Abstract
Background
Advanced glycation end products, selectins, and adiponectin play important roles in the development of atherosclerosis in individuals with diabetes. Sitagliptin has been shown to reduce the concentration of glycated hemoglobin in diabetic patients. However, its effects on soluble receptor for advanced glycation end products (sRAGEs), selectins, and adiponectin in these patients are poorly understood. This study was conducted to assess the effects of sitagliptin on the circulating levels of sRAGEs, monocyte chemoattractant protein-1 (MCP-1), selectins, and adiponectin in patients with type 2 diabetes.
Methods
Diabetic patients eligible for sitagliptin monotherapy or combination therapy (eg, sitagliptin plus a sulfonylurea) were administered sitagliptin (50 mg/day) for 6 months. Levels of soluble P-selectin (sP-selectin), soluble E-selectin (sE-selectin), soluble vascular cell adhesion molecule-1 (sVCAM-1), MCP-1, sRAGEs, and adiponectin were measured by ELISA at baseline and after 3 and 6 months of treatment.
Results
At baseline, the levels of MCP-1, sP-selectin, sE-selectin, and sVCAM-1 were higher and the level of adiponectin was lower in diabetic patients than in nondiabetic patients. Sitagliptin therapy for 3 and 6 months significantly reduced plasma levels of sP-selectin, sE-selectin, sVCAM-1, and MCP-1 relative to baseline, while significantly increasing adiponectin levels. sRAGEs did not exhibit a statistical significance, although there was an increasing tendency. Furthermore, the reductions in sP-selectin, sE-selectin, sVCAM-1, and MCP-1 during sitagliptin therapy were significantly greater in responders, defined as patients with a significant increase in adiponectin levels, than in nonresponders. In contrast, responders showed a significant increase in the plasma concentration of sRAGEs.
Conclusion
Sitagliptin shows an adiponectin-dependent anti-atherothrombotic effect, which may be beneficial for primary prevention of atherothrombosis, in patients with type 2 diabetes.
Introduction
The development of atherosclerosis in patients with type 2 diabetes mellitus (T2DM) may be due to hypercoagulability and platelet hyperaggregability,Citation1,Citation2 along with increased levels of platelet activation markers. These changes have been associated with increased risks of cardiovascular events.Citation3,Citation4 Diabetes is also characterized by the increased expression of cell adhesion molecules,Citation5 which may play a role in the microvascular complications of this disease. The first step in the process of leukocyte migration into the subendothelial space is the adhesion of circulating leukocytes to the endothelium, which may involve adhesion molecules such as P-selectin, E-selectin, and vascular cell adhesion molecule-1 (VCAM-1). These molecules have been associated with vascular complications, with higher serum levels of soluble P-selectin (sP-selectin), soluble E-selectin (sE-selectin), and soluble VCAM-1 (sVCAM-1) in patients with diabetes.Citation6,Citation7 Patients with postprandial hyperglycemia often have accompanying postprandial hyperinsulinemia. However, the postprandial increase in blood glucose level itself is now considered a risk factor for the progression of atherosclerosis.Citation8
Adiponectin, the most abundant adipose tissue-specific protein, is exclusively expressed in and secreted by adipose tissue.Citation9 Plasma adiponectin concentrations, which are normally high, have been shown to be reduced in obese individualsCitation9,Citation10 and those with T2DM,Citation11 and to be closely related to insulin sensitivity.Citation12 Adiponectin has been shown to stimulate nitric oxide (NO) production in vascular endothelial cells, ameliorating endothelial function.Citation13,Citation14 These observations suggest that the anti-atherogenic properties of adiponectin may involve its NO-dependent antiplatelet effects.
Advanced glycation end products (AGEs), the final products of the nonenzymatic glycation of proteins,Citation15 bind to activate receptor for AGEs (RAGEs), enhancing inflammation.Citation16,Citation17 RAGEs also promote chronic inflammation, as observed in diabetes and atherosclerosis.Citation18,Citation19 In contrast, soluble RAGE (sRAGE), a RAGE isoform lacking the transmembrane domain, is an inhibitor of AGE–RAGE-mediated pathological effects.Citation20 For example, sRAGE was shown to protect blood vessels against AGE–RAGE-mediated microvascular damage in patients with T2DM.Citation21
Dipeptidyl peptidase-4 is an enzyme involved in the degradation of the intact (active) incretin hormones, glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic peptide, to their inactive metabolites. GLP-1 and glucose-dependent insulinotropic peptide are released by the intestine into the circulation in response to a meal, and both the hormones increase glucose-dependent insulin secretion by inhibiting the degradation of active incretins. Sitagliptin is the first dipeptidyl peptidase-4 inhibitor that increases active incretin concentrations, thereby enhancing their glucoregulatory effects.Citation22–Citation24 Many clinical trials have shown that sitagliptin reduces glycated hemoglobin (HbA1c) concentrations.Citation25,Citation26 However, the effects of sitagliptin on sP-selectin, adiponectin, and sRAGE in patients with T2DM are poorly understood. To determine whether sitagliptin has anti-atherosclerotic effects, we assessed its effects on sP-selectin, adiponectin, and sRAGE concentrations in patients with T2DM.
Materials and methods
Patients
The study cohort included 72 nondiabetic and 113 diabetic patients (), selected from among those admitted to our hospital between April 2011 and March 2014 for the treatment of hypertension, hyperlipidemia, and diabetes. The study protocol was approved by the Institutional Review Board of Kansai Medical University, and written informed consent was obtained from each patient. Individuals were excluded if they had a history (within 3 months prior to enrollment) of inflammatory, coronary artery, or cerebrovascular disease; or if they had clinically detectable hepatic dysfunction (elevated transaminases), infection (fever or an elevated white blood cell count), or malignancy (detected on ultrasound or computed tomography). Of the included patients, 20 were taking aspirin because of a previous cerebral infarction or angina pectoris, 69 were taking angiotensin II receptor blockers, 47 were taking Ca antagonists, and 48 were taking statins (). The doses of these drugs were not adjusted, and there were no other changes to drug therapy, during the present study.
Table 1 Demographic and clinical characteristics of the diabetic patients and nondiabetic controls
Study design
Patients were eligible for sitagliptin monotherapy if their diet/exercise therapy had continued unchanged for 3 months. Patients who had used a biguanide (metformin), a sulfonylurea (glibenclamide or glimepiride), a thiazolidinedione (glitazone), or insulin for 3 months were eligible for combination therapy with that agent plus sitagliptin. Patients with almost normal renal function (serum creatinine [S-CRTN] <2.0 mg/dL) were administered sitagliptin (50 mg/day) once daily for 6 months. Clinical and biochemical data were obtained before and 3 and 6 months after starting sitagliptin treatment.
Measurement of soluble molecules and adiponectin
Blood samples from patients and controls under fasting conditions were collected into tubes with or without sodium citrate and allowed to clot at room temperature for a minimum of 1 hour. Citrated plasma or serum was isolated by centrifugation at 1,000× g for 20 minutes at 4°C and stored at −30°C until analyzed. Plasma concentrations of sP-selectin, sE-selectin, sVCAM-1, and monocyte chemoattractant protein-1 (MCP-1) were measured using monoclonal antibody-based ELISA kits (Invitrogen Inc., Camarillo, CA, USA), plasma adiponectin was measured with adiponectin ELISA kits (Otsuka Pharmaceutical Co., Ltd. Tokyo, Japan), and serum sRAGE was measured using sRAGE ELISA kits (Quantikine; R&D Systems, Minneapolis, MN, USA). The recombinant products and standard solutions provided with each kit were used as positive controls in each assay, and all the procedures were performed according to the manufacturers’ instructions.
Diabetic patients were divided into two subgroups based on their adiponectin responses to sitagliptin treatment. Responders were defined as patients showing a ≥1.5-fold increase in plasma adiponectin levels, relative to baseline, after sitagliptin treatment, whereas nonresponders were defined as those with a <1.5-fold increase in plasma adiponectin.
Statistics
Data were expressed as mean ± standard deviation and were analyzed using multivariate regression analysis, as appropriate. Between-group comparisons were analyzed using the Newman–Keuls test and Scheffe’s test. The correlation between uric acid concentration and continuous variables was assessed using multivariate linear regression analysis. The significance of differences among variables was determined by analysis of variance (ANOVA). P-values less than 0.05 were considered statistically significant. All the analyses were performed using the Stat Flex program (V 6.0).
Results
Patient demographic and clinical characteristics were similar in the diabetic and nondiabetic groups, except for fasting blood glucose and HbA1c concentrations ().
The levels of blood urea nitrogen, S-CRTN, C-reactive protein, MCP-1, sP-selectin, sE-selectin, and sVCAM-1 were higher in diabetic than in nondiabetic patients (). However, adiponectin was lower in diabetic than in nondiabetic patients ().
Table 2 Plasma levels of soluble factors, chemokines, and adiponectin in the nondiabetic controls and diabetic patients
Using univariate and multivariate regression analyses, we investigated the associations between 16 variables and HbA1c concentration in diabetic patients (). Univariate analysis showed that body mass index, angina pectoris, low-density lipoprotein cholesterol, sP-selectin, sE-selectin, sVCAM-1, MCP-1, sRAGE, and adiponectin were factors significantly associated with HbA1c, whereas sP-selectin, sVCAM-1, MCP-1, and adiponectin were significantly correlated with HbA1c in multivariate analysis.
Table 3 Multiregression analysis on HbA1c in diabetic patients
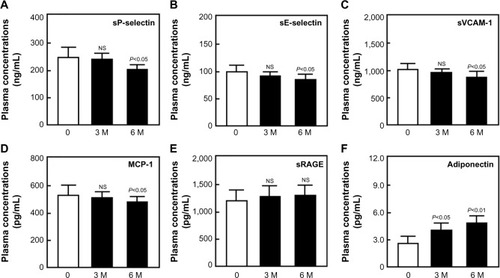
Renal function was almost normal (S-CRTN <2.0 mg/dL) in 65 of the 113 diabetic patients. Administration of sitagliptin to these 65 patients for 3 months significantly reduced fasting blood glucose and HbA1c (data not shown), and administration for 6 months significantly reduced plasma concentrations of sP-selectin, sE-selectin, sVCAM-1, and MCP-1 relative to baseline (P<0.05 each; ). Sitagliptin treatment significantly increased adiponectin concentrations after 3 (P<0.05) and 6 (P<0.01) months relative to baseline (). Sitagliptin also increased sRAGE concentration relative to baseline, although the differences were not statistically significant ().
Figure 1 Plasma concentrations of sP-selectin (A), sE-selectin (B), sVCAM-1 (C), MCP-1 (D), sRAGE (E), and adiponectin (F) before and after sitagliptin treatment in diabetic patients.
Abbreviations: sP-selectin, soluble P-selectin; sE-selectin, soluble E-selectin; sVCAM-1, soluble vascular cell adhesion molecule-1; MCP-1, monocyte chemoattractant protein-1; sRAGE, soluble receptor for advanced glycation end product; NS, not significant; SD, standard deviation.
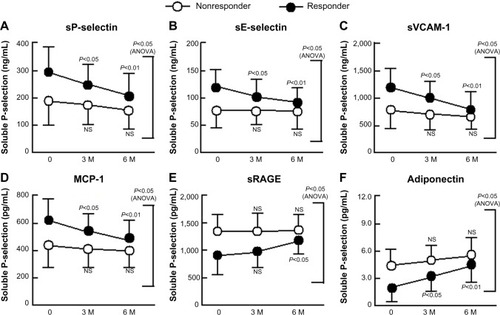
We divided diabetic patients into two subgroups according to their adiponectin response to sitagliptin treatment. Responders showed significant reductions in plasma concentrations of sP-selectin, sE-selectin, sVCAM-1, and MCP-1 relative to baseline (P<0.01 for each; ), and all the four concentrations were significantly lower in responders than in nonresponders after 6 months of sitagliptin treatment (two-factor ANOVA; P<0.05 each). However, responders showed a significant increase in plasma concentration of sRAGE and adiponectin ().
Figure 2 Changes in sP-selectin (A), sE-selectin (B), sVCAM-1 (C), MCP-1 (D), sRAGE (E), and adiponectin (F) in response to treatment with sitagliptin of patients with type 2 diabetes with and without significant improvements in adiponectin.
Abbreviations: sP-selectin, soluble P-selectin; ANOVA, analysis of variance; NS, not significant; sE-selectin, soluble E-selectin; sVCAM-1, soluble vascular cell adhesion molecule-1; MCP-1, monocyte chemoattractant protein-1; sRAGE, soluble receptor for advanced glycation end product; SD, standard deviation.
Discussion
Postprandial hyperglycemia is an early manifestation of T2DM and is caused by the loss of early-phase insulin response.Citation27 Chronic hyperglycemia is also associated with accumulation of AGEs.Citation28 Diabetic complications may be due to inflammatory and oxidative stresses to endothelial cells caused by AGEs, mainly through RAGE. Circulating concentrations of both cleaved-type sRAGE and endogenous secretory RAGE (esRAGE) are increased in diabetic patients.Citation29–Citation31 sRAGE concentrations are linked to reactions between AGE and RAGE, and are affected by various clinical features in T2DM.Citation31–Citation33 Interestingly, esRAGE concentration was reported to be negatively correlated with the development of atherosclerosis in patients with T2DM,Citation34 with esRAGE increasing after statin treatment.Citation35 This study showed that sitagliptin treatment tended to increase sRAGE, although the increase was not statistically significant. However, adiponectin responders showed a significant increase in sRAGE following sitagliptin treatment, in agreement with previous findings.Citation35 In addition, sitagliptin treatment also reduced the concentrations of the endothelial cell activation markers sE-selectin and sVCAM-1 in patients with diabetes, suggesting that sitagliptin has an effect on atherosclerosis related to sRAGE and endothelial cell activation. Further investigations are needed, because our assay of sRAGE could not measure esRAGE specifically.
Postprandial hyperglycemia in diabetic patients may be associated with the activation of endothelial cells. Postprandial hyperglycemia induces oxidative stress via various biochemical pathways, generating superoxide, which reacts with NO to form peroxynitrite.Citation36 The resulting decreases in NO concentrations and activity may accelerate vascular inflammation by enhancing the expression of various cytokines and growth factors.Citation37 Treatment with sitagliptin was shown to significantly reduce body mass index and waist circumference,Citation38,Citation39 as well as to prevent nephropathic complications in patients with T2DM.Citation40 Thus, our results suggest that sitagliptin causes the improvement of endothelial dysfunction and atherosclerosis.
Plasma concentrations of adiponectin are lower in obese than in nonobese individualsCitation9 and are closely related to whole-body insulin sensitivity.Citation11 Plasma adiponectin concentrations are also reduced in patients with T2DM.Citation11 Adiponectin has been reported to suppress the attachment of monocytes to endothelial cellsCitation37 and to play a role in protection against vascular injury, suggesting that hypoadiponectinemia is associated with endothelial dysfunction.Citation41 Hypoadiponectinemia has also been associated with platelet activation. The level of NO, which regulates platelet activation, is reduced by hypoadiponectinemia, because adiponectin stimulates NO production by vascular endothelial cells.Citation13,Citation14,Citation42 Thus, platelet activation due to low NO concentrations occurs in individuals with hypoadiponectinemia. Therefore, the sitagliptin-induced increase in adiponectin may have an antiplatelet effect by enhancing NO production,Citation43 as shown by the significant reduction in sP-selectin in diabetic patients treated with sitagliptin.
Various posttranslational modifications, including the glycosylation of lysine residues, have been shown to be necessary for the multimerization of adiponectin.Citation44 These intracellular posttranslational processes may be affected by hyperglycemia, leading to functional impairment at the organ level in diabetic patients.Citation45–Citation47 Therefore, the sitagliptin-induced improvement in postprandial hyperglycemia may alter the posttranslational modification of adiponectin.
This study also found that sitagliptin reduced sP-selectin, sE-selectin, sVCAM-1, and MCP-1 concentrations. Grouping of the diabetic patients into two subgroups according to the adiponectin response to sitagliptin treatment showed that the plasma levels of these four markers were significantly reduced in adiponectin responders, suggesting that sitagliptin induces adiponectin-dependent improvements in the plasma levels of sP-selectin, sE-selectin, sVCAM-1, and MCP-1 in diabetic patients.
The exact mechanism by which sitagliptin treatment increases circulating adiponectin concentrations remains unclear, although the gut-derived incretin hormone GLP-1 is likely involved. GLP-1-based therapies have been shown to reduce glucose concentrations and have antiobesity effects in patients with T2DM.Citation48 Sitagliptin was found to enhance the secretion of active GLP-1, suggesting that the antidiabetic properties of sitagliptin depend, in part, on GLP-1.Citation49 In addition, GLP-1 was shown to promote adiponectin secretion.Citation50,Citation51 Our findings suggest that the effects of sitagliptin on sP-selectin, sE-selectin, sVCAM-1, and MCP-1 concentrations depend on adiponectin. Therefore, sitagliptin may inhibit the progression of atherothrombosis by promoting adiponectin-dependent reductions in plasma sP-selectin, sE-selectin, sVCAM-1, and MCP-1. However, further studies are necessary to elucidate the effects of sitagliptin itself on adiponectin production.
This study had two potential strengths. First, despite the treatment of many T2DM patients with sitagliptin, no previous study had assessed the effects of sitagliptin on serum markers of the disease. Second, it showed that investigation of appropriate serum markers can be used to address atherosclerosis. However, this study also had several limitations. First, changes in clinical parameters such as body mass index were not routinely recorded. Second, we could not identify causative differences in groups of adiponectin responders and nonresponders. Responders had poorer serum values at the onset of sitagliptin treatment, suggesting a genetic/environmental factor associated with these differences. Third, we could not clarify the significance of sRAGE relative to atherosclerosis after sitagliptin treatment. Confirmation of these findings in larger and more particular studies would be useful.
In conclusion, sitagliptin increased circulating sRAGE and adiponectin concentrations in patients with T2DM. In addition, sitagliptin treatment reduced sP-selectin, sE-selectin, sVCAM-1, and MCP-1 levels. Sitagliptin may be beneficial in the primary prevention of atherothrombosis in patients with T2DM.Citation52 However, large clinical trials are required to test this hypothesis.
Acknowledgments
This study was partly supported by a grant from the Japan Foundation of Neuropsychiatry and Hematology Research, a Research Grant for Advanced Medical Care from the Ministry of Health and Welfare of Japan, and a Grant (13670760 to SN) from the Ministry of Education, Science and Culture of Japan.
Disclosure
The authors report no conflicts of interest in this work.
References
- SchaferAIThe hypercoagulable statesAnn Intern Med19851026814 8283158262
- FradeLJGde la CalleHAlavaINavarroJLCreightonLJGaffineyPJDiabetes as a hypercoagulable state: its relationship with fibrin fragments and vascular damageThromb Res1987475533 5403118498
- SeshasalSRKaptogeSThompsonADiabetes mellitus, fasting glucose, and risk of cause-specific deathN Engl J Med20113649829 84121366474
- ScheenAJCardiovascular effects of gliptinsNat Rev Cardiol201310273 8423296071
- CominaciniLPasiniAFGarbinUElevated levels of soluble E-selectin in patients with IDDM and NIDDM: relation to metabolic controlDiabetologia19953891122 11248591829
- LimYCSnappKKansasGSCamphausenRDingHLuscinskasFWImportant contributions of P-selectin glycoprotein ligand-1-mediated secondary capture to human monocyte adhesion to P-selectin, E-selectin, and TNF-α-activated endothelium under flow in vitroJ Immunol199816152501 25089725249
- NomuraSShouzuAOmotoSNishikawaMFukuharaSSignificance of chemokines and activated platelets in patients with diabetesClin Exp Immunol20001213437 44310971508
- NakagamiTHyperglycaemia and mortality from all cause and from cardiovascular disease in five populations of Asian originDiabetologia2004473385 39414985967
- OuchiNKiharaSAritaYAdiponectin, an adipocyte-derived plasma protein, inhibits endothelial NF-kappa B signaling through a cAMP-dependent pathwayCirculation2000102111296 130110982546
- AritaYKiharaSOuchiNParadoxical decrease of an adipose-specific protein, adiponectin, in obesityBiochem Biophys Res Com1999257179 8310092513
- HottaKFunahashiTAritaYPlasma concentrations of a novel, adipose-specific protein, adiponectin, in type 2 diabetes patientsArterioscler Thromb Vasc Biol20002061595 159910845877
- WeyerCFunahashiTTanakaSHypoadiponectinemia in obesity and type 2 diabetes: close association with insulin resistance and hyperinsulinemiaJ Clin Endocrinol Metab20018651930 193511344187
- ChenHMontagnaniMFunahashiTShimomuraIQuonMJAdiponectin stimulates production of nitric oxide in vascular endothelial cellsJ Biol Chem20032784545021 4502612944390
- HattoriYSuzukiMHattoriSKasaiKGlobular adiponectin upregulates nitric oxide production in vascular endothelial cellsDiabetologia200346111543 154914551684
- ZiemanSJMelenovskyVClattenburgLAdvanced glycation end product crosslink breaker (alagebrium) improves endothelial function in patients with isolated systolic hypertensionJ Hypertens2007253577 58317278974
- HofmannMADrurySFuCRAGE mediates a novel proinflammatory axis: a central cell surface receptor for S100/calgranulin polypeptidesCell1999977889 90110399917
- HaslbeckKMSchleicherEBierhausAThe AGE/RAGE/NF-kB pathway may contribute to the pathogenesis of polyneuropathy in impaired glucose tolerance (IGT)Exp Clin Endocrinol Diabetes20051135288 29115926115
- BierhausANawrothPPMultiple levels of regulation determine the role of the receptor for AGE (RAGE) as common soil in Kalousova M inflammation, immune responses and diabetes mellitus and its complicationsDiabetologia200952112251 226319636529
- YanSFYanSDRamasamyRSchmidtAMTempering the wrath of RAGE: an emerging therapeutic strategy against diabetic complications, neurodegeneration, and inflammationAnn Med2009416408 42219322705
- KalousováMJáchymováMMestekOReceptor for advanced glycation end products-soluble form and gene polymorphisms in chronic haemodialysis patientsNephrol Dial Transplant20072272020 202617347281
- GrossinNWautierMPMeasTGuillausseauPJMassinPWautierJLSeverity of diabetic microvascular complications is associated with a low soluble RAGE levelDiabetes Metab2008344392 39518701333
- HermanGAStevensCVan DyckKPharmacokinetics and pharmacodynamics of sitagliptin, an inhibitor of dipeptidyl peptidase IV, in healthy subjects: results from two randomized, double-blind, placebo-controlled studies with single oral dosesClin Pharmacol Ther2005786675 68816338283
- HermanGABergmanAStevensCEffect of single oral doses of sitagliptin, a dipeptidyl peptidase-4 inhibitor, on incretin and plasma glucose levels after an oral glucose tolerance test in patients with type 2 diabetesJ Clin Endocrinol Metab200691114612 461916912128
- HeransenKKipnesMLuoEEfficacy and safety of the dipeptidyl peptidase-4 inhibitor, sitagliptin, in patients with type 2 diabetes mellitus inadequately controlled on glimepiride alone or on glimepiride and metforminDiabetes Obes Metab200795733 74517593236
- IwamotoYTaniguchiTNonakaKDose-ranging efficacy of sitagliptin, a dipeptidyl peptidase-4 inhibitor, in Japanese patients with type 2 diabetes mellitusEndocr J2010575383 39420332588
- KimSAShimWHLeeEHPredictive clinical parameters for the therapeutic efficacy of sitagliptin in Korean type 2 diabetes mellitusDiabetes Metab J2011353159 16521738898
- PolonskyKSGivenBDHirschLJAbnormal patterns of insulin secretion in non-insulin-dependent diabetes mellitusN Engl J Med1988318191231 12393283554
- WendtTTanjiNGuoJGlucose, glycation, and RAGE: implications for amplification of cellular dysfunction in diabetic nephropathyJ Am Soc Nephrol20031451383 139512707408
- Soro-PaavonenAWatsonAMLiJReceptor for advanced glycation end products (RAGE) deficiency attenuates the development of atherosclerosis in diabetesDiabetes20085792461 246918511846
- ThomasMCSöderlundJLehtoMSoluble receptor for AGE (RAGE) is a novel independent predictor of all-cause and cardiovascular mortality in type 1 diabetesDiabetologia201154102669 267721607631
- KoyamaHShojiTYokoyamaHPlasma level of endogenous secretory RAGE is associated with components of the metabolic syndrome and atherosclerosisArterioscler Thromb Vasc Biol200525122587 259316224056
- GohdaTTanimotoMMoonJYIncreased serum endogenous secretory receptor for advanced glycation end-product (esRAGE) levels in type 2 diabetic patients with decreased renal functionDiabetes Res Clin Pract2008812196 20118550199
- SembaRDFerrucciLFinkJCAdvanced glycation end products and their circulating receptors and level of kidney function in older community-dwelling womenAm J Kidney Dis200953151 5818789567
- KatakamiNMatsuhisaMKanetoHYamasakiYSerum endogenous secretory RAGE levels are inversely associated with carotid IMT in type 2 diabetic patientsAtherosclerosis2007190122 2316876804
- TamHLShiuSWWongYChowWSBetteridgeDJTanKCEffects of atorvastatin on serum soluble receptors for advanced glycation end-products in type 2 diabetesAtherosclerosis20102091173 17719733353
- KurowskaEMNitric oxide therapies in vascular diseasesCurr Pharm Des200283155 16611812265
- OuchiNKiharaSAritaYNovel modulator for endothelial adhesion molecules: adipocyte-derived plasma protein, adiponectinCirculation1999100252473 247610604883
- MaedaHKubotaATanakaYTakeuchiYMatsubaIThe safety, efficacy and predictors for HbA1c reduction of sitagliptin in the treatment of Japanese type 2 diabetesDiabetes Res Clin Pract2012951e20 e2222055835
- HongESKhangARYoonJWComparison between sitagliptin as add-on therapy to insulin and insulin dose-increase therapy in uncontrolled Korean type 2 diabetes: CSI studyDiabetes Obes Metab2012149795 80222443183
- MoriHOkadaYAraoTTanakaYSitagliptin improves albuminuria in patients with type 2 diabetes mellitusJ Diabetes Invest201453313 319
- ShimabukuroMHigaNAsahiTHypoadiponectinemia is closely linked to endothelial dysfunction in manJ Clin Endocr Metab20038873236 324012843170
- NomuraSShouzuAOmotoSCorrelation between adiponectin and reduction of cell adhesion molecules after pitavastatin treatment in hyperlipidemic patients with type 2 diabetes mellitusThromb Res2008122139 4517920663
- GuptaAKVermaAKKailashiyaJSitagliptin: antiplatelet effect in diabetes and healthy volunteersPlatelets2012238565 57022950787
- WangYLamKSChanLPost-translational modifications of the four conserved lysine residues within the collagenous domain of adiponectin are required for the formation of its high molecular weight oligomeric complexJ Biol Chem20062812416391 1640016621799
- FülöpNMarchaseRBChathamJCRole of protein O-linked N-acetyl-glucosamine in mediating cell function and survival in the cardiovascular systemCardiovasc Res2007732288 29716970929
- FülöpNMasonMMDuttaKImpact of type 2 diabetes and aging on cardiomyocyte function and O-linked N-acetylglucosamine levels in the heartAm J Physiol Cell Physiol20072924C1370 C137817135297
- OchiaiHOokaHShidaCIshikawaTInoueDOkazakiRAcarbose treatment increases serum total adiponectin levels in patients with type 2 diabetesEndocr J2008553549 55618480556
- KnopFKVilsbøllTLarsenSMadsbadSHolstJJKrarupTNo hypoglycemia after subcutaneous administration of glucagons-like peptide-1 in lean type 2 diabetic patients and in patients with diabetes secondary to chronic pancreatitisDiabetes Care20032692581 258712941722
- IshibashiYMatsuiTTakeuchiMYamagishiSSitagliptin augments protective effects of GLP-1 against advanced glycation end product receptor axis in endothelial cellsHorm Metab Res2011431731 73421932180
- PocaiACarringtonPEAdamsJRGlucagon-like peptide 1/glucagons receptor dual agonism reverses obesity in miceDiabetes200958102258 226619602537
- Kim Chung leTHosakaTYoshidaMExendin-4, a GLP-1 receptor agonist, directly induces adiponectin expression through protein kinase A pathway and prevents inflammatory adipokine expressionBiochem Biophys Res Comm20093903613 61819850014
- DhindsaSJialalIPotential anti-atherosclerotic effects of dipeptidyl peptidase-4 inhibitors in type 2 diabetes mellitusCurr Diabetes Rep2014142463
