Abstract
The risk for developing type 2 diabetes (T2DM) is greater among obese individuals. Following onset of the disease, patients with T2DM become more likely to be afflicted with diabetic micro- and macrovascular complications. Decreasing body weight has been shown to lower glycosylated hemoglobin and improve other metabolic parameters in patients with T2DM. Medications used to lower blood glucose may increase body weight in patients with T2DM and this has been repeatedly shown to be the case for conventional, human insulin formulations. Insulin detemir is a neutral, soluble, long-acting insulin analog in which threonine-30 of the insulin B-chain is deleted, and the C-terminal lysine is acetylated with myristic acid, a C14 fatty acid chain. Insulin detemir binds to albumin, a property that enhances its pharmacokinetic/pharmacodynamic profile. Results from clinical trials have demonstrated that treatment with insulin detemir is associated with less weight gain than either insulin glargine or neutral protamine Hagedorn insulin. There are many potential reasons for the lower weight gain observed among patients treated with insulin detemir, including lower risk for hypoglycemia and therefore decreased defensive eating due to concern about this adverse event, along with other effects that may be related to the albumin binding of this insulin that may account for lower within-patient variability and consistent action. These might include faster transport across the blood–brain barrier, induction of satiety signaling in the brain, and preferential inhibition of hepatic glucose production versus peripheral glucose uptake. Experiments in diabetic rats have also indicated that insulin detemir increases adiponectin levels, which is associated with both weight loss and decreased eating.
Introduction
The population of overweight (body mass index [BMI] 25.0–29.9 kg/m2) and obese (BMI ≥ 30 kg/m2) individuals in the USA continues to increase, and these conditions are a major clinical problem with respect to the development and treatment of type 2 diabetes mellitus (T2DM).Citation1,Citation2 It has been estimated that 82%–87% of individuals with diabetes are either overweight or obese,Citation3 and it is also well known that cardiovascular risk factors beyond hyperglycemia, such as overweight/obesity, elevated blood pressure, and abnormal lipid levels, are important predictors of all-cause and cardiovascular mortality in patients with diabetes.Citation4 Weight gain is also an important concern as a potential side effect of treatment for patients with T2DM receiving certain oral therapies or insulin;Citation5,Citation6 it has been suggested that the increase in body weight associated with antidiabetes therapy may blunt the clinical benefit of improved glycemic control associated with such therapy.Citation7 Weight gain in patients with T2DM can also contribute to patient frustration with treatment, may negatively impact their adherence to therapeutic regimens, and could deter their motivation to adhere to lifestyle modifications.Citation8
Results from numerous clinical trials have demonstrated that weight reduction decreases cardiovascular risk factors, and it is likely that this benefit extends to patients with T2DM.Citation9–Citation12 In addition, multiple studies have shown that obesity is an important contributor to insulin resistance and thus has the potential to blunt the benefit of oral secretagogues and insulin therapy.Citation13–Citation15 Data from the weight loss arm of the Trials of Hypertension Prevention study showed that even modest weight loss (−4.4 kg at 6 months, −2.0 kg at 18 months, and −0.2 kg at 36 months) led to clinically significant long-term reductions in blood pressure (BP) (−3.7, −1.8, and −1.3 mm Hg for systolic BP; and −2.7, −1.3, and −0.9 mm Hg for diastolic BP at 6, 18, and 36 months, respectively) and reduced risk for hypertension by 42% at 6 months, 22% at 18 months, and 19% at 36 months. Subjects who lost ≥4.5 kg at 6 months and maintained this weight reduction for the next 30 months had the greatest reduction in BP and a 65% reduction in the risk for hypertension.Citation16 A 2-year weight reduction study (via weight-loss diets) in moderately obese individuals showed that a diet that leads to weight loss favorably modifies metabolic parameters related to cardiovascular risk (ie, low density lipoprotein cholesterol, triglycerides, high-density lipoprotein cholesterol).Citation17 One-year results from the Action for Health in Diabetes (Look AHEAD) trial showed that clinically significant weight loss in patients with T2DM was associated with improved glycemic control and an improved cardiovascular risk profile.Citation18 Recently published 4-year results from the Look AHEAD trial indicated that patients receiving intensive lifestyle intervention had a significantly greater percentage of weight loss than participants who received only diabetes education (−6.15% versus −0.88%, P < 0.001), greater reductions in glycosylated hemoglobin (A1C) levels (−0.36% versus −0.09%, P < 0.001), greater decreases in systolic and diastolic BP and triglycerides, and significantly greater increases in high density lipoprotein cholesterol.Citation9
The results from Look AHEAD and other trials are consistent with the suggestion that the care of individuals with T2DM may be too glucocentric and that patient management must consider a wider range of factors that may contribute to the development of micro- and macrovascular disease, including obesity, hypertension, and dyslipidemia.Citation18,Citation19 The benefit of a more multifaceted and integrated approach to the management of patients with T2DM that simultaneously addressed multiple cardiovascular risk factors has been demonstrated by results from the Steno-2 trial.Citation20 In this study, 160 patients with T2DM and microalbuminuria received either intensive multifaceted treatment or conventional therapy. Patients in Steno-2 were followed for 13.3 years; over this period, 24 patients in the intensive therapy group died versus 40 in the conventional treatment group (hazard ratio [HR] = 0.54, P = 0.02). Intensive therapy was associated with a lower risk of death from cardiovascular causes (HR = 0.43, P = 0.04), cardiovascular events (HR = 0.41, P < 0.001), diabetic nephropathy (relative risk [RR] = 0.44, P = 0.004), and requirement for retinal photocoagulation (RR = 0.45, P = 0.02).Citation20
While it has been shown to improve the long-term prognosis in patients with T2DM, treatment with insulin therapy is complicated by weight gain, which may delay initiation of this treatment in patients not maintaining glycemic control on oral drugs and/or intensification of dosing to maintain control in those already receiving insulin.Citation21 Because insulin detemir is associated with less weight gain than insulin glargine,Citation22,Citation23 the aim of this paper is to review the role of insulin detemir in the treatment of patients with T2DM, with a focus on efficacy in obese patients and effects on body weight.
Cost of agents is always a consideration. However, the question of cost is a complex topic requiring a thorough balance between direct and indirect costs and short term versus long-term costs. Such an analysis is beyond the scope of this paper. However, reports do suggest a cost advantage of long-acting insulin analogs over neutral protamine Hagedorn (NPH) insulin.Citation24
Insulin therapy and body weight
A very large percentage of patients with T2DM ultimately require insulin to maintain control over blood glucose, due to the progressive nature of the disease and the exhaustion of pancreatic β-cells.Citation25,Citation26 Results from 61,890 patients with T2DM indicated that after more than 20 years following diagnosis, 50% were receiving insulin.Citation27 Insulin, when properly dosed, is the most effective drug currently available to achieve optimal glycemic control and avoid long-term disease complications. Although there is no maximum dose or ceiling effect, insulin treatment is commonly associated with weight gain brought on by multiple factors. Mechanisms believed to be involved in weight gain associated with insulin therapy include reduced glycosuria and associated loss of caloric intake; stimulation of fatty acid conversion into triglycerides in adipose tissue, which favors an increase in adipose mass; and inhibition of muscle proteolysis, resulting in a positive nitrogen balance and an increase in lean mass.Citation28 The anabolic effect of insulin (inhibition of muscle proteolysis) is reflected by the fact that patients with diabetes who are receiving insulin gain lean as well as fat mass. Nevertheless, results from one evaluation indicated that nearly two-thirds of the weight gain associated with insulin treatment was fat mass.Citation29 Defensive snacking behaviors, driven by fears of hypoglycemia, can also contribute to weight gain in patients using insulin.Citation30
The type of insulin used for treatment also influences weight gain. For example, results from multiple clinical trials have demonstrated that patients with T2DM treated with NPH insulin gained between 0.3 and 2.8 kg.Citation31–Citation33 The development of insulin analogs has the potential to significantly ameliorate the weight gain associated with basal insulin therapy; this has been shown most clearly and consistently for insulin detemir. In fact, although improvements in A1C were not significantly different between patients treated with insulin glargine (−1.46% ± 1.09%) or insulin detemir (−1.54% ± 1.11%, between group P = 0.149), those treated with insulin detemir had significantly less weight gain (difference: 0.77 kg, P < 0.001).Citation23 Similar differences in weight gain were found in earlier studies as well;Citation22 however, no studies have yet been designed to assess whether such lower amounts of weight gain can translate into improvements in long-term outcomes, such as morbidity and mortality. The evidence that exists from both the Diabetes Control and Complications Trial and the UK Prospective Diabetes Study supports the concept that better glucose control does relate to less morbidity and mortality,Citation34 although there is no established link between improved morbidity and mortality and insulin detemir.
Insulin detemir
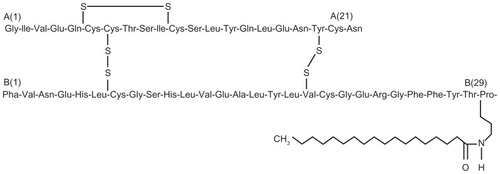
Insulin detemir is a neutral, soluble, long-acting insulin analog in which threonine is deleted from position B30 of the insulin B-chain and the ɛ-amino group of lysine B29 is acetylated with a 14-carbon myristoyl fatty acid ().Citation35–Citation37 This fatty acid modification allows insulin detemir to reversibly bind to the long-chain fatty acid binding sites of albumin, and this contributes to the extended time action profile for this analog.Citation38 Insulin detemir is soluble at neutral pH, which enables it to remain in a liquid form following subcutaneous injection. This property contrasts with NPH insulin, which is a preformed crystalline precipitate suspension, and insulin glargine, which is an acidic solution that precipitates in the subcutaneous tissue after administration. The solubility of insulin detemir may contribute to its low variability in pharmacokinetic and pharmacodynamic properties.Citation35,Citation36
Figure 1 Structure of insulin detemir.Citation37
Pharmacokinetics/Pharmacodynamics
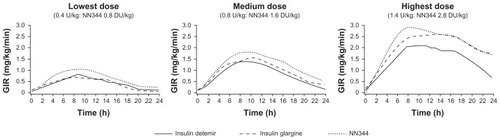
Once-daily insulin detemir provides 24-hour control over blood glucose equivalent to that for insulin glargine, the other long-acting insulin analog.Citation39 Insulin detemir administered twice daily reaches steady state after the second injection and shows a constant metabolic effect over time under steady-state conditions.Citation40 It has a duration of action of about 24 hours and concentrations in blood that increase with dose ().Citation41–Citation43 The effects of body weight on the pharmacokinetics are not known due to the fact that published studies concerned with studying pharmacodynamics of insulin detemir have typically included nonobese subjects and because insulin detemir was dosed on the basis of body weight.Citation40,Citation44
Figure 2 Pharmacodynamic profile for insulin detemir (glucose infusion rate in euglycemic glucose clamp experiments).Citation42
Abbreviation: GIR, glucose infusion rate.
Multiple studies have provided clear support for a once-daily dosing regimen with insulin detemir. For example, results from the European cohort of the Predictable Results and Experience in Diabetes through Intensification and Control to Target: an International Variability Evaluation (PREDICTIVETM) study showed that switching from twice daily NPH insulin to once daily insulin detemir resulted in significant 12-week reductions in A1C (P < 0.001).Citation45 A 52-week trial included patients with T2DM who were receiving oral antidiabetes drugs and insulin detemir once or twice daily as needed or once daily insulin glargine, reported that A1C decreased from 8.6% to 7.1% with insulin detemir among patients who completed the study, whether or not they received one or two doses per day, and from 8.6% to 7.1% for all patients who received insulin glargine.Citation22
Clinical experience with basal insulin analogs: focus on weight gain
Results from multiple controlled clinical trials of patients with T2DM have shown that insulin detemir results in less weight gain than NPH insulin when used as monotherapy, when added to oral antidiabetes drugs, and when employed as part of basal bolus therapy.Citation46–Citation48 Additional controlled clinical studies have also demonstrated that insulin detemir is associated with less weight gain than insulin glargine when used as part of basal bolus treatmentCitation49,Citation50 and when added to oral antidiabetes drugs.Citation22 Results from a 20-week comparison of insulin detemir versus NPH insulin added to oral agents indicated that weight gain was 1.2, 0.7, and 1.6 kg with morning detemir, evening detemir, and NPH insulin, respectively (P = 0.005 for evening detemir versus NPH insulin).Citation48 Another comparison of insulin detemir versus NPH insulin, each combined with mealtime insulin aspart for 26 weeks in overweight patients (mean BMI = 31.8 kg/m2), indicated that weight gain was 0.4 kg for insulin detemir and 1.9 kg for NPH insulin (P ≤ 0.0001).Citation46 A comparison of insulins detemir and glargine as part of basal bolus therapy in patients with T2DM indicated that weight gain was 1.2 kg for insulin detemir versus 2.7 kg for insulin glargine at the end of 26 weeks of treatment (P = 0.001).Citation50 Results from a 52-week, multinational, open-label, parallel-group, noninferiority, treat-to-target trial comparing both once and twice daily insulin detemir with once daily insulin glargine in a basal plus regimen with oral antidiabetes drugs in patients with T2DM indicated that mean weight gain at 52 weeks was significantly lower among those who received insulin detemir than insulin glargine (3.0 versus 3.9 kg, P < 0.001), and further analysis revealed there was even less weight gain among those who received insulin detemir once a day (2.3 kg).Citation22 Therefore, once daily dosing with insulin detemir may be advantageous in obese or overweight patients with T2DM to minimize weight gain.
The effects of insulin detemir on weight gain have been studied across a wide range of patients, including those who are overweight or obese. Meta-analysis of five parallel group randomized controlled trials of at least 20 weeks’ duration that compared once daily evening insulin glargine or insulin detemir with a common comparator, NPH insulin (evening administration), showed that patients with T2DM (n = 2092) who received evening insulin detemir gained significantly less weight (−1.22 kg) than those treated with insulin glargine (−0.29 kg, P = 0.010).Citation51 Importantly, this analysis focused on insulin naïve, T2DM patients poorly controlled with oral antidiabetes drugs, which are the patients most likely to be switched to insulin therapy in clinical practice. The beneficial effects of insulin detemir on weight change appear greatest in patients with high body weight or BMI at the initiation of treatment.Citation33,Citation52 Results from the Treat to target with once-daily Insulin Therapy: Reduce AIC by Titrating Effectively (TITRATETM) study, in which insulin detemir was added to oral antidiabetes drugs, indicated that body weight changes from baseline after insulin detemir treatment were related to baseline BMI. Patients with baseline BMI ≤ 25 kg/m2 experienced weight increases from 1.35 to 1.38 kg and those with baseline BMI > 40 kg/m2 at baseline experienced weight decreases from −0.14 to −0.33 kg.Citation53
Pooled analyses have also investigated whether weight changes with insulin detemir were linked to baseline BMI in patients with diabetes. Data were collected from three randomized, parallel group trials of 22–26 weeks duration that included 1416 patients aged ≥65 years and 880 patients aged 18–64 years, all of whom were treated with insulin.Citation54 At the end of the study, A1C control and fasting plasma glucose were similar for both age groups, regardless of whether they received insulin detemir or NPH insulin. However, both older patients (mean weight at baseline: insulin detemir = 81 kg, NPH insulin = 79 kg) and younger patients (mean weight at baseline: insulin detemir = 85 kg, NPH insulin = 85 kg) gained statistically significantly less body weight with insulin detemir than NPH insulin (0.69 versus 1.62 kg for older persons, P < 0.001; 0.80 versus 1.93 kg for younger persons, P < 0.001).Citation54 A report of data pooled from two randomized, parallel group trials of 22 and 24 weeks’ duration that included 900 insulin treated patients with T2DM who had their treatment intensified to basal bolus therapy.Citation47 Patients received once or twice daily insulin detemir or NPH insulin in conjunction with insulin aspart or human soluble insulin at mealtimes. Although there was no between group difference in A1C, indicating comparable glycemic control, patients treated with insulin detemir had minimal weight gain (mean < 1 kg), regardless of their BMI at entry (estimated slope −0.032); whereas, in patients treated with NPH insulin, weight gain increased as baseline BMI rose (estimated slope 0.075, P = 0.025). Patients receiving NPH insulin with BMI > 35 kg/m2 gained the most weight (mean of ~2.4 kg) while insulin detemir-treated patients with the same BMI range lost weight (~0.5 kg).Citation47
Can reduced risk for hypoglycemia explain decreased weight gain with insulin detemir?
Although insulin glargine can also provide less of a risk for hypoglycemia than NPH insulin,Citation55 insulin detemir has even less of an impact on weight gain than other basal or intermediate-acting insulin formulations, and therefore it is important to discuss mechanisms that allow this to be manifested. Several potential mechanisms that have been proposed are addressed below.
Reduced risk of hypoglycemia with insulin detemir, coupled with a more consistent and reliable delivery of the desired dose than is available with traditional basal insulin, such as NPH insulin, has been proposed as a possible mechanism that could decrease defensive snacking by patients and help to limit weight gain.Citation56 Insulin detemir has been repeatedly shown to have lower risk for hypoglycemia than NPH insulin when administered either onceCitation48 or twiceCitation33 daily. A recent meta-analysis has shown that insulin detemir is associated with significantly lower overall risk for severe hypoglycemia versus NPH insulin (RR = 0.74, 95% confidence interval [CI] = 0.58–0.96, P < 0.05). Insulin detemir is also associated with significantly lower risk for nocturnal hypoglycemia versus NPH insulin (RR = 0.92, 95% CI = 0.85–0.98, P < 0.05).Citation57 Results from another meta-analysis of studies comparing insulin detemir with NPH insulin that focused on patient age indicated that the RR for all hypoglycemic episodes (insulin detemir/NPH insulin) was 0.59 (95% CI = 0.42–0.83) for older persons and 0.75 (95% CI = 0.59–0.96) for younger patients.Citation54 Results from the TITRATE study, which had aggressive glucose targets, indicated that overall rates of hypoglycemia episodes were low and were comparable between insulin detemir treatment groups (7.73 and 5.27 events/subject/year for the 70–90 mg/dL and 80–110 mg/dL fasting plasma target groups, respectively). A single event of major hypoglycemia was reported in the 70–90 mg/dL target group.Citation53 Results from the PREDICTIVE BMI study showed that the incidence of hypoglycemia was lower with insulin detemir versus NPH insulin (RR = 0.62 for all events and 0.43 for nocturnal events, P < 0.0001 for both comparisons).Citation46
Although the earlier described studies and meta-analysis show that the risk for hypoglycemia is significantly decreased with insulin detemir versus NPH insulin, and it is reasonable to expect that this may result in less defensive eating, decreased weight gain with insulin detemir is not completely explained by decreased risk for hypoglycemia. A 26-week, randomized, multicenter, open label, parallel group trial compared glycemic control, hypoglycemia, and weight change between insulin detemir and NPH insulin (administered in the morning and evening) in a total of 476 insulin naïve patients with T2DM who were also being treated with one or two oral antidiabetes drugs.Citation58 Weight gain data from this study were analyzed as a function of hypoglycemia frequency. Weight gain with insulin detemir was 1.2 kg versus 2.8 kg with NPH insulin (P < 0.001), and the overall risk of hypoglycemia was 47% lower with insulin detemir (P < 0.001). No significant relationship between hypoglycemia and weight gain was seen with insulin detemir (P = 0.2), but such a relationship was demonstrated for NPH insulin (P = 0.003). These results are consistent with observations from healthy volunteers showing that insulin detemir treatment results in increased awareness during hypoglycemia.Citation59 Thus, results from these two studies, when considered together, suggest that decreased awareness of hypoglycemia and less defensive eating cannot fully explain less weight gain with insulin detemir.
Alternative explanations for decreased weight gain with insulin detemir
There are several potential reasons for decreased weight gain with insulin detemir beyond a potential reduction in defensive eating secondary to decreased risk for hypoglycemia.
Hepatic influences
Due to its novel method of prolonging action via albumin binding, insulin detemir may influence hepatic glucose metabolism to a greater extent than peripheral tissues. The association of insulin detemir with nonesterified fatty acid binding sites on albumin may limit its transfer from the circulation into the extravascular extracellular space in adipose tissue and muscle, due to the capillary endothelial cell barrier. In the liver, the open sinusoids may expose hepatocytes to insulin detemir, enabling it to have a greater effect in the liver than in peripheral tissues. Results from a study in humans indicated that at and below plasma glucose concentrations of 54 mg/dL, suppression of endogenous glucose production was greater with insulin detemir than with NPH insulin, whereas stimulation of peripheral glucose uptake was greater with NPH insulin than with insulin detemir.Citation60 These authors suggested that decreased peripheral glucose uptake may contribute to lower weight gain with insulin detemir versus NPH insulin.
CNS influences
Another possibility that might explain less weight gain with insulin detemir versus other insulins is that it facilitates the actions of central nervous system mechanisms associated with satiety. Insulin detemir may be more effective than regular human insulin in communicating satiety signals within the central nervous system due to increased ability to cross the blood–brain barrier resulting from albumin binding. Results from a study in which insulin was administered intranasally to healthy subjects indicated that this treatment, which increases insulin levels in the cerebrospinal fluid, produced significant elevations in serum leptins and weight loss in men.Citation61 This suggests that insulin may provide a negative feedback signal that regulates adiposity. Results from studies in human patients who received insulin injections indicated that this treatment can rapidly alter electroencephalographic activity.Citation62 This effect suggests that insulin can influence hypothalamic circuits involved in satiety and feeding.Citation61 A study in 15 healthy volunteers showed that a bolus injection of insulin detemir during hyperinsulinemic-euglycemic clamp produced a change in the electroencephalogram not observed after injection of regular human insulin.Citation62 Results from this study also showed that insulin detemir significantly decreased subsequent food intake by about 300 kcal versus regular human insulin (P < 0.04). However, it is important to note that other recent studies have suggested that insulin detemir is not transported across the blood–brain barrier,Citation63 and that acute intracerebroventricularly injected human insulin does not significantly inhibit food intake in rats.Citation64
Results from studies in experimental animals also support the suggestion that actions other than decreasing the risk of hypoglycemia may be involved in the effects of insulin detemir on body weight in patients with T2DM. It has been shown that administration of insulin detemir to Zucker diabetic fatty rats (an animal model for diabetes) results in smaller increases in fat mass than either insulin glargine or NPH insulin.Citation65 This result prompted the authors of this study to speculate that the reduced weight gain in patients treated with insulin detemir versus other intermediate or long-acting insulins to be due to decreased adiposity. A second study in the same strain of diabetic rats showed further that insulin detemir increases adiponectin levels to a greater extent than NPH insulin.Citation66 It is well known that adiponectin is decreased in obesity, and that higher adiponectin concentrations are associated with both weight loss and anorexia.Citation67 Both adiponectin and leptin are derived from adipose tissue and they influence food intake, insulin resistance, and lipolysis.Citation68,Citation69 Comparison of the effects of insulin detemir and human insulin on 3T3-L1 preadipocytes indicated that human insulin, but not insulin detemir, was associated with clonal expansion and that insulin detemir had reduced adipogenic effects versus human insulin.Citation70 Thus, while the mechanisms underlying lower weight gain with insulin detemir versus other insulins are not clear, it is reasonable to speculate that they may involve albumin binding, preferential activity in brain and liver, and favorable effects on adipocytes.Citation71 Further studies are needed to determine which, of these or other mechanisms, underlie the positive effects of insulin detemir versus other insulin formulations.
Conclusion
Most patients with T2DM progress to treatment with insulin. The benefit of better glucose control offered by insulin can be offset by the higher weight gain and the hypoglycemia that occurs most commonly with human insulins than some analog formulations. This may lead to poor adherence to treatment by some patients. Results from multiple clinical trials with insulin detemir have shown that its use results in less weight gain than older types of insulin, including NPH insulin. In addition, insulin detemir causes less weight gain among patients with high baseline weight or BMI. This trend for less weight gain may have a clinical impact on an individual basis. Multiple mechanisms may contribute to less weight gain with insulin detemir versus other insulins, and they all may be linked to the unique structure of the molecule, which includes a C14 fatty acid chain that enables binding to albumin.
Acknowledgments
The author wishes to thank Robert W Rhoades of MedVal Scientific Information Services, LLC, for providing medical writing and editorial assistance. This manuscript was prepared according to the International Society for Medical Publication Professionals’ Good Publication Practice for Communicating Company-Sponsored Medical Research: the GPP2 Guidelines.
Disclosure
Funding to support the preparation of this manuscript was provided by Novo Nordisk Inc. The author declares no other conflicts of interest in this work.
References
- National Diabetes Information Clearinghouse National Diabetes Statistics 2007 Available from: http://diabetes.niddk.nih.gov/dm/pubs/statistics/#allages Accessed October 6, 2011
- Geiss LS Pan L Cadwell B Gregg EW Benjamin SM Engelgau MM Changes in incidence of diabetes in US adults, 1997–2003 Am J Prev Med 2006 30 5 371 377 16627124
- Bays HE Chapman RH Grandy S The relationship of body mass index to diabetes mellitus, hypertension and dyslipidaemia: comparison of data from two national surveys Int J Clin Pract 2007 61 5 737 747 17493087
- Wei M Gaskill SP Haffner SM Stern MP Effects of diabetes and level of glycemia on all-cause and cardiovascular mortality. The San Antonio Heart Study Diabetes Care 1998 21 7 1167 1172 9653614
- Peters AL Patient and treatment perspectives: Revisiting the link between type 2 diabetes, weight gain, and cardiovascular risk Cleve Clin J Med 2009 76 Suppl 5 S20 S27 19952300
- Campbell IW Comparing the actions of older and newer therapies on body weight: to what extent should these effects guide the selection of antidiabetic therapy? Int J Clin Pract 2010 64 6 791 801 20518953
- Russell-Jones D Khan R Insulin-associated weight gain in diabetes – causes, effects and coping strategies Diabetes Obes Metab 2007 9 6 799 812 17924864
- Pi-Sunyer FX The impact of weight gain on motivation, compliance, and metabolic control in patients with type 2 diabetes mellitus Postgrad Med 2009 121 5 94 107 19820278
- Wing RR Long-term effects of a lifestyle intervention on weight and cardiovascular risk factors in individuals with type 2 diabetes mellitus: four-year results of the Look AHEAD trial Arch Intern Med 2010 170 17 1566 1575 20876408
- Hollander PA Elbein SC Hirsch IB Role of orlistat in the treatment of obese patients with type 2 diabetes. A 1-year randomized double-blind study Diabetes Care 1998 21 8 1288 1294 9702435
- Esposito K Pontillo A Di Palo C Effect of weight loss and lifestyle changes on vascular inflammatory markers in obese women: a randomized trial JAMA 2003 289 14 1799 1804 12684358
- Cox KL Puddey IB Morton AR Burke V Beilin LJ McAleer M Exercise and weight control in sedentary overweight men: effects on clinic and ambulatory blood pressure J Hypertens 1996 14 6 779 790 8793702
- Fernandez-Veledo S Nieto-Vazquez I Vila-Bedmar R Garcia-Guerra L Alonso-Chamorro M Lorenzo M Molecular mechanisms involved in obesity-associated insulin resistance: therapeutical approach Arch Physiol Biochem 2009 115 4 227 239 19673658
- Zeyda M Stulnig TM Obesity, inflammation, and insulin resistance – a mini-review Gerontology 2009 55 4 379 386 19365105
- Yki-Jarvinen H Ryysy L Kauppila M Effect of obesity on the response to insulin therapy in noninsulin-dependent diabetes mellitus J Clin Endocrinol Metab 1997 82 12 4037 4043 9398709
- Stevens VJ Obarzanek E Cook NR Long-term weight loss and changes in blood pressure: results of the Trials of Hypertension Prevention, phase II Ann Intern Med 2001 134 1 1 11 11187414
- Shai I Schwarzfuchs D Henkin Y Weight loss with a low-carbohydrate, Mediterranean, or low-fat diet N Engl J Med 2008 359 3 229 241 18635428
- Pi-Sunyer X Blackburn G Brancati FL Reduction in weight and cardiovascular disease risk factors in individuals with type 2 diabetes: one-year results of the Look AHEAD trial Diabetes Care 2007 30 6 1374 1383 17363746
- Gaede P Pedersen O Multi-targeted and aggressive treatment of patients with type 2 diabetes at high risk: what are we waiting for? Horm Metab Res 2005 37 Suppl 1 76 82 15918115
- Gaede P Lund-Andersen H Parving HH Pedersen O Effect of a multifactorial intervention on mortality in type 2 diabetes N Engl J Med 2008 358 6 580 591 18256393
- Carver C Insulin treatment and the problem of weight gain in type 2 diabetes Diabetes Educ 2006 32 6 910 917 17102158
- Rosenstock J Davies M Home PD Larsen J Koenen C Schernthaner G A randomised, 52-week, treat-to-target trial comparing insulin detemir with insulin glargine when administered as add-on to glucose-lowering drugs in insulin-naive people with type 2 diabetes Diabetologia 2008 51 3 408 416 18204830
- Swinnen SG Dain MP Aronson R A 24-week, randomized, treat-to-target trial comparing initiation of insulin glargine once-daily with insulin detemir twice-daily in patients with type 2 diabetes inadequately controlled on oral glucose-lowering drugs Diabetes Care 2010 33 6 1176 1178 20200301
- Valentine WJ Palmer AJ Erny-Albrecht KM Cost-effectiveness of basal insulin from a US health system perspective: comparative analyses of detemir, glargine, and NPH Adv Ther 2006 23 2 191 207 16751153
- DeFronzo RA Banting Lecture. From the triumvirate to the ominous octet: a new paradigm for the treatment of type 2 diabetes mellitus Diabetes 2009 58 4 773 795 19336687
- Meece J Dispelling myths and removing barriers about insulin in type 2 diabetes Diabetes Educ 2006 32 1 Suppl 9S 18S 16439485
- Eliasson B Eeg-Olofsson K Cederholm J Nilsson PM Gudbjornsdottir S Antihyperglycaemic treatment of type 2 diabetes: results from a national diabetes register Diabetes Metab 2007 33 4 269 276 17499541
- Laville M Andreelli F Mechanisms for weight gain during blood glucose normalization Diabetes Metab 2000 26 Suppl 3 42 45 10945152
- Groop L Widen E Franssila-Kallunki A Different effects of insulin and oral antidiabetic agents on glucose and energy metabolism in type 2 (non-insulin-dependent) diabetes mellitus Diabetologia 1989 32 8 599 605 2506091
- Hartman I Insulin analogs: impact on treatment success, satisfaction, quality of life, and adherence Clin Med Res 2008 6 2 54 67 18801953
- Russell-Jones D Simpson R Hylleberg B Draeger E Bolinder J Effects of QD insulin detemir or neutral protamine Hagedorn on blood glucose control in patients with type I diabetes mellitus using a basal-bolus regimen Clin Ther 2004 26 5 724 736 15220016
- Home P Bartley P Russell-Jones D Insulin detemir offers improved glycemic control compared with NPH insulin in people with type 1 diabetes: a randomized clinical trial Diabetes Care 2004 27 5 1081 1087 15111525
- Hermansen K Davies M Derezinski T Ravn GM Clauson P Home P A 26-week, randomized, parallel, treat-to-target trial comparing insulin detemir with NPH insulin as add-on therapy to oral glucose-lowering drugs in insulin-naive people with type 2 diabetes Diabetes Care 2006 29 6 1269 1274 16732007
- Skyler JS Bergenstal R Bonow RO Intensive glycemic control and the prevention of cardiovascular events: implications of the ACCORD, ADVANCE, and VA diabetes trials: a position statement of the American Diabetes Association and a scientific statement of the American College of Cardiology Foundation and the American Heart Association Diabetes Care 2009 32 1 187 192 19092168
- Chapman TM Perry CM Insulin detemir: a review of its use in the management of type 1 and 2 diabetes mellitus Drugs 2004 64 22 2577 2595 15516157
- Philips JC Scheen A Insulin detemir in the treatment of type 1 and type 2 diabetes Vasc Health Risk Manag 2006 2 3 277 283 17326333
- Levemir® (insulin detemir [rDNA origin] injection) Prescribing information Princeton, NJ Novo Nordisk Inc 11 2010
- Havelund S Plum A Ribel U The mechanism of protraction of insulin detemir, a long-acting, acylated analog of human insulin Pharm Res 2004 21 8 1498 1504 15359587
- King AB Once-daily insulin detemir is comparable to once-daily insulin glargine in providing glycaemic control over 24 h in patients with type 2 diabetes: a double-blind, randomized, crossover study Diabetes Obes Metab 2009 11 1 69 71 19120433
- Bott S Tusek C Jacobsen LV Insulin detemir under steady-state conditions: no accumulation and constant metabolic effect over time with twice daily administration in subjects with type 1 diabetes Diabet Med 2006 23 522 528 16681561
- Plank J Bodenlenz M Sinner F A double-blind, randomized, dose-response study investigating the pharmacodynamic and pharmacokinetic properties of the long-acting insulin analog detemir Diabetes Care 2005 28 5 1107 1112 15855574
- Klein O Lynge J Endahl L Damholt B Nosek L Heise T Albumin-bound basal insulin analogues (insulin detemir and NN344): comparable time-action profiles but less variability than insulin glargine in type 2 diabetes Diabetes Obes Metab 2007 9 3 290 299 17391154
- Heise T Pieber TR Towards peakless, reproducible and long-acting insulins. An assessment of the basal analogues based on isoglycaemic clamp studies Diabetes Obes Metab 2007 9 5 648 659 17645556
- Heise T Nosek L Ronn BB Lower within-subject variability of insulin detemir in comparison to NPH insulin and insulin glargine in people with type 1 diabetes Diabetes 2004 53 6 1614 1620 15161770
- Sreenan S Virkamaki A Zhang K Hansen JB Switching from NPH insulin to once-daily insulin detemir in basal-bolus-treated patients with diabetes mellitus: data from the European cohort of the PREDICTIVE study Int J Clin Pract 2008 62 12 1971 1980 19166444
- Fajardo Montanana C Hernandez Herrero C Rivas Fernandez M Less weight gain and hypoglycaemia with once-daily insulin detemir than NPH insulin in intensification of insulin therapy in overweight type 2 diabetes patients – the PREDICTIVE™ BMI clinical trial Diabet Med 2008 25 8 916 923 18959604
- Raslova K Tamer SC Clauson P Karl D Insulin detemir results in less weight gain than NPH insulin when used in basal-bolus therapy for type 2 diabetes mellitus, and this advantage increases with baseline body mass index Clin Drug Invest 2007 27 4 279 285
- Philis-Tsimikas A Charpentier G Clauson P Martinez Ravn G Roberts VL Thorsteinsson B Comparison of once-daily insulin detemir with NPH insulin added to a regimen of oral antidiabetic drugs in poorly controlled type 2 diabetes Clin Ther 2006 28 10 1569 1581 17157113
- Hollander P Cooper J Bregnhoj J Pedersen CB A 52-week, multinational, open-label, parallel-group, noninferiority, treat-to-target trial comparing insulin detemir with insulin glargine in a basal-bolus regimen with mealtime insulin aspart in patients with type 2 diabetes Clin Ther 2008 30 11 1976 1987 19108786
- Raskin P Gylvin T Weng W Chaykin L Comparison of insulin detemir and insulin glargine using a basal-bolus regimen in a randomized, controlled clinical study in patients with type 2 diabetes Diabetes Metab Res Rev 2009 25 6 542 548 19565569
- Fakhoury W Lockhart I Kotchie RW Aagren M Lereun C Indirect comparison of once daily insulin detemir and glargine in reducing weight gain and hypoglycaemic episodes when administered in addition to conventional oral anti-diabetic therapy in patients with type-2 diabetes Pharmacology 2008 82 2 156 163 18679040
- Philis-Tsimikas A Zhang Q Walker C Glycemic control with insulin glargine as part of an ethnically diverse, community-based diabetes management program Am J Ther 2006 13 6 466 472 17122525
- Blonde L Merilainen M Karwe V Raskin P Patient-directed titration for achieving glycaemic goals using a once-daily basal insulin analogue: an assessment of two different fasting plasma glucose targets – the TITRATE study Diabetes Obes Metab 2009 11 6 623 631 19515182
- Garber AJ Clauson P Pedersen CB Kolendorf K Lower risk of hypoglycemia with insulin detemir than with neutral protamine hagedorn insulin in older persons with type 2 diabetes: a pooled analysis of phase III trials J Am Geriatr Soc 2007 55 11 1735 1740 17979896
- Home PD Fritsche A Schinzel S Massi-Benedetti M Meta-analysis of individual patient data to assess the risk of hypoglycaemia in people with type 2 diabetes using NPH insulin or insulin glargine Diabetes Obes Metab 2010 12 9 772 779 20649629
- Hermansen K Davies M Does insulin detemir have a role in reducing risk of insulin-associated weight gain? Diabetes Obes Metab 2007 9 3 209 217 17391147
- Singh SR Ahmad F Lal A Yu C Bai Z Bennett H Efficacy and safety of insulin analogues for the management of diabetes mellitus: a meta-analysis CMAJ 2009 180 4 385 397 19221352
- Davies MJ Derezinski T Pedersen CB Clauson P Reduced weight gain with insulin detemir compared to NPH insulin is not explained by a reduction in hypoglycemia Diabetes Technol Ther 2008 10 4 273 277 18715200
- Tschritter O Schafer SA Klett J Insulin detemir causes increased symptom awareness during hypoglycaemia compared to human insulin Diabetes Obes Metab 2009 11 11 1017 1026 19650876
- Hordern SV Wright JE Umpleby AM Shojaee-Moradie F Amiss J Russell-Jones DL Comparison of the effects on glucose and lipid metabolism of equipotent doses of insulin detemir and NPH insulin with a 16-h euglycaemic clamp Diabetologia 2005 48 420 426 15729576
- Hallschmid M Benedict C Schultes B Fehm HL Born J Kern W Intranasal insulin reduces body fat in men but not in women Diabetes 2004 53 11 3024 3029 15504987
- Hallschmid M Jauch-Chara K Korn O Euglycemic infusion of insulin detemir compared to human insulin appears to increase direct current brain potential response and reduces food intake while inducing similar systemic effects Diabetes 2010 59 4 1101 1107 20068139
- Banks WA Morley JE Lynch JL Lynch KM Mooradian AD Insulin detemir is not transported across the blood-brain barrier Peptides 2010 31 12 2284 2288 20868713
- Jessen L Clegg DJ Bouman SD Evaluation of the lack of anorectic effect of intracerebroventricular insulin in rats Am J Physiol Regul Integr Comp Physiol 2010 298 1 R43 R50 19864335
- Fledelius C Damgaard J Vinterby A Ribel U Petersen JS Sturis J Insulin detemir reduces gain in bodyweight and fat mass in ZDF rats when compared with both NPH and insulin glargine Diabetologia 2008 51 Suppl 1 S392 (abstr 975)
- Fledelius C Olsen GS Jensen AB The weight sparing effect of insulin detemir is associated with increased adiponectin levels and decreased adiposity in the diabetic ZDF rat Diabetologia 2009 52 Suppl 1 S285 (abstr 727)
- Haluzik M Parizkova J Haluzik MM Adiponectin and its role in the obesity-induced insulin resistance and related complications Physiol Res 2004 53 2 123 129 15046547
- Boden G Laakso M Lipids and glucose in type 2 diabetes: what is the cause and effect? Diabetes Care 2004 27 9 2253 2259 15333497
- Jung CH Rhee EJ Choi JH The relationship of adiponectin/leptin ratio with homeostasis model assessment insulin resistance index and metabolic syndrome in apparently healthy korean male adults Korean Diabetes J 2010 34 4 237 243 20835341
- Bohm A Staiger H Hennige AM Haas C Machicao F Haring HU Effect of insulin detemir, compared to human insulin, on 3T3-L1 adipogenesis Regul Pept 2008 151 1–3 160 163 18571747
- Tibaldi J Actions of insulin beyond glycemic control: a perspective on insulin detemir Adv Ther 2007 24 4 868 882 17901036