Abstract
Chronic hyperglycemia and the corresponding glucotoxicity are the main pathogenic mechanisms of diabetes and its complications. Streptozotocin (STZ)-induced diabetic animal models are useful platforms for the understanding of β cell glucotoxicity in diabetes. As diabetes induced by a single STZ injection is often referred to as type 1 diabetes that is caused by STZ’s partial destruction of pancreas, one question often being asked is whether the STZ type 1 diabetes animal model is a good model for studying the mitochondrial mechanisms of β cell glucotoxicity. In this mini review, we provide evidence garnered from the literature that the STZ type 1 diabetes is indeed a suitable model for studying mitochondrial mechanisms of diabetic β cell glucotoxicity. Evidence presented includes: 1) continued β cell derangement is due to chronic hyperglycemia after STZ is completely eliminated out of the body; 2) STZ diabetes can be reversed by insulin treatment, which indicates that β cell responds to treatment and shows ability to regenerate; and 3) STZ diabetes can be ameliorated or alleviated by administration of phytochemicals. In addition, mechanisms of STZ action and fundamental gaps in understanding mitochondrial mechanisms of β cell dysfunction are also discussed.
Introduction
Diabetes mellitus and its complications are chronic glucotoxicity diseases. The concept of β cell glucotoxicity (and other cells as well) implicates that persistent excessive glucose can exert adverse or toxic effect on β cell function after the establishment of diabetes induced by either genetic or environmental factors.Citation1 Hence, diabetic glucotoxicity is believed to play a forceful role in driving secondary β cell failure in both type 1 and type 2 diabetes.Citation2,Citation3 Despite intensive and extensive studies on β cell glucotoxicity, detailed mitochondrial mechanisms still remain poorly understood. In this regard, experimental animal models of diabetes have been indispensable to research in diabetes and its complications.
Streptozotocin-induced animal models of diabetes
Streptozotocin (STZ) is a widely used chemical for the induction of experimental diabetes in rodents.Citation4,Citation5 Since the initial report of its diabetogenic properties in 1963,Citation6 STZ has been used alone or in combination with other chemicals or with dietary manipulations for induction of either type 1 or type 2 diabetes.Citation7,Citation8 Type 1 diabetes can be induced in rodents by a single STZ injection,Citation9,Citation10 while type 2 diabetes can be induced by at least three approaches, which include STZ injection after administration of nicotinamide,Citation11,Citation12 high fat diet (HFD) feeding followed by a low-dose STZ injection,Citation13 and STZ injection during the neonatal period.Citation14,Citation15 All these STZ-involved diabetic animal models have been very useful in elucidating the mechanisms of diabetic pathogenesis and in screening artificial chemicals, natural products, and pharmacological agents that are potentially capable of lowering blood glucose levels.Citation16,Citation17
Mechanisms of STZ action
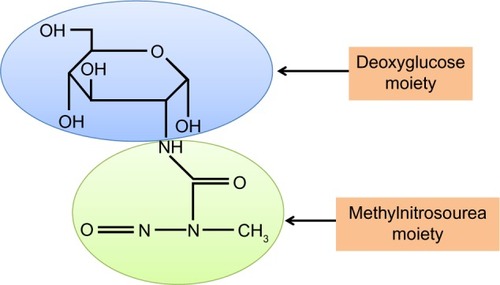
STZ is an antibiotic produced by the bacterium Streptomyces achromogens and possesses a broad spectrum of antibacterial properties.Citation18 It contains a glucose molecule (in deoxy form) that is linked to a highly reactive methylnitrosourea moiety () that is thought to exert STZ’s cytotoxic effects, while the glucose moiety directs the chemical to the pancreatic β cells.Citation19 STZ recognizes the GLUT2 receptor that is abundant on β cell plasma membranes.Citation5 Therefore, pancreatic β cell is a specific target of STZ. As GLUT2 also exists in liver and kidney to a less extent,Citation20 high doses of STZ could also impair the functions of liver and kidney.Citation20 Upon ingestion, STZ is rapidly metabolized in the liver and quickly eliminated by renal excretion;Citation21 therefore, STZ has really a short life (with a half-life of 15 minutes in the serum after IV injectionCitation22) and its acute toxicity to the liver and the kidney can be neglected after persistent hyperglycemia is obtained.Citation23 After STZ is eliminated out of the body, any further functional impairment of the liver and the kidney may be attributed to the effects of diabetic hyperglycemia. This is the basis for studying the mechanisms of STZ diabetic complications in these organsCitation23 as well as other organs such as the brain, the heart, and the muscles.Citation24
Potential mechanisms of diabetic β cell glucotoxicity
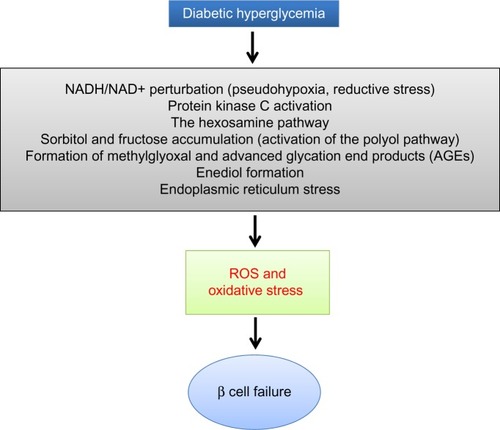
When there is a status of persistent hyperglycemia as opposed to episodic hyperglycemia, cellular metabolic system is under constant pressure due to glucose overloading.Citation25 This glucose overloading can activate many metabolic or signaling pathways that not only attempt to dispose excessive glucose but also generate more reactive oxygen species, leading to oxidative stress and β cell failure.Citation1 As shown in , these hyperglycemia-stimulated pathways include an increased NADH/NAD+ ratio linked to pseudohypoxia and reductive stress,Citation25 the hexosamine pathway responsible for protein O-GlcNAc modifications,Citation26 protein kinase C activation,Citation27 activation of the polyol pathway resulting in accumulation of sorbitol and fructose,Citation28 formation of methylglyoxal and advanced glycation products,Citation29 enediol formation,Citation30 and endoplasmic reticulum stress.Citation31 The establishment that all these pathways culminate in reactive oxygen species production,Citation32 together with the evidence of a low-level antioxidant capacity in β cells,Citation1 is thought to be responsible for secondary diabetic β cell failure.Citation3 Nonetheless, the mitochondrial mechanisms of β cell glucotoxicity in diabetes still remain poorly understood.
Figure 2 Mechanisms by which diabetic hyperglycemia can impose glucotoxicity on β cells.
Abbreviations: STZ, streptozotocin; ROS, reactive oxygen species.
Glucose combustion, mitochondrial ATP production, and β cell insulin secretion
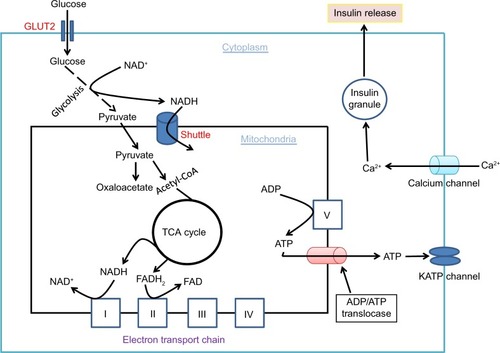
It is well established that glucose-stimulated insulin secretion is tightly linked to glucose-driven ATP production by β cell mitochondria.Citation33,Citation34 As β cell has a very low level of lactate dehydrogenase,Citation35 most of the pyruvate generated by the glycolytic pathway is transported into mitochondria to generate the reducing compounds NADH and FADH2, along with a complete combustion of pyruvate to CO2.Citation36 This process is achieved not only by formation of acetyl-CoA that feeds into the Krebs cycle but also by formation of oxaloacetate that replenishes the intermediates in the Krebs cycle.Citation37 When blood glucose gets higher after a meal, ATP production in β cell mitochondria also gets higher (). This leads to an elevated level of ATP/ADP ratio in the cytoplasm and a consequent closure of the kATP channels on the cell membrane. The closure of the kATP channels then depolarizes the membranes and renders the opening of the calcium channel, resulting in calcium influx that triggers insulin granule exocytosis and insulin releaseCitation38–Citation40 (). In diabetes, however, this episodic process of glucose-stimulated insulin secretion is believed to be impaired.Citation41,Citation42
Figure 3 Glucose combustion is tightly coupled to insulin secretion in pancreatic β cells.
Abbreviation: TCA, tricarboxylic acid.
Redox imbalance and mitochondrial deregulation in β cell dysfunction
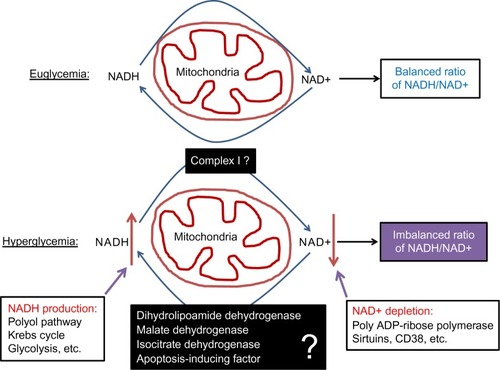
Although pancreatic β cell function operates by a supply driven mechanism by which glucose or nutrient metabolism is coupled to insulin secretion,Citation43 persistent stimulation of β cell by a high level of glucose could eventually lead to β cell exhaustion and cell death.Citation3,Citation44 This glucotoxicity has been thought to originate from redox imbalance between NADH and NAD+.Citation45 On one hand, NADH can be overproduced by hyperglycemia and the activation of polyol pathway that makes NADH from NADPH;Citation25 on the other hand, NAD+ can be depleted by the activation of enzymes such as poly ADP-ribosylase, sirtuins, and CD38 that use NAD+ as their substrate.Citation46 Therefore, exceedingly accumulation of NADH and potential depletion of NAD+ indicate a severe problem in NADH/NAD+ recycling under diabetic conditions.Citation46 As mitochondria is the major cellular site for maintaining redox balance by oxidizing NADH via complex I and FADH2 via complex II, mitochondrial election transport chain plays a critical role in β cell function and dysfunction.Citation36 Nonetheless, the role of each component of the electron transport chain, in particular, that of complex I (), in diabetic β cell glucotoxicity remains largely unknown. Additionally, the roles of those mitochondrial dehydrogenases in making NADH from NAD+ () are also unknown. From this perspective, STZ diabetic animal models should provide a broad platform for exploring the mitochondrial mechanism of diabetic glucotoxicity, implicating each component of the electron transport chain.
Figure 4 Role of redox imbalance between NADH and NAD+ in β cell dysfunction.
STZ-induced type 1diabetes as an animal model for further exploring the mitochondrial mechanisms of diabetic β cell glucotoxicity
Our laboratory recently embarked on studies of mitochondrial mechanisms of β cell glucotoxicity using animal models of diabetes induced by a single intraperitoneal STZ injection. While there is no doubt that STZ-induced type 2 diabetes are good models for studying mitochondrial glucotoxicity in β cells, one question often being asked is that whether the STZ type 1 diabetes model is suitable for studying β cell diabetic glucotoxicity as STZ partially destructs pancreas and reduces β cell mass.Citation47 After reviewing the literature, we firmly believe that the single STZ injection diabetic animal model is appropriate for studying the mitochondrial mechanisms of β cell glucotoxicity. Specifically, our belief is based on the following experimental evidence reported in the literature.
STZ is rapidly eliminated after ingestion. For intraperitoneal injection, STZ can be eliminated within 48 hours of ingestionCitation21 and its DNA methylating effect quickly diminishes as no further increase in DNA methylation can be detected after 24 hours of STZ exposure.Citation48,Citation49 These pieces of evidence indicate that STZ acute toxicity is very short-lived after ingestion. Yet, β cell function continues to deteriorate in the absence of detectable STZ.Citation50 It is now known that it is the hyperglycemic state established by acute STZ toxicity that drives further β cell derangement.Citation51,Citation52 Therefore, after induction of diabetes, β cell dysfunction is maintained by persistent hyperglycemia, and such β cell can be explored to provide insights into the mitochondrial mechanisms of glucotoxicity.
STZ diabetes can be reversed by insulin treatment. Grossman et al have reported that glycemic control in STZ diabetic mice by insulin can promote β cell regeneration in the diabetic pancreas,Citation53 demonstrating again that further β cell dysfunction, in the absence of STZ, is due to diabetic hyperglycemia. Once this hyperglycemic status is reversed to euglycemia by insulin supplement, β cell function improves via β cell regeneration.Citation53 In fact, while animals show insulin deficiency after STZ ingestion, they do not require insulin treatment for survival.Citation54 Therefore, many diabetic animals can live beyond 24 weeks without any interventions after a single STZ injection,Citation24 indicating that the dosage of STZ given as a single injection only partially destructs islets and β cellsCitation54,Citation55 and that diabetes created as such is due to β cell glucotoxicity rather than STZ acute toxicity. The results of Grossman’s study agree well with those of other studies that β cell function indeed responds to treatment and may have limited regeneration after partial pancreatic destruction by STZ.Citation52,Citation56,Citation57 On the other hand, the use of high STZ doses (eg, 100 mg/kg for rats) could lead to nearly a complete destruction of β cells, which is often associated with a quick demise of the animals.Citation54 However, the use of a very high STZ dose is never the case for studies of drug-screening and of glucotoxicity in diabetes and diabetic complications.
β cell dysfunction in STZ diabetes can be alleviated by plant natural products or phytochemicals. In fact, this is the basis for screening diabetic drugs or compounds using STZ diabetic animal models. In addition to DNA alkylation thought to be involved in STZ β cell toxicity,Citation4,Citation5 oxidative damage by reactive oxygen or reactive nitrogen species has also been implicated in β cell destruction by STZ.Citation4,Citation5 Therefore, numerous studies have demonstrated that plant extracts or phytochemicals having antioxidant properties can ameliorate β cell dysfunction in STZ diabetes. For example, grape seed proanthocyanidins,Citation58 curcumin,Citation59 resveratrol,Citation60 and pycnogenolCitation61 all have been reported to improve β cell function in STZ diabetic animals. The underlying mechanisms of these phytochemicals are likely due to their abilities to attenuate hyperglycemic glucotoxicity by decreasing blood glucose levels and/or facilitating glucose combustion, leading to an eventual rebalanced redox state between NADH and NAD+,Citation62–Citation64 which is conducive to proliferation of surviving β cell or regeneration of β cell from other type of cells such as acinar and ductal cells.Citation10,Citation20,Citation56 It is unlikely that hyperglycemiaimpaired β cell would be a direct target for repairing by these phytochemicals or other glucose lowering agents.
Summary and conclusion
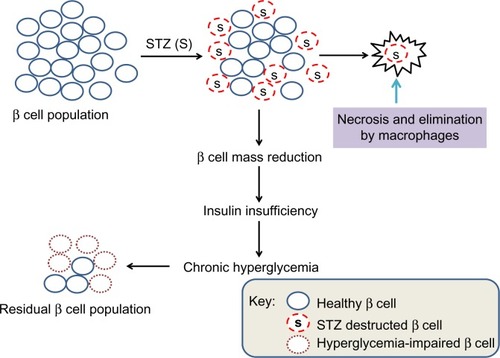
In summary, as the initial STZ toxicity to β cells is short-livedCitation21 and further impairment of the surviving β cell’s function is due to hyperglycemic toxicity,Citation24,Citation51,Citation65 the mitochondrial mechanisms of β cell glucotoxicity in these surviving β cells can thus be elucidated (). Additionally, as STZ-destructed cells undergo necrosis and are eliminated by macrophages,Citation66,Citation67 no intact mitochondria may be isolated from these STZ-destructed cells; thus, mitochondria isolated from STZ-exposed pancreas should be from those surviving or hyperglycemia-impaired β cells (). Based on these findings, we believe that animal models of diabetes induced by single STZ injection can serve as a platform for studying the mitochondrial mechanisms of β cell glucotoxicity. Of course, no mitochondria isolation and functional analysis should be performed right after STZ injection in order to avoid any acute toxic effects of STZ on mitochondria. Rather, mitochondria should be isolated when overt diabetes develops after STZ is completely eliminated out of the body. The point here is that it should be distinguished between STZ acute toxicity and diabetic glucotoxicity when needed for given STZ diabetes settings.
Figure 5 Scheme showing partial destruction of β cell population by STZ and reduction in β cell mass that induces insulin insufficiency and chronic hyperglycemia.
Abbreviation: STZ, streptozotocin.
In conclusion, STZ-induced type 1 diabetes in rodents is a well-established and well-accepted practice for the studies of pathogenesis of diabetes and its complications. However, it should be pointed out that this STZ model has both advantages and disadvantages over other STZ-involved experimental models, including STZ-neonatal and STZ-HFD. On one hand, when compared with the STZ-neonatal and STZ-HFD models that can also be used to study the mitochondrial mechanisms of β cell glucotoxicity, the single STZ injection model is much less expensive and much less time-consuming. For example, in the STZ-HFD model, HFD feeding usually takes a few weeks or a few months followed by low-dose STZ injections.Citation13,Citation16,Citation68 Similarly, the STZ-neonatal model also takes a longer time for diabetes to develop in addition to caring and manipulating neonatal animals.Citation15,Citation69 On the other hand, the disadvantage of the single STZ injection model is that it does not create the insulin resistance pathophysiologyCitation70 that can be observed in the STZ-neonatal and STZ-HFD models. Additionally, no standard protocols exist for STZ preparation and injections, and the diabetic state can be highly variable due to factors such as age of animals, sex, body weight, species, and strains.Citation71 Moreover, it is by no means that the STZ type 1 diabetic animal model would be equivalent to the human disease state. Nonetheless, the single STZ injection model should continue to provide a cost-effective, time-saving, convenient platform for the study of the pathophysiological mitochondrial mechanisms of β cell derangement induced by diabetic glucotoxicity.
Acknowledgments
This publication was supported in part by National Institute of Neurological Disorders and Stroke, Grant R01NS079792.
Disclosure
The authors declare that there is no conflict of interest.
References
- PoitoutVRobertsonRPGlucolipotoxicity: fuel excess and beta-cell dysfunctionEndocr Rev2008293351 36618048763
- KorsgrenOJanssonLSandlerSAnderssonAHyperglycemia-induced B cell toxicity. The fate of pancreatic islets transplanted into diabetic mice is dependent on their genetic backgroundJ Clin Invest19908662161 21682254465
- PoitoutVRobertsonRPMinireview: secondary beta-cell failure in type 2 diabetes – a convergence of glucotoxicity and lipotoxicityEndocrinology20021432339 34211796484
- SzkudelskiTThe mechanism of alloxan and streptozotocin action in B cells of the rat pancreasPhysiol Res2001506537 54611829314
- LenzenSThe mechanisms of alloxan- and streptozotocin-induced diabetesDiabetologia2008512216 22618087688
- RakietenNRakietenMLNadkarniMRStudies on the diabetogenic action of streptozotocin (NSC-37917)Cancer Chemother Rep19632991 98
- GajdosikAGajdosikovaAStefekMNavarovaJHozovaRStreptozotocin-induced experimental diabetes in male Wistar ratsGen Physiol Biophys19991854 6210703720
- GhasemiAKhalifiSJediSStreptozotocin-nicotinamide-induced rat model of type 2 diabetes (review)Acta Physiol Hung20141014408 42025532953
- JunodALambertAEStauffacherWRenoldAEDiabetogenic action of streptozotocin: relationship of dose to metabolic responseJ Clin Invest196948112129 21394241908
- YinDTaoJLeeDDRecovery of islet beta-cell function in streptozotocin-induced diabetic mice: an indirect role for the spleenDiabetes200655123256 326317130468
- WuMSLiangJTLinYDWuETTsengYZChangKCAminoguanidine prevents the impairment of cardiac pumping mechanics in rats with streptozotocin and nicotinamide-induced type 2 diabetesBr J Pharmacol20081544758 76418376420
- SzkudelskiTStreptozotocin-nicotinamide-induced diabetes in the rat. Characteristics of the experimental modelExp Biol Med (Maywood)20122375481 49022619373
- SkovsoSModeling type 2 diabetes in rats using high fat diet and streptozotocinJ Diabetes Investig201454349 358
- PatilMASuryanarayanaPPutchaUKSrinivasMReddyGBEvaluation of neonatal streptozotocin induced diabetic rat model for the development of cataractOxid Med Cell Longev2014201446326425505935
- PorthaBBlondelOSerradasPThe rat models of non-insulin dependent diabetes induced by neonatal streptozotocinDiabete Metab198915261 752525491
- SrinivasanKViswanadBAsratLKaulCLRamaraoPCombination of high-fat diet-fed and low-dose streptozotocin-treated rat: a model for type 2 diabetes and pharmacological screeningPharmacol Res2005524313 32015979893
- KumarSVasudevaNSharmaSGC-MS analysis and screening of antidiabetic, antioxidant and hypolipidemic potential of Cinnamomum tamala oil in streptozotocin induced diabetes mellitus in ratsCardiovasc Diabetol2012119522882757
- VavraJJDeboerCDietzAHankaLJSokolskiWTStreptozotocin, a new antibacterial antibioticAntibiot Annu19597230 23513841501
- JohanssonEBTjalveHStudies on the tissue-disposition and fate of [14C]streptozotocin with special reference to the pancreatic isletsActa Endocrinol1978892339 351151471
- BouwensLRoomanIRegulation of pancreatic beta-cell massPhysiol Rev20058541255 127016183912
- KarunanyakeEHHearseDJMellowsGThe synthesis of C14Streptozotocin and its distribution and excretion in the ratBiochem J1974142673 6834282704
- EleazuCOEleazuKCChukwumaSEssienUNReview of the mechanism of cell death resulting from streptozotocin challenge in experimental animals, its practical use and potential risk to humansJ Diabetes Metab Disord20131216024364898
- TeschGHAllenTJRodent models of streptozotocin-induced diabetic nephropathyNephrology2007123261 26617498121
- WeiMOngLSmithMTThe streptozotocin-diabetic rat as a model of the chronic complications of human diabetesHeart Lung Circ200312144 5016352106
- YanLJPathogenesis of chronic hyperglycemia: from reductive stress to oxidative stressJ Diabetes Res2014201413791925019091
- IssadTMassonEPagesyPO-GlcNAc modification, insulin signaling and diabetic complicationsDiabetes Metab2010366 pt 1423 43521074472
- NaruseKRask-MadsenCTakaharaNActivation of vascular protein kinase C-beta inhibits Akt-dependent endothelial nitric oxide synthase function in obesity-associated insulin resistanceDiabetes2006553691 69816505232
- WilliamsonJRChangKFrangosMHyperglycemic pseudohypoxia and diabetic complicationsDiabetes1993426801 8138495803
- QueisserMAYaoDGeislerSHyperglycemia impairs proteasome function by methylglyoxalDiabetes2010593670 67820009088
- WolffSPDeanRTGlucose autoxidation and protein modification. The potential role of ‘autoxidative glycosylation’ in diabetesBiochem J19872451243 2503117042
- LeibowitzGKaiserNCerasiEbeta-Cell failure in type 2 diabetesJ Diabetes Investig20112282 91
- RobertsonRPChronic oxidative stress as a central mechanism for glucose toxicity in pancreatic islet beta cells in diabetesJ Biol Chem20042794142351 4235415258147
- MaechlerPWollheimCBRole of mitochondria in metabolism-secretion coupling of insulin release in the pancreatic beta-cellBiofactors199883–4255 2629914827
- MalmgrenSNichollsDGTaneeraJTight coupling between glucose and mitochondrial metabolism in clonal beta-cells is required for robust insulin secretionJ Biol Chem20092844732395 3240419797055
- SekineNCirulliVRegazziRLow lactate dehydrogenase and high mitochondrial glycerol phosphate dehydrogenase in pancreatic beta-cells. Potential role in nutrient sensingJ Biol Chem199426974895 49028106462
- WiederkehrAWollheimCBMinireview: implication of mitochondria in insulin secretion and actionEndocrinology200614762643 264916556766
- SchuitFDe VosAFarfariSMetabolic fate of glucose in purified islet cells. Glucose-regulated anaplerosis in beta cellsJ Biol Chem19972723018572 185799228023
- MaechlerPMitochondrial function and insulin secretionMol Cell Endocrinol20133791–212 1823792187
- MaechlerPCarobbioSRubiBIn beta-cells, mitochondria integrate and generate metabolic signals controlling insulin secretionInt J Biochem Cell Biol2006385–6696 70916443386
- MaechlerPMitochondrial signal transduction in pancreatic beta-cellsBest Pract Res Clin Endocrinol Metab2012266739 75223168276
- ZouCYGongYLiangJMetabolic signaling of insulin secretion by pancreatic beta-cell and its derangement in type 2 diabetesEur Rev Med Pharmacol Sci201418152215 222725070829
- SatinLSButlerPCHaJShermanASPulsatile insulin secretion, impaired glucose tolerance and type 2 diabetesMol Aspects Med2015
- WiederkehrAWollheimCBMitochondrial signals drive insulin secretion in the pancreatic beta-cellMol Cell Endocrinol20123531–2128 13721784130
- BensellamMLaybuttDRJonasJCThe molecular mechanisms of pancreatic beta-cell glucotoxicity: recent findings and future research directionsMol Cell Endocrinol20123641–21 2722885162
- TeodoroJSRoloAPPalmeiraCMThe NAD ratio redox paradox: why does too much reductive power cause oxidative stress?Toxicol Mech Methods2013235297 30223256455
- LuoXLiRYanLJRoles of pyruvate, NADH, and mitochondrial complex I in redox balance and imbalance in β cell function and dysfunctionJ Diabetes Res2015
- GomesAVedasiromoniJRDasMSharmaRMGangulyDKAnti-hyperglycemic effect of black tea (Camellia sinensis) in ratJ Ethnopharmacol1995453223 2267623488
- MossmanBTIrelandCMFilipakMLeDouxSWilsonGLComparative interactions of streptozotocin and chlorozotocin with DNA of an insulin-secreting cell line (RINr)Diabetologia1986293186 1912938999
- PettepherCCLeDouxSPBohrVAWilsonGLRepair of alkali-labile sites within the mitochondrial DNA of RINr 38 cells after exposure to the nitrosourea streptozotocinJ Biol Chem199126653113 31171825207
- RerupCCDrugs producing diabetes through damage of the insulin secreting cellsPharmacol Rev1970224485 5184921840
- KullinMLiZHansenJBBjorkESandlerSKarlssonFAK(ATP) channel openers protect rat islets against the toxic effect of streptozotocinDiabetes20004971131 113610909969
- MatsudaMKawasakiFMikamiYRescue of beta-cell exhaustion by diazoxide after the development of diabetes mellitus in rats with streptozotocin-induced diabetesEur J Pharmacol20024531141 14812393069
- GrossmanEJLeeDDTaoJGlycemic control promotes pancreatic beta-cell regeneration in streptozotocin-induced diabetic micePLoS One201051e874920090914
- RodriguesBPoucheretPBattellMLMcneillJHStreptozotocin-induced diabetes: Induction, mechanism(s), and dose dependencyMcNeillJHExperimental Models of DiabetesBoca RatonCRC Press19993 18
- KozukaCSunagawaSUedaRGamma-oryzanol protects pancreatic beta-cells against endoplasmic reticulum stress in male miceEndocrinology201515641242 125025594697
- MarchandKCAranyEJHillDJEffects of atorvastatin on the regeneration of pancreatic {beta}-cells after streptozotocin treatment in the neonatal rodentAm J Physiol Endocrinol Metab20102991E92 E10020388824
- HammingNAReynoldsWADNA synthesis in pancreatic islet and acinar cells in rats with streptozotocin-induced diabetesHorm Metab Res197792114 116140841
- DingYZhangZDaiXGrape seed proanthocyanidins ameliorate pancreatic beta-cell dysfunction and death in low-dose streptozotocin- and high-carbohydrate/high-fat diet-induced diabetic rats partially by regulating endoplasmic reticulum stressNutr Metab20131051
- ChanpooMPetchpiboonthaiHPanyarachunBAnupunpisitVEffect of curcumin in the amelioration of pancreatic islets in streptozotocin-induced diabetic miceJ Med Assoc Thai201093Suppl 6S152 S15921280528
- KuCRLeeHJKimSKLeeEYLeeMKLeeEJResveratrol prevents streptozotocin-induced diabetes by inhibiting the apoptosis of pancreatic beta-cell and the cleavage of poly (ADP-ribose) polymeraseEndocr J2012592103 10922068111
- ParveenKIshratTMalikSKausarMASiddiquiWAModulatory effects of Pycnogenol in a rat model of insulin-dependent diabetes mellitus: biochemical, histological, and immunohistochemical evidencesProtoplasma20132501347 36022660838
- KannadhasanRAshwini AnjanaAJPhytochemical and antioxidant activity of polyherbal hydroethanolic extract in streptozotocin induced β cell destruction in ratsInt J Pharm Sci Drug Res20124125 30
- ErejuwaOOSulaimanSAWahabMSSirajudeenKNSallehMSGurtuSAntioxidant protection of Malaysian tualang honey in pancreas of normal and streptozotocin-induced diabetic ratsAnn Endocrinol2010714291 296
- NitureNTAnsariAANaikSRAnti-hyperglycemic activity of rutin in streptozotocin-induced diabetic rats: an effect mediated through cytokines, antioxidants and lipid biomarkersIndian J Exp Biol2014527720 72725059040
- Mellado-GilJMAguilar-DiosdadoMHigh glucose potentiates cytokine- and streptozotocin-induced apoptosis of rat islet cells: effect on apoptosis-related genesJ Endocrinol20041831155 16215525583
- FujimotoHHiraseTMiyazakiYIL-27 inhibits hyperglycemia and pancreatic islet inflammation induced by streptozotocin in miceAm J Pathol201117952327 233621925473
- O’BrienBAHarmonBVCameronDPAllanDJBeta-cell apoptosis is responsible for the development of IDDM in the multiple low-dose streptozotocin modelJ Pathol19961782176 1818683386
- ZhangMLvXYLiJXuZGChenLThe characterization of high-fat diet and multiple low-dose streptozotocin induced type 2 diabetes rat modelExp Diabetes Res2008200870404519132099
- TakadaJMachadoMAPeresSBNeonatal streptozotocin-induced diabetes mellitus: a model of insulin resistance associated with loss of adipose massMetabolism2007567977 98417570261
- LarsenMOBeta-cell function and mass in type 2 diabetesDan Med Bull2009563153 16419728971
- DeedsMCAndersonJMArmstrongASSingle dose streptozotocin-induced diabetes: considerations for study design in islet transplantation modelsLab Anim2011453131 14021478271