Abstract
Background
Obesity and type 2 diabetes mellitus are associated with elevated risk of limb bone fracture. Incidences of these conditions are on the rise worldwide. Genistein, a phytoestrogen, has been shown by several studies to demonstrate bone-protective properties and may improve bone health in obese type 2 diabetics.
Methods
In this study, we test the effects of genistein treatment on limb bone and growth plate cartilage histomorphometry in obese, hyperglycemic ob/ob mice. Six-week-old ob/ob mice were divided into control and genistein-treated groups. Genistein-treated mice were fed a diet containing 600 mg genistein/kg for a period of 4 weeks. Cross-sectional geometric and histomorphometric analyses were conducted on tibias.
Results
Genistein-treated mice remained obese and hyperglycemic. However, histomorphometric comparisons show that genistein-treated mice have greater tibial midshaft diameters and ratios of cortical bone to total tissue area than the controls. Genistein-treated mice also exhibit decreased growth plate thickness of the proximal tibia.
Conclusion
Our results indicate that genistein treatment affects bone of the tibial midshaft in the ob/ob mouse, independent of improvements in the hyperglycemic state and body weight.
Introduction
More than one-third of US adults are obese.Citation1 According to the Centers of Disease Control and Prevention, obesity-related conditions such as hypertension, heart disease, cancer, and type 2 diabetes mellitus (T2DM) are listed as the leading causes of preventable death. Obesity is also a significant risk factor for the development of diseases that affect the skeleton, such as T2DM. In a report by the World Health Organization, ~85% of patients with T2DM were found to be obese. Patients with T2DM also have elevated rates of limb bone fractures, even when controlling for weight and other risk factors.Citation2–Citation5 T2DM-related loss of cortical limb bone without sufficient compensatory growth of cancellous bone reduces limb bone resistance to bending and elevates bone fracture prevalence in the T2DM population.Citation6,Citation7
The relationship between obesity-related T2DM and skeletal health is multifaceted. One important influence on this relationship is leptin, an adipokine released in response to insulin that helps regulate adipose and bone metabolism.Citation8,Citation9 Leptin resistance due to hyperleptinemia has been implicated in the pathophysiology of insulin resistance and obesity-related T2DM.Citation10–Citation14 Following onset, obese adolescents and obese females with T2DM display significant relative hypoleptinemia in comparison with obese individuals without T2DM.Citation15,Citation16 Ob/ob mice that have a mutation in the gene encoding leptin display elevated insulin levels and T2DM-like hyperglycemia, as well as decreased cortical bone mass and size.Citation17,Citation18 Ob/ob leptin-deficient mice also exhibit reduced metabolism of epiphyseal plates during growth.Citation19 Further confirming the role of leptin in the maintenance of bone health is the observation that leptin administration decreases the presence of adipocytes in rodent bone marrow, leading to a reduced production of inflammatory cytokines that promote osteoclastogenesis, thereby promoting bone formation and the maintenance of bone mass.Citation20 Thus, increased fracture risk associated with T2DM could, in part, be related to the role that leptin plays in skeletal tissue health and maintenance.
It is well documented that estrogen replacement therapy (ERT) has been effective in reducing or reversing postmenopausal bone loss and in reducing the risk of osteoarthritis (OA).Citation21–Citation24 Although the exact mechanisms are still unclear, it has been suggested that estrogen may interact in an indirect fashion with leptin to affect fat utilization.Citation25 However, recent studies have shown that ERT is correlated with increased risk of breast, ovarian, and endometrial cancers, as well as cardiovascular disorders.Citation26,Citation27 Genistein is a phytoestrogen found in soybean products currently being studied as a potential alternative treatment to ERT due to its reported positive effects on postmenopausal depression, bone metabolism, and tumor growth, as well as its use as a dietary supplement to attenuate symptoms of menopause.Citation28–Citation31 Several studies have raised concerns about the effectiveness of genistein treatment on postmenopausal bone maintenance and the relationship of genistein treatment with cancer risk and other health problems, while others suggest that genistein increases bone mineral density (BMD), and any deleterious effects are related to age and dose dependent or drug interactions with cancer interventions.Citation32–Citation36 Longitudinal studies lasting a year or more have shown that genistein treatment may exhibit tumor-suppressing qualities, does not alter the expression of the breast cancer susceptibility genes, and may decrease the prevalence of chromosomal aberrations, along with other health benefits.Citation32,Citation37–Citation40
Structurally, genistein resembles estrogen and can bind to estrogen receptors with an affinity 100- to 1,000-fold less than that of estradiol.Citation41 While the mechanism of action of genistein on bone is not yet fully understood, its positive effects on bone are most likely a direct result of greater binding affinity for ER-α compared with ER-β, leading to the mineralization phase of bone formation.Citation42,Citation43 With previous animal studies demonstrating increased BMD and increased bone fracture strength with genistein treatment, as well as clinical studies demonstrating increased BMD in postmenopausal women with phytoestrogen treatment, it can be presumed that genistein, when ingested, aids in the regulation of bone formation and resorption.Citation44–Citation50 In addition to increased bone loss and fracture risk, diabetes has also been linked to OA. OA is a degenerative disease characterized by the loss of collagens and proteoglycans as the main structural molecules of articular cartilage.Citation51 There has yet to be conclusive evidence labeling diabetes as a risk factor for OA; however, obesity has shown to be a risk factor for both conditions, highlighting the need for interventions that can address both OA and diabetes. Epidemiological studies have shown that the prevalence, incidence, symptoms, and severity of OA increase after menopause and are more severe in women.Citation52–Citation55 These findings indicate a common relationship between OA and estrogen and suggests that genistein treatment could be beneficial to the health of cartilage.
To the best of our knowledge, genistein as a dietary treatment to counteract the negative effects on bone and cartilage seen in obese individuals with T2DM has yet to be explored. The aim of this study is to investigate the effects of genistein treatment on tibial bone remodeling in female leptin-deficient ob/ob mice, which exhibit the type 2 diabetic phenotype.
Methods
Animals
The study utilized female ob/ob mice (B6.V-Lep/J) purchased from Jackson Laboratory (Bar Harbor, ME, USA) at 4–5 weeks of age. These mice display leptin deficiency, severe obesity, hyperinsulinemia, and hyperglycemia in a phenotype similar to human patients with T2DM. All mice consumed food and water ad libitum and were housed in an animal facility maintained at a room temperature of 22°C with a 12-hour light/dark cycle. Body weight was measured weekly during the study, and the general health was monitored biweekly. The Institutional Animal Care and Use Committee at Midwestern University approved the study. Animal care followed the guidelines set forth in the National Institutes of Health’s The Guide for the Care and Use of Laboratory Animals published in 2011.
Study design
We followed the methodology previously described by Al-Nakkash et al.Citation56 Briefly, mice were randomly assigned to a genistein-containing or standard rodent diet for a period of 4 weeks. The genistein-treated group consisted of ten mice, and the control group consisted of nine mice; one tibia was excluded due to fracture during the embedding process. The specially formulated genistein-containing diet was prepared in powder form by Dyets Inc., (Bethlehem, PA, USA), which contains 600 mg genistein/kg. Diets had an energy content estimate of 16.28 kJ/g and contained 20.3 g protein, 66 g carbohydrate, and 5 g fat. Mice were allowed to eat ad libitum. Genistein at the concentration of 600 mg/kg was chosen due to its high bioavailability and maintenance of plasma concentrations comparable to those of soy-based human diets. This is based on earlier work demonstrating that genistein in humans is readily absorbed, with genistein concentrations in plasma of ~2 μmol/L.Citation57–Citation59 Furthermore, it has previously been reported that the consumption of 750 mg/L genistein also generates plasma genistein concentrations of ~2 μmol/L in mice.Citation60 Mice were monitored during the experiment, and no mice exhibited distress indicative of treatment intolerance. After 4 weeks of dietary treatment, mice were sacrificed with CO2 asphyxiation followed by bilateral pneumothorax, and the hind limb bones were dissected. Blood was collected and centrifuged at 14,500 rpm for 5 minutes, stored at −80°C, and later analyzed for glucose using a commercially available kit (Autokit Glucose; Wako Pure Chemical Industries, Osaka, Japan).
Cross-sectional geometry of the tibia
Following sacrifice, tibias were collected, cleaned, and measured using a digital caliper. Tibias were fixed in formalin, dehydrated in 70% and 85% alcohol, and cleared using Histoclear (National Diagnostics, Atlanta, GA, USA), with each step involving two changes 24 hours apart. Infiltration step 1 was performed using Osteo-Bed Resin A, two changes 48 hours apart.
Infiltration step 2 was performed using Catalyzed Osteo-Bed Resin (100 mL Osteo-Bed Resin A, 1.40 g benzoyl peroxide), two changes 48 hours apart. Embedding solution (100 mL Osteo-Bed Resin A, 3.5 g benzoyl peroxide) was prepared ahead of time. Small amounts were poured into 19 vials that were then placed in a bead bath maintained at 32°C for 48 hours. After polymerization, tibias were removed from infiltration, marked at the midshaft, placed in each vile, and covered with embedding solution. The samples were again placed in a bead bath maintained at 32°C for 48 hours to polymerize. A single transverse section was taken at the midshaft of the tibias with an Isomet low-speed saw (Buehler, Lake Bluff, IL, USA). The sections were manually ground to a thickness of 75 μm and digitally captured under microscopy at 4× magnification using a Nikon Eclipse 55i (Nikon Instruments, Melville, NY, USA). Histomorphometric properties of bone were calculated using the ImageJ v1.44 plugin Moment MacroJ (authored by M Warfel and revised by S Serafin). These properties included the minimum and maximum second areas of moment (I min and I max, respectively), polar moment of area (J), and area of cortical bone (CtAr). These cross-sectional geometric data are indicative of a bone’s ability to resist deforming under mechanical loading. CtAr measures resistance to compressive loading, I min and I max measure resistance to bending loads in the minor and major axes, respectively, and J approximates torsional rigidity. Body mass, maximum radius (MaxRad), periosteal perimeter (PsPm), endosteal perimeter (EsPm), the location of the geometric mean on the X-axis (Xbar) and Y-axis (Ybar), medullary area (MAr), total area of the cross-section (TtAr), ratios of cortical area to total area of the cross-section (CtAr/TtAr), and marrow area relative to the total area (MAr/TtAr) were recorded as well.
Histomorphometry of the tibia
After the mice were sacrificed, tibia bones were dissected free of soft tissue and tibial length was measured with a digital caliper. The proximal half of the tibias were decalcified and fixed (Surgipath Decalcifier I; Surgipath Medical Industries, Grayslake, IL, USA) for 4 days. Once decalcified, liquid nitrogen was used to snap-freeze the tibias, and 12 μm-thick coronal sections were made using a cryostat. After staining with aqueous fast green and toluidine blue (Sigma-Aldrich, Co., St Louis, MO, USA), the sections were digitally captured at 40× magnification (Nikon Instruments). Thickness of the growth plate and the calcified growth plate layer were measured in the middling of the joint using ImageJ.
Statistics
Data were shown as mean ± standard error. The t-tests were used to identify significant differences between treatment groups. Kolmogorov–Simonov test for normality and Levene’s tests of homogeneity of the variances were used to ensure assumptions of the t-tests were not violated. Cross-sectional geometric comparisons were adjusted for body mass. SPSS 19 was used for all statistical analyses (StataCorp LP, College Station, TX, USA). Values of P<0.05 were considered significant.
Results
Genistein does not affect body mass or serum glucose
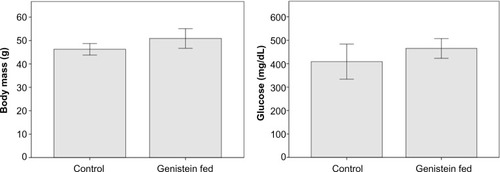
While the genistein-treated mice had lower body mass overall in comparison with the control group, the difference was not significant (P=0.06). Genistein-treated mice had serum glucose levels that were 12.9% lower than controls, but again this difference was not statistically significant (P=0.22, ).
Figure 1 The effects of genistein treatment on body mass and serum glucose in ob/ob mice.
Abbreviation: SE, standard error.
Genistein treatment increased bone mass of the tibia
Genistein treatment had no effect on tibial length (). Nor did genistein treatment affect CtAr, I max, I min, J, or the periosteal and endosteal perimeters. However, CtAr/TtAr and MaxRad were significantly elevated in genistein-treated mice in comparison with controls (P<0.05). MAr was also not significantly elevated or decreased, but MAr/TtAr was significantly reduced in genistein-treated mice in comparison with controls (P<0.05). Xbar was not significantly different between treatment groups, but Ybar was found to be significantly greater in genistein-treated mice (P<0.05), indicating a change in location of the geometric mean of the sections.
Table 1 The effects of genistein treatment on cross-sectional geometric properties of the tibial midshaft in ob/ob mice
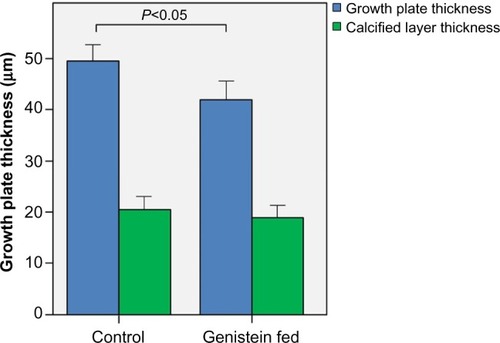
Genistein treatment decreases growth plate thickness in the proximal femur
shows micrographs of proximal tibial growth plates of control and genistein-fed mice. Growth plate thickness in genistein-treated mice was found to be significantly smaller in comparison with control mice (P<0.05). Genistein treatment had no effect on calcified cartilage thickness ().
Figure 2 Micrographs of the proximal tibial growth plate of (A) control and (B) genistein-fed ob/ob mice.
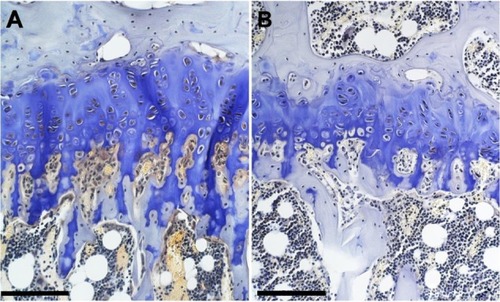
Figure 3 Growth plate thickness in control and genistein fed mice.
Abbreviation: SE, standard error.
Discussion
Earlier studies on the effect of leptin on skeletal tissue were inconsistent; however, a clearer picture is emerging that suggests leptin increases the expression of genes that promotes bone formation and decreases the expression of genes that promotes resorption of bone.Citation10,Citation61–Citation65 As a result, leptin-deficient ob/ob mice exhibit reductions in bone mass and bone formation rates, mimicking the phenotype seen in human diabetic patients.Citation66 Although leptin-deficient mice that undergo leptin treatment recover osteogenic capabilities, human diabetic patients are often found to have hyperleptinemia and are nonresponsive to leptin treatments due to leptin resistance, rendering leptin an ineffective antidiabetic treatment.Citation67,Citation68
ERT is another mode of treatment that has been considered due to its effectiveness at ameliorating hyperandrogenicity and improving glucose homeostasis and lipoprotein profile in postmenopausal women with T2DM.Citation69 ERT has also been documented as reducing or reversing postmenopausal bone loss and reducing the risk of OA.Citation21–Citation24 However, due to the many risk factors that have been linked to chronic ERT, there has been increased interest and research on the effects of phytoestrogens as a potential antidiabetic treatment.Citation29,Citation30 Previous nutritional intervention studies performed on animals and humans suggest that ingestion of phytoestrogens found in soy products can improve glucose control and insulin resistance.Citation70 Genistein is a phytoestrogen that has been suggested to be a potential natural antidiabetic agent that directly modulates pancreatic β-cell function via activation of the cAMP/PKA-dependent ERK1/2 signaling pathway as well as positively affect bone formation.Citation41,Citation42,Citation71 Increased BMD has been seen with genistein treatment in previous animal studies along with increased BMD in postmenopausal women with phytoestrogen treatment.Citation44,Citation45,Citation47–Citation50 Genistein has also been noted to have a unique role in not only dampening osteoclastic markers but also stimulating osteoblastic markers in comparison with other available osteoporosis therapies that simply inhibit osteoclastic markers.Citation46 The aim of our study was to examine the effect of dietary genistein treatment on attenuating bone loss in ob/ob mice. This is the first known study to examine the effects of genistein treatment on bone in the diabetic phenotype.
Genistein treatment did improve some indicators of bone strength in the ob/ob-treated mice of our study, but had little effect on others. Bone geometry did not adapt in a manner that improved cross-sectional geometric indicators of resistance to bending or torsional loads as indicated by a lack of significant differences in I max, I min, and J. However, mice fed a genistein-rich diet demonstrated significantly greater maximum radius of the tibia from the geometric mean, MaxRad, suggesting increased periosteal growth at the tibial midshaft. Additionally, MAr/TtAr was significantly decreased in genistein-treated groups, suggesting endosteal growth reduced the size of the medullary cavity. It is unclear how these findings may translate to humans. Several clinical trials of genistein treatment in postmenopausal women have reported no effect on bone growth or maintenance.Citation34,Citation35 However, we did not directly measure genistein consumption by the ob/ob mice, so it is unknown how genistein intake in our study compares to that of the postmenopausal women in these studies. Additionally, female subjects in these studies were not obese diabetics, potentially confounding direct comparisons with our findings. Finally, ob/ob mice are hyogonadal, and it is unclear if the estrogen-like effects of genistein treatment on bone are directly related to the diabetic state. More research is needed to determine how our findings translate to human T2DM patients.
Cartilage has been documented as an estrogen-targeted tissue, suggesting that phytoestrogens could pose as a potential treatment for OA.Citation72 The effect of genistein on cartilage has minimally been explored in the literature. To our knowledge, this is the first known study to examine the effect of genistein on cartilage in ob/ob mice. Our study found that genistein treatment significantly reduced growth plate thickness, but did not reduce tibial length. This is consistent with the findings of the recent study that the expression of the main components of the extracellular matrix cartilage (collagen type II and aggrecan) and chondrocyte proliferation decreased significantly in genistein-treated mice.Citation73 This is most likely due to genistein competing with local estrogen, leading to a decreased need for autocrine estrogen in cartilage metabolism.Citation24 Since genistein is a phytoestrogen and has a weaker effect, this leads to suppression of extracellular matrix synthesis and chondrocyte proliferation. Although the growth plate differences we identified between treatment groups were not associated with decreases in tibial length, any treat ment that impairs growth in diabetic children would warrant caution. If further investigation links genistein treatment to impaired growth due to disruption of the growth plate, its clinical value as a treatment for T2DM would diminish.
The results of our investigation support the hypothesis that genistein may slightly attenuate bone fracture risks seen in T2DM. Genistein treatment at 600 mg/kg increased periosteal and endosteal growth and led to greater cortical bone mass relative to total area of the midshaft cross-section. However, genistein treatment did not promote growth of growth plate cartilage.
Acknowledgments
We thank Ms Monica Castro, Ms Kelly Ezell, and Ms Lana Leung for their help. We also wish to thank Midwestern University, Diabetes Action Research and Education Foundation, and Soy Health Research Program, which helped fund this research.
Disclosure
The authors report no conflicts of interest in this work.
References
- Ogden CL Carroll MD Kit BK Flegal KM Prevalence of childhood and adult obesity in the United States, 2011–2012 JAMA 2014 311 806 814 24570244
- de Liefde II van der Klift M de Laet CE van Daele PL Hofman A Pols HA Bone mineral density and fracture risk in type-2 diabetes mellitus: the Rotterdam Study Osteoporos Int 2005 16 1713 1720 15940395
- Dede AD Tournis S Dontas I Trovas G Type 2 diabetes mellitus and fracture risk Metabolism 2014 63 1480 1490 25284729
- Bonds DE Larson JC Schwartz AV Risk of fracture in women with type 2 diabetes: the Women’s Health Initiative Observational Study J Clin Endocrinol Metab 2006 91 3404 3410 16804043
- Garg R Chen Z Beck T Hip geometry in diabetic women: implications for fracture risk Metabolism 2012 61 1756 1762 22726843
- Burghardt AJ Issever AS Schwartz AV High-resolution peripheral quantitative computed tomographic imaging of cortical and trabecular bone microarchitecture in patients with type 2 diabetes mellitus J Clin Endocrinol Metab 2010 95 5045 5055 20719835
- Leslie WD Rubin MR Schwartz AV Kanis JA Type 2 diabetes and bone J Bone Miner Res 2012 27 2231 2237 23023946
- Hamrick MW Ferrari SL Leptin and the sympathetic connection of fat to bone Osteoporos Int 2008 19 905 912 17924050
- Wauters M Considine RV Yudkin JS Peiffer F De Leeuw I Van Gaal LF Leptin levels in type 2 diabetes: associations with measures of insulin resistance and insulin secretion Horm Metab Res 2003 35 92 96 12734788
- Donahue RP Prineas RJ Donahue RD Is fasting leptin associated with insulin resistance among nondiabetic individuals? The Miami Community Health Study Diabetes Care 1999 22 1092 1096 10388973
- Zimmet P Boyko EJ Collier GR de Courten M Etiology of the metabolic syndrome: potential role of insulin resistance, leptin resistance, and other players Ann N Y Acad Sci 1999 892 25 44 10842650
- Lewis GF Carpentier A Adeli K Giacca A Disordered fat storage and mobilization in the pathogenesis of insulin resistance and type 2 diabetes Endocr Rev 2002 23 201 229 11943743
- Sethi JK Vidal-Puig AJ Thematic review series: adipocyte biology. Adipose tissue function and plasticity orchestrate nutritional adaptation J Lipid Res 2007 48 1253 1262 17374880
- Correia ML Rahmouni K Role of leptin in the cardiovascular and endocrine complications of metabolic syndrome Diabetes Obes Metab 2006 8 603 610 17026484
- Gu X Chen Z El Bayoumy I Serum leptin levels in obese women with and without type 2 diabetes mellitus Minerva Endocrinol 2014 39 223 229 24819932
- Reinehr T Woelfle J Wiegand S Leptin but not adiponectin is related to type 2 diabetes mellitus in obese adolescents Pediatr Diabetes Epub 2015 4 16
- Hamrick MW Pennington C Newton D Xie D Isales C Leptin deficiency produces contrasting phenotypes in bones of the limb and spine Bone 2004 34 376 383 15003785
- Muzzin P Eisensmith RC Copeland KC Woo SL Correction of obesity and diabetes in genetically obese mice by leptin gene therapy Proc Natl Acad Sci USA 1996 93 14804 14808 8962136
- Kishida Y Hirao M Tamai N Leptin regulates chondrocyte differentiation and matrix maturation during endochondral ossification Bone 2005 37 607 621 16039170
- Bartell SM Rayalam S Ambati S Central (ICV) leptin injection increases bone formation, bone mineral density, muscle mass, serum IGF-1, and the expression of osteogenic genes in leptin-deficient ob/ob mice J Bone Miner Res 2011 26 1710 1720 21520275
- Fitzpatrick LA Estrogen therapy for postmenopausal osteoporosis Arq Bras Endocrinol Metabol 2006 50 705 719 17117296
- Nevitt MC Cummings SR Lane NE Association of estrogen replacement therapy with the risk of osteoarthritis of the hip in elderly white women Arch Intern Med 1996 156 2073 2080 8862099
- Zhang Y McAlindon TE Hannan MT Estrogen replacement therapy and worsening of radiographic knee osteoarthritis: the Framingham Study Arthritis Rheum 1998 41 1867 1873 9778229
- Spector TD Nandra D Hart DJ Doyle DV Is hormone replacement therapy protective for hand and knee osteoarthritis in women? The Chingford Study Ann Rheum Dis 1997 56 432 434 9486006
- Pelleymounter MA Baker MB McCaleb M Does estradiol mediate leptin’s effects on adiposity and body weight? Am J Physiol 1999 276 955 963
- Beral V Bull D Doll R Breast cancer and hormone replacement therapy: collaborative reanalysis of data from 51 epidemiological studies of 52, 705 women with breast cancer and 108, 411 women without breast cancer Lancet 1997 350 1047 1059 10213546
- Beral V Banks E Reeves G Breast cancer and hormone-replacement therapy: the Million Women Study Lancet 2003 362 1330 1331
- Kim HK Nelson-Dooley C Della-Fera MA Genistein decreases food intake, body weight, and fat pad weight and causes adipose tissue apoptosis in ovariectomized female mice J Nutr 2006 136 409 414 16424120
- Clarkson TB Anthony MS Williams JK Honoré EK Cline JM The potential of soybean phytoestrogens for postmenopausal hormone replacement therapy Proc Soc Exp Biol Med 1998 217 365 368 9492349
- Brzezinski A Debi A Phytoestrogens: the “natural” selective estrogen receptor modulators? Eur J Obstet Gynecol Reprod Biol 1999 85 47 51 10428321
- Atteritano M Mazzaferro S Bitto A Genistein effects on quality of life and depression symptoms in osteopenic postmenopausal women: a 2-year randomized, double-blind, controlled study Osteoporos Int 2014 25 1123 1129 24114397
- Bouker KB Hilakivi-Clarke L Genistein: does it prevent or promote breast cancer? Environ Health Perspect 2000 108 701 708 10964789
- Seo HS DeNardo DG Jacquot Y Stimulatory effect of genistein and apigenin on the growth of breast cancer cells correlates with their ability to activate ER alpha Breast Cancer Res Treat 2006 99 121 134 16541309
- Levis S Strickman-Stein N Ganjei-Azar P Xu P Doerge DR Krischer J Soy isoflavones in the prevention of menopausal bone loss and menopausal symptoms: a randomized, double-blind trial Arch Intern Med 2011 171 1363 1369 21824950
- Tai TY Tsai KS Tu ST The effect of soy isoflavone on bone mineral density in postmenopausal Taiwanese women with bone loss: a 2-year randomized double-blind placebo-controlled study Osteoporos Int 2012 23 1571 1580 21901480
- Hu XJ Xie MY Kluxen FM Diel P Genistein modulates the anti-tumor activity of cisplatin in MCF-7 breast and HT-29 colon cancer cells Arch Toxicol 2014 88 625 635 24504162
- Atteritano M Pernice F Mazzaferro S Effects of phytoestrogen genistein on cytogenetic biomarkers in postmenopausal women: 1 year randomized, placebocontrolled study Eur J Pharmacol 2008 589 22 26 18541232
- Marini H Bitto A Altavilla D Breast safety and efficacy of genistein aglycone for postmenopausal bone loss: a follow-up study J Clin Endocrinol Metab 2008 93 4787 4796 18796517
- Bitto A Polito F Atteritano M Genistein aglycone does not affect thyroid function: results from a three-year, randomized, double-blind, placebocontrolled trial J Clin Endocrinol Metab 2010 95 3067 3072 20357174
- Russo M Russo GL Daglia M Understanding genistein in cancer: the “good” and the “bad” effects: a review Food Chem 2016 196 589 600 26593532
- McClain RM Wolz E Davidovich A Edwards J Bausch J Reproductive safety studies with genistein in rats Food Chem Toxicol 2007 45 1319 1332 17433519
- Messina M Ho S Alekel DL Skeletal benefits of soy isoflavones: a review of the clinical trial and epidemiologic data Curr Opin Clin Nutr Metab Care 2004 7 649 658 15534433
- McCarty MF Isoflavones made simple – genistein’s agonist activity for the beta-type estrogen receptor mediates their health benefits Med Hypotheses 2006 66 1093 1114 16513288
- Chanawirat A Khemapech S Patumraj S Siriviriyakul P Genistein replacement therapy on endothelial dysfunction and bone loss in bilateral ovariectomized rats Clin Hemorheol Microcirc 2006 34 309 314 16543651
- Erlandsson MC Islander U Moverare S Ohlsson C Carlsten H Estrogenic agonism and antagonism of the soy isoflavone genistein in uterus, bone and lymphopoiesis in mice APMIS 2005 113 317 323 16011657
- Bitto A Burnett BP Polito F Effects of genistein aglycone in osteoporotic, ovariectomized rats: a comparison with alendronate, raloxifene and oestradiol Br J Pharmacol 2008 155 896 905 18695641
- Chen YM Ho SC Lam SS Ho SS Woo JL Beneficial effect of soy isoflavones on bone mineral content was modified by years since menopause, body weight, and calcium intake: a double-blind, randomized, controlled trial Menopause 2004 11 246 254 15167303
- Wu J Oka J Higuchi M Cooperative effects of isoflavones and exercise on bone and lipid metabolism in postmenopausal Japanese women: a randomized placebo-controlled trial Metabolism 2006 55 423 433 16546471
- Morabito N Crisafulli A Vergara C Effects of genistein and hormone replacement therapy on bone loss in early postmenopausal women: a randomized double blind placebo – controlled study J Bone Miner Res 2002 17 1904 1912 12369794
- Marini H Minutoli L Polito F Effects of the phytoestrogen genistein on bone metabolism in osteopenic postmenopausal women: a randomized trial Ann Intern Med 2007 146 839 847 17577003
- Claassen H Briese V Manapov F Nebe B Schünke M Kurz B The phytoestrogens daidzein and genistein enhance the insulin-stimulated sulfate uptake in articular chondrocytes Cell Tissue Res 2008 333 71 79 18509682
- Kim RP Edelman SV Kim DD Musculoskeletal complications of diabetes mellitus Clin Diabetes 2001 19 132 135
- Bergink AP van Meurs JB Loughlin J Estrogen receptor α gene haplotype is associated with radiographic osteoarthritis of the knee in elderly men and women Arthritis Rheum 2003 48 1913 1922 12847685
- Spector TD Campion GD Generalised osteoarthritis: a hormonally mediated disease Ann Rheum Dis 1989 48 523 2662920
- Ushiyama T Ueyama H Inoue K Nishioka J Ohkubo I Hukuda S Estrogen receptor gene polymorphism and generalized osteoarthritis J Rheumatol 1998 25 134 137 9458216
- Al-Nakkash L Clarke LL Rottinghaus GE Chen YJ Cooper K Rubin LJ Dietary genistein stimulates anion secretion across female murine intestine J Nutr 2006 136 2785 2790 17056801
- Barnes S Sfakianos J Coward L Kirk M Soy isoflavonoids and cancer prevention. Underlying biochemical and pharmacological issues Adv Exp Med Biol 1996 401 87 100 8886128
- Sfakianos J Coward L Kirk M Barnes S Intestinal uptake and biliary excretion of the isoflavone genistein in rats J Nutr 1997 127 1260 1268 9202077
- Xu X Wang HJ Murphy PA Cook L Hendrich S Daidzein is a more bioavailable soymilk isoflavone than is genistein in adult women J Nutr 1994 124 825 832 8207540
- Hsieh CY Santell RC Haslam SZ Helferich WG Estrogenic effects of genistein on the growth of estrogen receptor-positive human breast cancer (MCF-7) cells in vitro and in vivo Cancer Res 1998 58 3833 3838 9731492
- Ducy P Amling M Takeda S Leptin inhibits bone formation through a hypothalamic relay: a central control of bone mass Cell 2000 100 197 207 10660043
- Burguera B Hofbauer LC Thomas T Leptin reduces ovariectomy-induced bone loss in rats Endocrinology 2001 142 3546 3553 11459801
- Takeda S Elefteriou F Levasseur R Leptin regulates bone formation via the sympathetic nervous system Cell 2002 111 305 317 12419242
- Liu LF Shen WJ Ueno M Patel S Azhar S Kraemer FB Age-related modulation of the effects of obesity on gene expression profiles of mouse bone marrow and epididymal adipocytes PLoS One 2013 8 e72367 23967297
- Zhang J Li T Xu L Leptin promotes ossification through multiple ways of bone metabolism in osteoblast: a pilot study Gynecol Endocrinol 2013 29 758 762 23706140
- Turner RT Kalra SP Wong CP Peripheral leptin regulates bone formation J Bone Miner Res 2013 28 22 34 22887758
- Mittendorfer B Horowitz JF DePaoli AM McCamish MA Patterson BW Klein S Recombinant human leptin treatment does not improve insulin action in obese subjects with type 2 diabetes Diabetes 2011 60 1474 1477 21411512
- Andersson B Mattsson LA Hahn L Estrogen replacement therapy decreases hyperandrogenicity and improves glucose homeostasis and plasma lipids in postmenopausal women with noninsulin-dependent diabetes mellitus J Clin Endocrinol Metab 1997 82 638 643 9024268
- Bhathena SJ Velasquez MT Beneficial role of dietary phytoestrogens in obesity and diabetes Am J Clin Nutr 2002 76 1191 1201 12450882
- Fu Z Zhang W Zhen W Genistein induces pancreatic β-cell proliferation through activation of multiple signaling pathways and prevents insulin-deficient diabetes in mice Endocrinology 2010 151 3026 3037 20484465
- Naaz A Yellayi S Zakroczymski MA The soy isoflavone genistein decreases adipose deposition in mice Endocrinology 2003 144 3315 3320 12865308
- Yu SB Wang MQ Li YQ The effects of age and sex on the expression of oestrogen and its receptors in rat mandibular condylar cartilages Arch Oral Biol 2009 54 479 485 19264293
- Yu SB Xing XH Dong GY Weng XL Wang MQ Excess genistein suppresses the synthesis of extracellular matrix in female rat mandibular condylar cartilage Acta Pharmacol Sin 2012 33 918 923 22705728
