Abstract
Metabolic abnormalities are common in cancers, and targeting metabolism is emerging as a novel therapeutic approach to cancer management. Pituitary adenoma (PA) is a type of benign tumor. Impairment of tumor cells’ metabolism in PA seems not to be as apparent as that of other malignant tumor cells; however, aberrant hormone secretion is conspicuous in most PAs. Hormones have direct impacts on systemic metabolism, which in turn, may affect the progression of PA. Nowadays, conventional therapeutic strategies for PA do not include modalities of adjusting whole-body metabolism, which is most likely due to the current consideration of the aberrant whole-body metabolism of PA patients as a passive associated symptom and not involved in PA progression. Because systemic metabolic abnormalities are presented by 22.3%–52.5% PA patients and are closely correlated with disease progression and prognosis, we propose that assessment of metabolic status should be emphasized during the treatment of PA and that control of metabolic abnormalities should be added into the current therapies for PA.
Introduction and background
Tumor cross talks with its environment, macroenvironment, and microenvironment. The macroenvironment of tumors is a concept relative to that of tumor microenvironment. Al-Zhoughbi et alCitation1 defined “tumor macroenvironment” as the pathological interaction between the tumor cells, as well as the tumor microenvironment, with other organs and systems of the body, which emphasizes the influence of tumors on the whole body. A number of studies have been done to understand the role of tumor microenvironment in tumor progression. Changes in microenvironment such as inflammatory cytokines, angiogenic factors, integrins, matrix metalloproteinases, and hypoxis are known to play important roles in tumor growth.Citation2 Cytokines released by tumor cells can, on the one hand, remodel the microenvironment, and, on the other hand, also influence the homeostasis of the whole body. One of the most consequential changes of the whole body in response to impacts originating from tumors is the metabolic imbalance in tumor patients.
Alterations in metabolisms of protein, lipid, and carbohydrate are often associated with tumor progression. Fearon et alCitation3 found that in lung or colorectal cancer patients, the whole-body protein synthesis was decreased. Increase of muscle protein degradation was observed in noncachectic sarcoma patients, and leucine metabolic abnormalities are specific to tumor growth in humans with high-grade sarcomas.Citation4 Tumor can also directly affect the profile of plasma-free amino acids. Studies on different types of cancers such as breast cancer and non-small cell lung cancer have shown that each type of these cancers has its specific plasma-free amino acid profiles.Citation5,Citation6 Lipid is another important dysregulated metabolite in cancer patients. Dysregulated lipid metabolism is considered a hallmark of cancer.Citation7 Cancer cells can use exogenous fatty acids for membrane synthesis and oncogenic signaling, which can ultimately influence the plasma lipid profile.Citation8 Compared with body mass index (BMI)-matched healthy controls, hematological neoplasia patients exhibit lower concentrations of plasma cholesterol, high-density lipoprotein cholesterol (HDL-C), and low-density lipoprotein cholesterol (LDL-C), and higher concentrations of triglyceride (TG).Citation9 These observations confirm the influence of tumor on the whole-body metabolism.
The whole-body metabolic imbalance may affect the progression of cancer. Progression of cancer is also found to be associated with the so-called metabolic syndrome, which is defined as a syndrome composed of obesity, dyslipidemia, hyperglycemia associated with insulin resistance, and development of diabetes mellitus.Citation10 Obesity has been reported as a cancer risk in colon cancer and rectal cancer,Citation11 pancreatic cancer,Citation12 ovarian cancer,Citation13 breast cancer,Citation14 and liver cancer.Citation15 Recently, Bhaskaran et alCitation16 conducted a population-based cohort study on 5.24 million adults in the United Kingdom, and found that BMI was associated with 17 most common cancers, although the effects varied substantially by tumor site. Hyperglycemia is also linked to cancers independent of BMI. Levine et al’sCitation17 study in 1990 revealed the relationship of postload plasma glucose and 12-year cancer mortality, and the conclusion was that hyperglycemia was related to cancer mortality in men, but not in women. A significantly increased risk of pancreatic cancer and malignant melanoma was found in both male and female hyperglycemia patients.Citation18 Dyslipidemia is another metabolic factor associated with risks of several cancers. Dyslipidemia presents low HDL-C, high LDL-C, and high serum TG levels. Low HDL-C level was associated with non-Hodgkin lymphoma,Citation19 lung cancer,Citation20 prostate cancer,Citation21 and breast cancer.Citation22 High serum TG was associated with postmenopausal breast and prostate cancer in a few studies.Citation23,Citation24 The pathophysiological mechanisms by which metabolic abnormalities participate in tumor progression is complicated. Braun et alCitation25 summarized the potential causal factors. The key factors include insulin and insulin-like growth factor (IGF) system; inflammatory factors such as IL-6, TNF-α, and C-reactive protein; transcription factors such as NF-κB, HIF-1α, PPARs; and other factors such as COX-2, leptin, and adiponectin.Citation25 In general, these observations indicate that tumor-associated whole-body metabolic alterations are not a passive element, but an active participant in tumor progression. Thus, correcting the whole-body imbalance of metabolism may be an effective approach for tumor management.
Pituitary adenoma (PA) is known to be often associated with whole-body imbalance of metabolism due to its aberrant secretion of hormones. In addition to the mass effects, the oversecretion of pituitary hormones is another key clinical manifestation. Even though 39% of PA are hormone silent, namely the nonfunctioning pituitary adenomas (NFPAs),Citation26 yet because of the mass effects of NFPAs, the portal vessels and pituitary stalk can be compressed, which can lead to a decrease in dopamine delivery that can cause hyperprolactinemia in nearly 35% of NFPA patients.Citation27
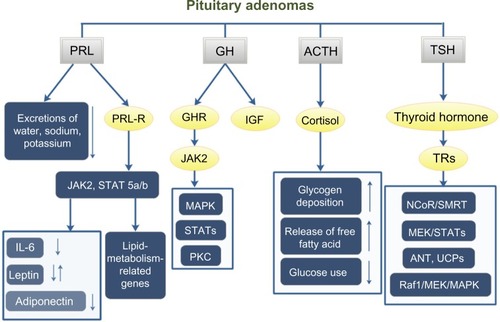
Pituitary hormones can affect whole-body metabolism directly or indirectly. The most common hormone-secreting pituitary tumors are prolactinomas (40%),Citation28 and 63% of prolactinoma patients have weight gain.Citation29 Prolactin can affect whole-body metabolism by regulating key enzymes and transporters that are associated with lipid and glucose metabolism. Prolactin can act as an autocrine or paracrine factor in human adipose tissue. Prolactin can either bind to its receptor, which induces the activation of the downstream pathways such as dimerization and phosphorylation of JAK2, recruitment of STAT 5a/b, finally alters the transcription of lipid-metabolism-related genes and the secretion of IL-6, adiponectin, and leptin.Citation30 In addition, prolactin can also reduce the excretions of water, sodium, and potassium from the kidney.Citation31 Growth hormone (GH) is another hormone that can significantly affect the whole-body metabolism. The predominant effect of GH is to stimulate lipid oxidation and lipolysis, switching energetic substrate from glucose and protein to lipid.Citation32 In acromegaly patients, hyperinsulinemia and impaired glucose tolerance are quite common. In an acromegaly registry clinical study conducted in France, the prevalence of diabetes in these patients was 22.3%,Citation33 and another study reported a 52.5% prevalence of diabetes in acromegaly patients.Citation34 Additionally, impaired glucose metabolism was also found in 66% of acromegaly patients.Citation35 GH acts through two pathways: one is to bind to GH receptors and then activate JAK2, MAPK, and STATs;Citation36 the other is to induce the production of IGFs that can stimulate proteoglycan, glycosaminoglycan and protein synthesis.Citation37 Adrenal corticosteroid is closely related to whole-body metabolic imbalance. Adrenocorticotropic hormone (ACTH) regulates the secretion of adrenal gland hormones directly. Glucocorticoids can increase blood glucose concentration by affecting glycogen, protein, and lipid metabolisms. Cortisol can stimulate glycogen deposition in the liver, inhibit glucose uptake and use in peripheral tissues, and activate lipolysis in adipose tissue, leading to the release of free fatty acids into circulation. High cortisol levels are thought to be related to the pathogenesis of metabolic syndrome.Citation38 A high prevalence of metabolic syndrome exists in Cushing’s syndrome patients.Citation39 95% of patients have obesity, 75% have hypertension, and 60% have glucose intolerance.Citation40 Prolonged and excessive glucocorticoid exposure and activation of the hypothalamic–pituitary–adrenal axis are involved in the pathogenesis of metabolic syndrome.Citation41 Polymorphisms of human glucocorticoid receptor gene are found to be associated with hypertension and obesity.Citation42 Metabolic syndrome is even considered to be a mild form of Cushing’s syndrome.Citation43 Interestingly, the prevalence of metabolic syndrome in NFPAs is up to 44.7%.Citation39 In a cross-sectional study, TSH in the upper normal range was found to be related to more obese, higher TGs, and increased likelihood of the metabolic syndrome.Citation44 And the thyroid hormone can bind to high-affinity thyroid hormone receptors, which can activate pathways such as NcoR/SMRT, MEK/STATs, ANT, UCPs, and Raf1/MEK/MAPK, thus affecting the synthesis, mobilization, and degradation of protein, carbohydrate, and lipid metabolism.Citation45,Citation46 Leptin is thought to play a role in the regulation of food intake and metabolism. A recent study shows that leptin-treated females and males had significantly elevated serum levels of luteinizing hormone and follicle-stimulating hormone, respectively ().Citation47
Figure 1 PA affects the whole-body metabolism through the pituitary hormones.
Abbreviations: GH, growth hormone; ACTH, adrenocorticotropic hormone; TSH, thyroid-stimulating hormone; GHR, growth hormone receptor; IGF, insulin-like growth factor; TRs, thyroid hormone receptors; PA, pituitary adenoma; NFPAs, nonfunctioning pituitary adenomas; FSH, follicle-stimulating hormone; LH, luteinizing hormone.
Currently, the first-line therapy for PA is surgery, which can be conducted transsphenoidally, transcranially, or in a combination of both. But prolactinomas are an exception.Citation48 Surgery can achieve local control completely or partly by releasing the compression of vital structures. Owing to infiltration to the adjacent structures, tumor tissues can be residual, which may elevate hormone levels and lead to a need for repetitive surgeries or pharmacological treatment. Dopamine agonists and somatostatin analogs are the most widely used medications to treat PA. But some patients with invasive PA are resistant at the beginning or develop resistance to these drugs.Citation28 When surgery and pharmacotherapy have failed, radiotherapy is the third-line therapy.Citation48 With the current procedures of managing PA, the recurrence rate is still not ideal, which is often related to tumor size and cavernous sinus extension.Citation49 In NFPA, the recurrence rate from nondetectable residual tumor is approximately 12%, and from the detectable tumor residuals is approximately 46%.Citation50 With successful surgeries, hormonal control cannot be achieved in every case. In Wang et al’sCitation51 study, the hormonal control rate was 69% of ACTH adenomas, 66% of GH adenomas, 85% of PRL adenomas, and 86% of TSH adenomas.
Imbalance of body metabolism can affect the prognosis of PA. A high rate of postoperative or postmedical therapy imbalance of body metabolism exists. Often, after 6 months of treatment with dopamine agonists, the BMI of prolactinoma patients does not change at all.Citation52 Acromegaly patients with abnormal glucose metabolism need more combined medical therapies than surgery alone.Citation53
Hypothesis
Based on the close association between PA and whole-body metabolic alterations, we propose that it is necessary to assess the status of whole-body metabolism and monitor the metabolic abnormalities before and after surgical or medical therapies, and control of imbalance of body metabolism should be added to the current procedures of treating PA.
Prospects
The metabolic abnormalities of PA patients should not be ignored or just considered as an associated symptom. The effect of pituitary hormones on body metabolism is apparent. But the relationship between the imbalance of whole-body metabolism and prognosis of PA has not gained enough attention. In fact, the prevalence rate of metabolic abnormalities in PA is high. Current therapies cannot relieve the metabolic abnormalities completely, and assessing the changes of metabolic status before and after therapies is not a standard practice. The change of metabolic indexes is a chronic process. Only after 12 months of treatment by dopamine agonists do the prolactinoma patients tend to lose weight, and can cabergoline induce weight loss.Citation52 In the treatment of acromegaly, different drugs affect the body metabolism to different degrees. Somatostatin analog Octreotide failed to change glucose tolerance significantly even though the insulin levels tended to be decreased by the treatment. Pegvisomant, a GH analog and functioning as a specific GH receptor antagonist, can effectively reduce plasma glucose concentrations.Citation54
Metabolic syndrome is a complicated disease. Treatment of metabolic syndrome-associated PA is not simple. Grundy has reviewed the therapies of metabolic syndrome.Citation55 Effective lifestyle therapies, including weight reduction, increasing physical activity, and antiatherogenic diet composition, are the first recommended way to control or slow down the progression of metabolic syndrome. Postponing the initiation of drug therapy as long as possible is preferred.
PA is benign, but has severe clinical complications. The mass effects of tumors can be severe or even fatal such as PA apoplexy, but they can always be remitted immediately by surgeries. Compared with its other life-threatening complications, hormonal disorders seem to be moderate. But even after surgeries, many patients will still suffer endocrine disorders. Postsurgery medical treatments are needed. As metabolic abnormalities are involved in all stages of PA development, the assessment and interference of whole-body metabolism before and after surgery should be considered in order to bring about better outcomes of PA patients.
Disclosure
The authors report no conflicts of interest in this work.
References
- Al-ZhoughbiWHuangJParamasivanGSTumor macroenvironment and metabolismSemin Oncol201441281 29524787299
- FingerECGiacciaAJHypoxia, inflammation, and the tumor microenvironment in metastatic diseaseCancer Metastasis Rev201029285 29320393783
- FearonKCHansellDTPrestonTInfluence of whole body protein turnover rate on resting energy expenditure in patients with cancerCancer Res1988482590 25953356019
- InculetRISteinTPPeacockJLAltered leucine metabolism in noncachectic sarcoma patientsCancer Res1987474746 47493621173
- CascinoAMuscaritoliMCangianoCPlasma amino acid imbalance in patients with lung and breast cancerAnticancer Res199515507 5107763031
- MaedaJHigashiyamaMImaizumiAPossibility of multivariate function composed of plasma amino acid profiles as a novel screening index for non-small cell lung cancer: a case control studyBMC Cancer20101069021176209
- NomuraDKCravattBFLipid metabolism in cancerBiochim Biophys Acta201318311497 149823921253
- LouieSMRobertsLSMulvihillMMLuoKNomuraDKCancer cells incorporate and remodel exogenous palmitate into structural and oncogenic signaling lipidsBiochim Biophys Acta201318311566 157223872477
- Kuliszkiewicz-JanusMMałeckiRMohamedASLipid changes occuring in the course of hematological cancersCell Mol Biol Lett200813465 47418463797
- JaggersJRSuiXHookerSPMetabolic syndrome and risk of cancer mortality in menEur J Cancer2009451831 183819250819
- LarssonSCWolkAObesity and colon and rectal cancer risk: a meta-analysis of prospective studiesAm J Clin Nutr200786556 56517823417
- AuneDGreenwoodDCChanDSBody mass index, abdominal fatness and pancreatic cancer risk: a systematic review and nonlinear dose-response meta-analysis of prospective studiesAnn Oncol201223843 85221890910
- OlsenCMNagleCMWhitemanDCBody size and risk of epithelial ovarian and related cancers: a population-based case-control studyInt J Cancer2008123450 45618449887
- JensenASharifHOlsenJHKjaerSKRisk of breast cancer and gynecologic cancers in a large population of nearly 50,000 infertile Danish womenAm J Epidemiol200816849 5718448441
- LarssonSCWolkAOverweight, obesity and risk of liver cancer: a meta-analysis of cohort studiesBr J Cancer2007971005 100817700568
- BhaskaranKDouglasIForbesHBody-mass index and risk of 22 specific cancers: a population-based cohort study of 5.24 million UK adultsLancet20143849945755 76525129328
- LevineWDyerARShekelleRBSchoenbergerJAStamlerJPost-load plasma glucose and cancer mortality in middle-aged men and women. 12-year follow-up findings of the Chicago Heart Association Detection Project in IndustryAm J Epidemiol1990154 62
- StattinPBjörOFerrariPProspective study of hyperglycemia and cancer riskDiabetes Care2007561 56717327321
- MorimotoYConroySMOllberdingNJErythrocyte membrane fatty acid composition, serum lipids, and non-Hodgkin’s lymphoma risk in a nested case-control study: the multiethnic cohortCancer Causes Control201231693 170322907421
- Kucharska-NewtonAMRosamondWDSchroederJCHDL-cholesterol and the incidence of lung cancer in the Atherosclerosis Risk in Communities (ARIC) studyLung Cancer200861292 30018342390
- KotaniKSekineYIshikawaSHigh-density lipoprotein and prostate cancer: an overviewJ Epidemiol201323313 31923985823
- Kucharska-NewtonAMRosamondWDMinkPJHDL-cholesterol and incidence of breast cancer in the ARIC cohort studyAnn Epidemiol200818671 67718794007
- GaardMTretliSUrdalPRisk of breast cancer in relation to blood lipids: a prospective study of 31,209 Norwegian womenCancer Causes Control19945501 5097827236
- WuermliLJoergerMHenzSHypertriglyceridemia as a possible risk factor for prostate cancerProstate Cancer Prostatic Dis20058316 32016158078
- BraunSBitton-WormsKLeRoithDThe link between the metabolic syndrome and cancerInt J Biol Sci201171003 101521912508
- BuurmanHSaegerWSubclinical adenomas in postmortem pituitaries: classification and correlations to clinical dataEur J Endocrinol2006154753 75816645024
- GreenmanYSternNNon-functioning pituitary adenomasBest Pract Res Clin Endocrinol Metab200923625 63819945027
- GillamMPMolitchMELombardiGColaoAAdvances in the treatment of prolactinomasEndocr Rev200627485 53416705142
- ColaoASarnoADCappabiancaPGender differences in the prevalence, clinical features and response to cabergoline in hyperprolactinemiaEur J Endocrinol2003148325 33112611613
- Ben-JonathanNHugoERBrandebourgTDLaPenseeCRFocus on prolactin as a metabolic hormoneTrends Endocrinol Metab200617110 11616517173
- HorrobinDFProlactin as a regulator of fluid and electrolyte metabolism in mammalsFed Proc1980392567 25706247216
- RabinowitzDZierlerKLA metabolic regulating device based on the actions of human growth hormone and of insulin, singly and together, on the human forearmNature1963199913 91514079908
- FieffeSMorangeIPetrossiansPDiabetes in acromegaly, prevalence, risk factors, and evolution: data from the French Acromegaly RegistryEur J Endocrinol2011164877 88421464140
- DrevalAVTrigolosovaIVMisnikovaIVPrevalence of diabetes mellitus in patients with acromegalyEndocr Connect2014393 9824692509
- BondanelliMBonadonnaSAmbrosioMRCardiac and metabolic effects of chronic growth hormone and insulin-like growth factor I excess in young adults with pituitary gigantismMetabolism2005541174 118016125529
- WatersMJBrooksAJChhabraYA new mechanism for growth hormone receptor activation of JAK2, and implications for related cytokine receptorsJak-Stat20143e2956925101218
- JonesJIClemmonsDRInsulin-like growth factors and their binding proteins: biological actionsEndocr Rev1995163 347758431
- ParkSBBlumenthalJALeeSYGeorgiadesAAssociation of cortisol and the metabolic syndrome in Korean men and womenJ Korean Med Sci201126914 91821738345
- WebbSMMoDLambertsSWMetabolic, cardiovascular, and cerebrovascular outcomes in growth hormone-deficient subjects with previous cushing’s disease or non-functioning pituitary adenomaJ Clin Endocrinol Metab201095630 63820022992
- Newell-PriceJBertagnaXGrossmanABNiemanLKCushing’s syndromeLancet20063671605 161716698415
- AnagnostisPAthyrosVGTziomalosKKaragiannisAMikhailidisDPClinical review: the pathogenetic role of cortisol in the metabolic syndrome: a hypothesisJ Clin Endocrinol Metab2009942692 270119470627
- MichailidouZCollAPKenyonCJPeripheral mechanisms contributing to the glucocorticoid hypersensitivity in proopiomelanocortin null mice treated with corticosteroneJ Endocrinol2007194161 17017592030
- KrikorianAKhanMIs metabolic syndrome a mild form of Cushing’s syndrome?Rev Endocr Metab Disord201011141 14520711679
- RuhlaSWeickertMOArafatAMA high normal TSH is associated with the metabolic syndromeClin Endocrinol (Oxf)201072696 70120447068
- BassettJHDHarveyCBWilliamsGRMechanisms of thyroid hormone receptor-specific nuclear and extra nuclear actionsMol Cell Endocrinol20032131 1115062569
- PucciEChiovatoLPincheraAThyroid and lipid metabolismInt J Obes Relat Metab Disord200024Suppl 2S109 S11210997623
- BarashIACheungCCWeigleDSLeptin is a metabolic signal to the reproductive systemEndocrinology19961373144 31478770941
- ColaoAGrassoLFSPivonelloRLombardiGTherapy of aggressive pituitary tumorsExpert Opin Pharmacother2011121561 157021434849
- ShirvaniMMotiei-LangroudiRTranssphenoidal surgery for growth hormone-secreting pituitary adenomas in 130 patientsWorld Neurosurg201481125 13023313263
- ChenYWangCDSuZPNatural history of postoperative non-functioning pituitary adenomas: a systematic review and meta-analysisNeuroendocrinology201296333 34222687984
- WangFZhouTWeiSEndoscopic endonasal transsphenoidal surgery of 1,166 pituitary adenomasSurg Endosc20142961270 128025270611
- Dos Santos SilvaCMBarbosaFRLimaGABMI and metabolic profile in patients with prolactinoma before and after treatment with dopamine agonistsObes (Silver Spring)201119800 805
- ChengSAl-AghaRAraujoPBMetabolic glucose status and pituitary pathology portend therapeutic outcomes in acromegalyPLoS One20138e7354324039977
- MøllerNJørgensenJOLEffects of growth hormone on glucose, lipid, and protein metabolism in human subjectsEndocr Rev200930152 17719240267
- GrundySMDrug therapy of the metabolic syndrome: minimizing the emerging crisis in polypharmacyNat Rev Drug Discov20065295 30916582875
