppaMAdvanced glycoxidation end products in commonly consumed foodsJ Am Diet Assoc200410481287 129115281050
- VlassaraHUribarriJCaiWStrikerGAdvanced glycation end product homeostasis: exogenous oxidants and innate defensesAnn N Y Acad Sci2008112646 5218448795
- VlassaraHStrikerGEAdvanced glycation endproducts in diabetes and diabetic complicationsEndocrinol Metab Clin North Am2013424697 71924286947
- AhmedNAdvanced glycation endproducts – role in pathology of diabetic complicationsDiabetes Res Clin Pract20056713 2115620429
- WuCHHuangSMLinJAYenGCInhibition of advanced glycation endproduct formation by foodstuffsFood Funct201125224 23421779560
- BierhausASternDMNawrothPPRAGE in inflammation: a new therapeutic target?Curr Opin Investig Drugs2006711985 991
- YonekuraHYamamotoYSakuraiSWatanabeTYamamotoHRoles of the receptor for advanced glycation endproducts in diabetes-induced vascular injuryJ Pharmacol Sci2005973305 31115750291
- GoldinABeckmanJASchmidtAMCreagerMAAdvanced glycation end products: sparking the development of diabetic vascular injuryCirculation20061146597 60516894049
- SternDYanSDYanSFSchmidtAMReceptor for advanced glycation endproducts: a multiligand receptor magnifying cell stress in diverse pathologic settingsAdv Drug Deliv Rev200254121615 162512453678
- KoschinskyTHeCJMitsuhashiTOrally absorbed reactive glycation products (glycotoxins): an environmental risk factor in diabetic nephropathyProc Natl Acad Sci U S A199794126474 64799177242
- VlassaraHCaiWCrandallJInflammatory mediators are induced by dietary glycotoxins, a major risk factor for diabetic angiopathyProc Natl Acad Sci U S A2002992415596 1560112429856
- UribarriJPeppaMCaiWDietary glycotoxins correlate with circulating advanced glycation end product levels in renal failure patientsAm J Kidney Dis2003423532 53812955681
- UribarriJPeppaMCaiWRestriction of dietary glycotoxins reduces excessive advanced glycation end products in renal failure patientsJ Am Soc Nephrol2003143728 73112595509
- HenleTAGEs in foods: do they play a role in uremia?Kidney Int Suppl200384S145 S14712694332
- ErbersdoblerHFFaistVMetabolic transit of Amadori productsNahrung2001453177 18111455784
- GugliucciABendayanMRenal fate of circulating advanced glycated end products (AGE): evidence for reabsorption and catabolism of AGE-peptides by renal proximal tubular cellsDiabetologia1996392149 1608635666
- MonnierVMSellDRNagarajRHMaillard reaction-mediated molecular damage to extracellular matrix and other tissue proteins in diabetes, aging, and uremiaDiabetes199241Suppl 236 411526333
- NiwaTKatsuzakiTMomoiTModification of beta 2 m with advanced glycation end products as observed in dialysis-related amyloidosis by 3-DG accumulating in uremic serumKidney Int1996493861 8678648931
- BucalaRMakitaZVegaGModification of low density lipoprotein by advanced glycation end products contributes to the dyslipidemia of diabetes and renal insufficiencyProc Natl Acad Sci U S A199491209441 94457937786
- JensenLJØstergaardJFlyvbjergAAGE-RAGE and AGE Cross-link interaction: important players in the pathogenesis of diabetic kidney diseaseHorm Metab Res200537Suppl 126 3415918107
- SaitoATakedaTSatoKSignificance of proximal tubular metabolism of advanced glycation end products in kidney diseasesAnn N Y Acad Sci20051043637 64316037287
- VlassaraHAdvanced glycation in health and disease: role of the modern environmentAnn N Y Acad Sci20051043452 46016037266
- VlassaraHStrikerLJTeichbergSFuhHLiYMSteffesMAdvanced glycation end products induce glomerular sclerosis and albuminuria in normal ratsProc Natl Acad Sci U S A1994912411704 117087972128
- SmedsrødBMelkkoJArakiNSanoHHoriuchiSAdvanced glycation end products are eliminated by scavenger-receptor mediated endocytosis in hepatic sinusoidal Kupffer and endothelial cellsBiochem J19973222567 5739065778
- MatsumotoKSanoHNagaiREndocytic uptake of advanced glycation end products by mouse liver sinusoidal endothelial cells is mediated by a scavenger receptor distinct from the macrophage scavenger receptor class ABiochem J20003521233 24011062078
- HansenBSvistounovDOlsenRNagaiRHoriuchiSSmedsrødBAdvanced glycation end products impair the scavenger function of rat hepatic sinusoidal endothelial cellsDiabetologia200245101379 138812378378
- SantosJCValentimIBde AraújoORAtaide TdaRGoulartMODevelopment of nonalcoholic hepatopathy: contributions of oxidative stress and advanced glycation end productsInt J Mol Sci2013141019846 1986624084729
- GohSYCooperMEClinical review: The role of advanced glycation end products in progression and complications of diabetesJ Clin Endocrinol Metab20089341143 115218182449
- SanduOSongKCaiWZhengFUribarriJVlassaraHInsulin resistance and type 2 diabetes in high-fat-fed mice are linked to high glycotoxin intakeDiabetes20055482314 231916046296
- CaiWRamdasMZhuLChenXStrikerGEVlassaraHOral advanced glycation endproducts (AGEs) promote insulin resistance and diabetes by depleting the antioxidant defenses AGE receptor-1 and sirtuin 1Proc Natl Acad Sci U S A20121093915888 1589322908267
- UribarriJCaiWRamdasMRestriction of advanced glycation end products improves insulin resistance in human type 2 diabetes: potential role of AGER1 and SIRT1Diabetes Care20113471610 161621709297
- NegreanMStirbanAStratmannBEffects of low- and high-advanced glycation endproduct meals on macro- and microvascular endothelial function and oxidative stress in patients with type 2 diabetes mellitusAm J Clin Nutr20078551236 124317490958
- StirbanANegreanMGöttingCDietary advanced glycation endproducts and oxidative stress: in vivo effects on endothelial function and adipokinesAnn N Y Acad Sci20081126276 27918448830
- MiyataTKurokawaKvan Ypersele de StrihouCRelevance of oxidative and carbonyl stress to long-term uremic complicationsKidney Int Suppl200076S120 S12510936808
- RajDSChoudhuryDWelbourneTCLeviMAdvanced glycation end products: a Nephrologist’s perspectiveAm J Kidney Dis2000353365 38010692262
- BuschMFrankeSMüllerAPotential cardiovascular risk factors in chronic kidney disease: AGEs, total homocysteine and metabolites, and the C-reactive proteinKidney Int2004661338 34715200442
- TanALForbesJMCooperMEAGE, RAGE, and ROS in diabetic nephropathySemin Nephrol2007272130 14317418682
- ChungACZhangHKongYZAdvanced glycation end-products induce tubular CTGF via TGF-beta-independent Smad3 signalingJ Am Soc Nephrol2010212249 26019959709
- CoreshJAstorBCGreeneTEknoyanGLeveyASPrevalence of chronic kidney disease and decreased kidney function in the adult US population: Third National Health and Nutrition Examination SurveyAm J Kidney Dis20034111 1212500213
- TanakaSAvigadGBrodskyBEikenberryEFGlycation induces expansion of the molecular packing of collagenJ Mol Biol19882032495 5053143838
- HaitoglouCSTsilibaryECBrownleeMCharonisASAltered cellular interactions between endothelial cells and nonenzymatically glucosylated laminin/type IV collagenJ Biol Chem19922671812404 124071618745
- GuLHagiwaraSFanQRole of receptor for advanced glycation end-products and signalling events in advanced glycation end-product-induced monocyte chemoattractant protein-1 expression in differentiated mouse podocytesNephrol Dial Transplant2006212299 31316263740
- PalimeriSPaliouraEPiperiCAdditive effects of dietary glycotoxins and androgen excess on the kidney of a female rat modelAlex J Med Epub
- SebekováKKupcováVSchinzelRHeidlandAMarkedly elevated levels of plasma advanced glycation end products in patients with liver cirrhosis – amelioration by liver transplantationJ Hepatol200236166 7111804666
- YagmurETackeFWeissCElevation of Nepsilon-(carboxymethyl) lysine-modified advanced glycation end products in chronic liver disease is an indicator of liver cirrhosisClin Biochem200639139 4516321365
- HyogoHYamagishiSIwamotoKElevated levels of serum advanced glycation end products in patients with non-alcoholic steatohepatitisJ Gastroenterol Hepatol20072271112 111917559366
- SatoTWuXShimogaitoNTakinoJYamagishiSTakeuchiMEffects of high-AGE beverage on RAGE and VEGF expressions in the liver and kidneysEur J Nutr20094816 1119083041
- CaiWHeJCZhuLReduced oxidant stress and extended lifespan in mice exposed to a low glycotoxin diet: association with increased AGER1 expressionAm J Pathol200717061893 190217525257
- PatelRBakerSSLiuWEffect of dietary advanced glycation end products on mouse liverPLoS One201274e3514322496902
- Diamanti-KandarakisEPiperiCKalofoutisACreatsasGIncreased levels of serum advanced glycation end-products in women with polycystic ovary syndromeClin Endocrinol (Oxf)200562137 4315638868
- Diamanti-KandarakisEPioukaALivadasSAnti-mullerian hormone is associated with advanced glycosylated end products in lean women with polycystic ovary syndromeEur J Endocrinol20091605847 85319208775
- Diamanti-KandarakisEKatsikisIPiperiCIncreased serum advanced glycation end-products is a distinct finding in lean women with polycystic ovary syndrome (PCOS)Clin Endocrinol (Oxf)2008694634 64118363886
- Diamanti-KandarakisEPiperiCPatsourisEImmunohistochemical localization of advanced glycation end-products (AGEs) and their receptor (RAGE) in polycystic and normal ovariesHistochem Cell Biol20071276581 58917205306
- Diamanti-KandarakisEPiperiCKorkolopoulouPAccumulation of dietary glycotoxins in the reproductive system of normal female ratsJ Mol Med (Berl)200785121413 142017694292
- KandarakiEChatzigeorgiouAPiperiCReduced ovarian glyoxalase-I activity by dietary glycotoxins and androgen excess: a causative link to polycystic ovarian syndromeMol Med2012181183 118922859292
- TantalakiEPiperiCLivadasSImpact of dietary modification of advanced glycation end products (AGEs) on the hormonal and metabolic profile of women with polycystic ovary syndrome (PCOS)Hormones (Athens)201413165 7324722128
- ChatzigeorgiouAKandarakiEPiperiCDietary glycotoxins affect scavenger receptor expression and the hormonal profile of female ratsJ Endocrinol20132183331 33723823020
- Diamanti-KandarakisEChatzigeorgiouAPapageorgiouEKoundourasDKoutsilierisMAdvanced glycation end-products and insulin signaling in granulosa cellsExp Biol Med Epub20155 7
- BoutziosGLivadasSPiperiCPolycystic ovary syndrome offspring display increased oxidative stress markers comparable to gestational diabetes offspringFertil Steril2013993943 95023200689
- SalahuddinPRabbaniGKhanRHThe role of advanced glycation end products in various types of neurodegenerative disease: a therapeutic approachCell Mol Biol Lett2014193407 43725141979
- CaiWUribarriJZhuLOral glycotoxins are a modifiable cause of dementia and the metabolic syndrome in mice and humansProc Natl Acad Sci U S A2014111134940 494524567379
- KandarakisSAPiperiCTopouzisFPapavassiliouAGEmerging role of advanced glycation-end products (AGEs) in the pathobiology of eye diseasesProg Retin Eye Res20144285 10224905859
- KandarakisSAPiperiCMoschonasDPKorkolopoulouPPapaloisAPapavassiliouAGDietary glycotoxins induce RAGE and VEGF up-regulation in the retina of normal ratsExp Eye Res20151371 1026026876
- SharafHMatou-NasriSWangQAdvanced glycation endproducts increase proliferation, migration and invasion of the breast cancer cell line MDA-MB-231Biochim Biophys Acta201518523429 44125514746
- Rodriguez-TejaMGronauJHBreitCAGE-modified basement membrane cooperates with Endo180 to promote epithelial cell invasiveness and decrease prostate cancer survivalJ Pathol20152354581 59225408555
- TakinoJYamagishiSTakeuchiMCancer malignancy is enhanced by glyceraldehyde-derived advanced glycation end-productsJ Oncol2010201073985220631911
- van HeijstJWNiessenHWHoekmanKSchalkwijkCGAdvanced glycation end products in human cancer tissues: detection of Nepsilon-(carboxymethyl)lysine and argpyrimidineAnn N Y Acad Sci20051043725 73316037299
- FosterDSpruillLWalterKRAGE metabolites: a biomarker linked to cancer disparity?Cancer Epidemiol Biomarkers Prev201423102186 219125053712
- JiaoLWeinsteinSJAlbanesDEvidence that serum levels of the soluble receptor for advanced glycation end products are inversely associated with pancreatic cancer risk: a prospective studyCancer Res201171103582 358921540233
- JiaoLTaylorPRWeinsteinSJAdvanced glycation end products, soluble receptor for advanced glycation end products, and risk of colorectal cancerCancer Epidemiol Biomarkers Prev20112071430 143821527578
- GroteVANietersAKaaksRThe associations of advanced glycation end products and its soluble receptor with pancreatic cancer risk: a case-control study within the prospective EPIC CohortCancer Epidemiol Biomarkers Prev2012214619 62822301828
- TurnerDPAdvanced glycation end-products: a biological consequence of lifestyle contributing to cancer disparityCancer Res201575101925 192925920350
- HofmannSMDongHJLiZImproved insulin sensitivity is associated with restricted intake of dietary glycoxidation products in the db/db mouseDiabetes20025172082 208912086936
- ZhengFHeCCaiWHattoriMSteffesMVlassaraHPrevention of diabetic nephropathy in mice by a diet low in glycoxidation productsDiabetes Metab Res Rev2002183224 23712112941
- VlassaraHStrikerGEAGE restriction in diabetes mellitus: a paradigm shiftNat Rev Endocrinol201179526 53921610689
- PouillartPMauprivezHAit-AmeurLStrategy for the study of the health impact of dietary Maillard products in clinical studies: the example of the ICARE clinical study on healthy adultsAnn N Y Acad Sci20081126173 17618448812
- VlassaraHCaiWGoodmanSProtection against loss of innate defenses in adulthood by low advanced glycation end products (AGE) intake: role of the antiinflammatory AGE receptor-1J Clin Endocrinol Metab200994114483 449119820033
- Birlouez-AragonISaavedraGTessierFJA diet based on high-heat-treated foods promotes risk factors for diabetes mellitus and cardiovascular diseasesAm J Clin Nutr20109151220 122620335546
- KellowNJSavigeGSDietary advanced glycation end-product restriction for the attenuation of insulin resistance, oxidative stress and endothelial dysfunction: a systematic reviewEur J Clin Nutr2013673239 24823361161
- BrownleeMVlassaraHKooneyAUlrichPCeramiAAminoguanidine prevents diabetes-induced arterial wall protein cross-linkingScience198623247581629 16323487117
- HammesHPBrownleeMEdelsteinDSaleckMMartinSFederlinKAminoguanidine inhibits the development of accelerated diabetic retinopathy in the spontaneous hypertensive ratDiabetologia199437132 358150227
- Soulis-LiparotaTCooperMPapazoglouDClarkeBJerumsGRetardation by aminoguanidine of development of albuminuria, mesangial expansion, and tissue fluorescence in streptozocin-induced diabetic ratDiabetes199140101328 13341834497
- NishizawaYWadaRBabaMTakeuchiMHanyu-ItabashiCYagihashiSNeuropathy induced by exogenously administered advanced glycation end-products in ratsJ Diabetes Investig201011–240 49
- ForbesJMSoulisTThallasVRenoprotective effects of a novel inhibitor of advanced glycationDiabetologia2001441108 11411206401
- Wilkinson-BerkaJLKellyDJKoernerSMALT-946 and aminoguanidine, inhibitors of advanced glycation, improve severe nephropathy in the diabetic transgenic (mREN-2)27 ratDiabetes200251113283 328912401720
- ChengGWangLLLongLBeneficial effects of C36, a novel breaker of advanced glycation endproducts cross-links, on the cardiovascular system of diabetic ratsBr J Pharmacol200715281196 120617965740
- OkamotoTYamagishiSInagakiYAngiogenesis induced by advanced glycation end products and its prevention by cerivastatinFASEB J200216141928 193012368225
- StittAGardinerTAAldersonNLThe AGE inhibitor pyridoxamine inhibits development of retinopathy in experimental diabetesDiabetes20025192826 283212196477
- DegenhardtTPAldersonNLArringtonDDPyridoxamine inhibits early renal disease and dyslipidemia in the streptozotocin-diabetic ratKidney Int2002613939 95011849448
- VasanSZhangXZhangXAn agent cleaving glucose-derived protein crosslinks in vitro and in vivoNature19963826588275 2788717046
- CandidoRForbesJMThomasMCA breaker of advanced glycation end products attenuates diabetes-induced myocardial structural changesCirc Res2003927785 79212623881
- CoughlanMTForbesJMCooperMERole of the AGE crosslink breaker, alagebrium, as a renoprotective agent in diabetesKidney Int Suppl2007106S54 S6017653212
- KassDAShapiroEPKawaguchiMImproved arterial compliance by a novel advanced glycation end-product crosslink breakerCirculation2001104131464 147011571237
- LittleWCZileMRKitzmanDWHundleyWGO’BrienTXDegroofRCThe effect of alagebrium chloride (ALT-711), a novel glucose cross-link breaker, in the treatment of elderly patients with diastolic heart failureJ Card Fail2005113191 19515812746
- AldiniGVistoliGStefekMMolecular strategies to prevent, inhibit, and degrade advanced glycoxidation and advanced lipoxidation end productsFree Radic Res201347Suppl 193 13723560617
- WinMTYamamotoYMunesueSRegulation of RAGE for attenuating progression of diabetic vascular complicationsExp Diabetes Res2012201289460522110482
- YamagishiSNakamuraKMatsuiTUedaSFukamiKOkudaSAgents that block advanced glycation end product (AGE)-RAGE (receptor for AGEs)-oxidative stress system: a novel therapeutic strategy for diabetic vascular complicationsExpert Opin Investig Drugs2008177983 996
- ParkLRamanKGLeeKJSuppression of accelerated diabetic atherosclerosis by the soluble receptor for advanced glycation endproductsNat Med1998491025 10319734395
- BucciarelliLGWendtTQuWRAGE blockade stabilizes established atherosclerosis in diabetic apolipoprotein E-null miceCirculation2002106222827 283512451010
- BarileGRPachydakiSITariSRThe RAGE axis in early diabetic retinopathyInvest Ophthalmol Vis Sci20054682916 292416043866
- KajiYUsuiTIshidaSInhibition of diabetic leukostasis and blood-retinal barrier breakdown with a soluble form of a receptor for advanced glycation end productsInvest Ophthalmol Vis Sci2007482858 86517251488
- LiFDrelVRSzabóCStevensMJObrosovaIGLow-dose poly(ADP-ribose) polymerase inhibitor-containing combination therapies reverse early peripheral diabetic neuropathyDiabetes20055451514 152215855340
- PacherPLiaudetLSorianoFGMableyJGSzabóESzabóCThe role of poly(ADP-ribose) polymerase activation in the development of myocardial and endothelial dysfunction in diabetesDiabetes2002512514 52111812763
- UedaSYamagishiSTakeuchiMOral adsorbent AST-120 decreases serum levels of AGEs in patients with chronic renal failureMol Med2006127–8180 18417088950
- Diamanti-KandarakisEKatsikisIPiperiCAlexandrakiKPanidisDEffect of long-term orlistat treatment on serum levels of advanced glycation end-products in women with polycystic ovary syndromeClin Endocrinol (Oxf)2007661103 10917201808
- PiperiCAdamopoulosCDalagiorgouGDiamanti-KandarakisEPapavassiliouAGCrosstalk between advanced glycation and endoplasmic reticulum stress: emerging therapeutic targeting for metabolic diseasesJ Clin Endocrinol Metab20129772231 224222508704
