Abstract
Saxagliptin (Onglyza™) is a potent, selective, once-daily dipeptidyl peptidase-4 (DPP-4) inhibitor indicated for improving glycemic control in patients with type 2 diabetes (T2D). By blocking DPP-4, saxagliptin increases and prolongs the effects of incretins, a group of peptide hormones released by intestinal cells after meals, which stimulate glucose-dependent insulin secretion to lower blood glucose. In controlled clinical trials, saxagliptin administered as monotherapy or in combination with metformin, glyburide, or a thiazolidinedione improved glycemic control in a clinically significant manner, reflected by significant decreases in glycated hemoglobin (monotherapy, −0.5%; add-on to metformin, thiazolidinedione, or sulfonylurea, −0.6% to 0.9%; initial combination with metformin, −2.5%), fasting plasma glucose, and postprandial glucose compared with controls. Additionally, saxagliptin improved β-cell function, reflected as increases in homeostasis model assessment (HOMA)-2β. Saxagliptin was generally well tolerated; it did not increase hypoglycemia compared with controls, and was weight neutral. A meta-analysis of Phase II and III trials showed that saxagliptin did not increase the risk of major cardiovascular events. Professional organizations have updated their guidelines for T2D to include a DPP-4 inhibitor as an early treatment option—either as initial therapy in combination with metformin, or as add-on therapy for patients whose glycemia is inadequately controlled by a single oral antidiabetic drug.
Introduction
Saxagliptin (Onglyza™, Bristol-Myers Squibb, Princeton, NJ; AstraZeneca Pharmaceuticals LP, Wilmington, DE, USA) is a selective dipeptidyl peptidase-4 (DPP-4) inhibitor approved for the management of type 2 diabetes (T2D) along with diet and exercise.Citation1 In addition to saxagliptin, sitagliptin is currently approved in the United States and various regions worldwide.Citation2 Vildagliptin is not yet approved in the United States.Citation3
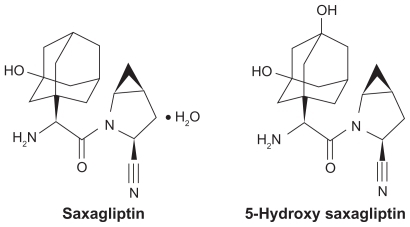
Structurally, saxagliptin is a cyanopyrrolidine derivative designed to provide extended inhibition of the DPP-4 enzyme ().Citation1,Citation4,Citation5 Saxagliptin has been evaluated in initial therapy of T2D both as a single agent and in combination with metformin,Citation6–Citation8 as well as add-on therapy for patients whose glycemia had not been adequately controlled by metformin, a sulfonylurea (glyburide), or a thiazolidinedione (pioglitazone or rosiglitazone).Citation9–Citation11 In each treatment setting, saxagliptin significantly improved glycemic control relative to the comparator group, and exhibited a safety and tolerability profile comparable with that observed in the control arms.Citation6–Citation11 The purpose of this article is to review the clinical profile of saxagliptin in the manage ment of patients with T2D. After providing the rationale for DPP-4 inhibition, the pharmacokinetics of saxagliptin will be considered, including its use in special patient populations, followed by the results of controlled clinical trials conducted with saxagliptin in the first-line and add-on treatment settings.
Role of incretins
The incretins are peptide hormones secreted by the intestines following food consumption. They bind to specific G-protein–coupled receptors, thereby stimulating glucose-dependent insulin secretion ().Citation12–Citation16 The best characterized incretins are glucagon-like peptide-1 (GLP-1) secreted by intestinal L-cells in the distal ileum and colon, and glucose-dependent insulinotropic peptide (GIP) produced by intestinal K-cells in the duodenum and jejunum. Together, these incretins account for approximately 50% to 70% of the postprandial insulin response in healthy individuals.Citation13 In addition to stimulating insulin secretion, GLP-1 has other actions that help to control blood glucose levels. GLP-1 reduces postprandial glucagon secretion from pancreatic α-cells to lower hepatic glucose production, slows gastric emptying to reduce glucose absorption after meals, and promotes satiety to reduce further food intake.Citation15,Citation17
Figure 2 The incretins are released from intestinal cells after a meal, and act on pancreatic β-cells to stimulate insulin secretion. GLP-1 also acts on pancreatic α-cells to inhibit glucagon secretion and thereby reduce hepatic glucose production. The actions of the incretins are limited by DPP-4, which rapidly degrades GLP-1 and GIP into inactive fragments.Citation12–Citation16 The presence of a DPP-4 inhibitor, such as saxagliptin, blocks degradation of the incretins, thereby increasing and prolonging their effects in the pancreas. As a result, blood glucose is lowered.Citation12,Citation15 Reprinted from Gastroenterology, 132, Baggio LL, Biology of incretins: GLP-1 and GIP, 2131–57, © 2007,Citation16 with permission from the AGA Institute.
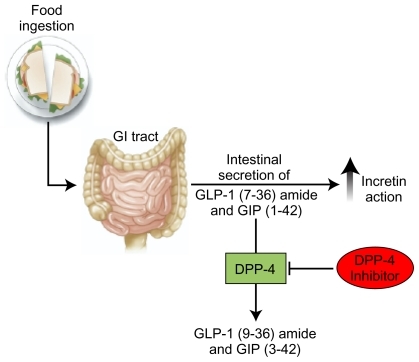
The actions of the incretins, however, are short-lived because they are rapidly inactivated by DPP-4, a serine protease that is widely distributed in human tissues, including the intestines, pancreas, lungs, kidneys, adrenals, brain, and lymphocytes.Citation13 DPP-4 predominantly cleaves peptides containing either an alanine or proline at position 2 of the N-terminus.Citation17 The half-life (t1/2) of biologically active GLP-1 and GIP, following exogenous administration, is <2 and 5–7 minutes, respectively, which reflects in part the rapid inactivation by DPP-4.Citation17 The secretion of GLP-1 following meals is reduced modestly in patients with T2D compared with healthy individuals, but its actions in stimulating insulin secretion are preserved.Citation17 In contrast, meal-stimulated GIP secretion is normal but its biologic activity is reduced in T2D.Citation17 By blocking inactivation of the incretins, DPP-4 inhibitors are designed to increase and prolong the effects of the incretins, particularly GLP-1, in patients with T2D, thereby reducing plasma glucose levels ().Citation12,Citation15
DPP-4 inhibition with saxagliptin
In vitro studies have shown that saxagliptin is a highly potent, selective, reversible, and competitive DPP-4 inhibitor. The enzyme-inhibitor dissociation constant (Ki) of saxagliptin for DPP-4 at 37°C is 1.3 nM, making it approximately 10-fold more potent for blocking DPP-4 than sitagliptin.Citation18,Citation19 DPP-4 belongs to a family of ubiquitous atypical serine proteases, which have physiologic functions that go beyond incretin degradation to include effects on endocrine and immune systems.Citation19 This family includes the intracytosolic members, DPP-8 and DPP-9, the specific physiologic function of which remains unclear. Saxagliptin is selective for DPP-4 relative to these other DPP-4 family members; the Ki values for blocking DPP-8 and DPP-9 are 508 and 100 nM, respectively, or approximately 400-fold and 75-fold higher than the concentrations needed to block DPP-4. Moreover, saxagliptin displays >4000-fold higher selectivity for DPP-4 inhibition compared with a panel of other proteases.Citation13,Citation18–Citation20 Saxagliptin has an active metabolite (5-hydroxy saxagliptin; BMS-510849) that is two-fold less active than the parent drug as a DPP-4 inhibitor (Ki = 2.6 nM), but displays approximately two-fold greater selectivity.Citation18,Citation20 Saxagliptin dissociates slowly from the DPP-4 active site with a t1/2 of 50 minutes, whereas slow dissociation has not been seen from any other enzymes tested, including DPP-8 and DPP-9.Citation13,Citation20 This tight but reversible binding to DPP-4 helps to explain why saxagliptin provides prolonged enzyme inhibition.Citation15 Saxagliptin inhibited plasma DPP-4 activity in a dose-related manner over the dose range of 2.5–400 mg once daily in healthy subjects and patients with T2D. DPP-4 activity remained inhibited by 50% and 79% when measured at 24 hours after the 2.5 and 400 mg doses, respectively.Citation21 After an oral glucose load or meal, inhibition of the DPP-4 enzyme by saxagliptin resulted in a two- to three-fold increase in circulating levels of active GLP-1 and GIP, and increased glucose-dependent insulin secretion.Citation1
The effect of saxagliptin on β-cell function was explored in a study of 36 treatment-naïve patients with T2D who received either saxagliptin 5 mg or placebo for 12 weeks.Citation22 Saxagliptin increased β-cell responsiveness to glucose in both the fasting and postprandial states, as evidenced by significant increases from baseline in the insulin secretion rate compared with placebo (P = 0.02 in the fasting state; P = 0.035 following oral glucose load). This was accompanied by a reduction in postprandial glucagon secretion. Moreover, saxagliptin increased and prolonged peak incretin levels, particularly GLP-1, after the oral glucose tolerance test (OGTT).Citation22 These results indicate that saxagliptin blocks DPP-4, slows inactivation of the incretins, in particular GLP-1, and prolongs their biologic actions.Citation1
Dosage, administration, and formulations
Saxagliptin is indicated as an adjunct to diet and exercise to improve glycemic control in adults with T2D. Saxagliptin should not be used in patients with type 1 diabetes or diabetic ketoacidosis, because the pathophysiology of these conditions, as well as the drug’s mechanism of action, would not confer benefit in these settings.Citation1
As indicated by the US label for saxagliptin, the recommended dose of saxagliptin for patients with T2D is 2.5 or 5 mg orally once daily, which can be taken without regard to the timing of meals.Citation1 No dosage adjustment is recommended based on age, gender, or race, or for patients with hepatic impairment or mild renal insuff iciency (ie, creatinine clearance [CrCl] >50 mL/min).Citation1,Citation23,Citation24 However, the dose of saxagliptin should be adjusted to 2.5 mg for those patients with moderate-to-severe renal impairment (CrCl ≤50 mL/min) or end-stage renal disease to achieve optimal saxagliptin plasma concentrations. Because saxagliptin is removed by hemodialysis, doses of saxagliptin should be given after, not before, hemodialysis sessions.Citation1 In addition, although dosage adjustment is not necessary based on age alone, it is important to recognize that elderly patients often have decreased renal function and, therefore, care should be exercised in selecting the dose of saxagliptin for elderly patients whose renal function may be compromised. Citation1 Dose adjustment to 2.5 mg is also recommended for patients who are concomitantly taking ketoconazole or another strong cytochrome P450 (CYP) 3A4/5 inhibitor (eg, atazanavir, clarithromycin, indinavir, itraconazole, nefazodone, nelfinavir, ritonavir, saquinavir, or telithromycin). However, dosage adjustment is not necessary for patients taking moderate CYP 3A4/5 inhibitors, such as diltiazem, verapamil, erythromycin, or grapefruit juice.Citation1
Pharmacokinetics
Absorption and exposure
The pharmacokinetics of saxagliptin were explored in two randomized, double-blind, placebo-controlled, sequential ascending-dose trials.Citation21 The first trial evaluated saxagliptin at doses from 2.5 to 50 mg once daily for 14 days in patients with T2D; the second trial evaluated doses from 100 to 400 mg once daily for 14 days in healthy volunteers. No differences in the pharmacokinetics of saxagliptin or 5-hydroxy saxagliptin were apparent between the patients with T2D and healthy volunteers.Citation21 Saxagliptin was well absorbed following oral administration, with time to peak plasma concentrations (Tmax) of saxagliptin and 5-hydroxy saxagliptin reached by ≤2 and ≤4 hours, respectively. Systemic exposure (area under the plasma concentration-time curve [AUC]) to saxagliptin and 5-hydroxy saxagliptin was proportional to the saxagliptin dose over the entire dose range from 2.5 to 400 mg, with no substantial accumulation or reduction in systemic exposure seen with the once-daily dosing regimen.Citation1,Citation21 On a molar basis, systemic exposure to 5-hydroxy saxagliptin was several-fold higher than for saxagliptin.Citation21 For example, following a 5 mg dose, the mean plasma AUC values for saxagliptin and 5-hydroxy saxagliptin were 78 and 214 ng.h/mL, respectively.Citation1 The mean t1/2 range of saxagliptin was 2.2 to 3.8 hours, and the t1/2 range of 5-hydroxy saxagliptin was 3.0 to 7.4 hours; both were independent of the saxagliptin dose. Approximately 70% of the administered dose was recovered in urine as the parent drug or 5-hydroxy saxagliptin.Citation21
When administered with a high-fat meal, the Tmax was delayed by 20 minutes and was associated with a 27% increase in the AUC of saxagliptin compared with dosing in a fasted condition. These findings indicated that saxagliptin can be taken with or without food.Citation25
Metabolism and elimination
Saxagliptin is eliminated by both renal and hepatic pathways. Following a single 50 mg dose of [14C]-saxagliptin, 24% and 36% of the administered dose was excreted in the urine as saxagliptin and 5-hydroxy saxagliptin, respectively. Active renal secretion of saxagliptin occurs, in so far as the mean renal clearance of the drug (~230 mL/min) was greater than the estimated glomerular filtration rate (~120 mL/min). Overall, 75% of the administered radioactivity was recovered in the urine and 22% in the feces, the latter representing the fraction of the dose excreted in bile or unabsorbed from the gastrointestinal tract.Citation1
The metabolism of saxagliptin is primarily mediated by CYP 3A4/5 and, consequently, the coadministration of saxagliptin with strong CYP 3A4/5 inhibitors or inducers could alter the pharmacokinetics of saxagliptin and 5-hydroxy saxagliptin.Citation1 When the strong CYP 3A4/5 inhibitor, ketoconazole (200 mg at 12-hourly intervals for nine days) was coadministered with a single 100 mg dose of saxagliptin in healthy subjects, it significantly increased the time to peak concentration (Cmax) and AUC from zero to infinity (AUC∞) of saxagliptin by 62% and 145%, respectively, compared with administration of saxagliptin alone. Exposure to 5-hydroxy saxagliptin, however, was reduced by 88%.Citation26 Based on these findings, reducing the dose of saxagliptin to 2.5 mg once daily for patients who are concomitantly taking ketoconazole or another strong CYP 3A4/5 inhibitor is recommended.Citation1
Concomitant administration of long-acting diltiazem (360 mg once daily for nine days), a moderate CYP 3A4/5 inhibitor, with a single 10 mg dose of saxagliptin significantly increased the Cmax and AUC∞ of saxagliptin by 63% and 109%, respectively, compared with the administration of saxagliptin alone.Citation27 Given the magnitude of the increase in exposure, dosage adjustment of saxagliptin is not needed when coadministered with diltiazem or other moderate CYP 3A4/5 inhibitors.Citation1 Coadministration of the CYP 3A4/5 substrate simvastatin (40 mg once daily for four days) with saxagliptin (10 mg once daily for four days) did not produce a clinically significant effect on systemic exposure to either drugCitation28 and, therefore, no dosage adjustment is needed when saxagliptin is given with mild CYP 3A4/5 inhibitors or substrates.Citation27
Coadministration of metformin 1000 mg, glyburide 5 mg, or pioglitazone 45 mg once daily for four days with single doses of saxagliptin revealed no clinically significant drug-drug interactions.Citation29–Citation31 Similarly, clinically significant interactions were not evident between saxagliptin and digoxin, famotidine, omeprazole, or the antacid containing aluminum hydroxide + magnesium hydroxide + simethicone.Citation1,Citation32,Citation33 Therefore, dosage adjustments are not needed when saxagliptin is coadministered with any of these agents.
Clinical trials
The efficacy and safety of saxagliptin were evaluated in a series of well-controlled clinical trials that enrolled treatment-naïve patients with T2D (),Citation6,Citation7 or those patients unable to achieve or maintain glycemic control with metformin,Citation9 a sulfonylurea (glyburide),Citation10 or a thiazolidinedione. Citation11 Pregnant women, as well as children and adolescents aged <18 years, were excluded from these trials. Saxagliptin carries a pregnancy category B label, reflecting that adequate and well-controlled trials have not been conducted in pregnant women. Consequently, saxagliptin, like many other antidiabetic medications, should be used during pregnancy only if clearly needed.Citation1
Table 1 Summary of the effects of saxagliptin 5 mg on glycemic parameters in controlled 24-week clinical trials
Efficacy of saxagliptin monotherapy
The efficacy of saxagliptin monotherapy was initially evaluated in a multicenter, randomized, double-blind, placebo-controlled, parallel-group, dose-ranging trial conducted at 152 US sites.Citation8 A total of 338 treatment-naïve patients with T2D with inadequate glycemic control (baseline glycated hemoglobin [HbA1c] 6.8%–9.7%) were randomly allocated to treatment with saxagliptin 2.5, 5, 10, 20, or 40 mg once daily or placebo for 12 weeks, and an additional cohort of 85 patients were randomized to saxagliptin 100 mg once daily or placebo for six weeks.Citation8 Starting from a relatively low mean baseline HbA1c of 7.9%, saxagliptin at doses of 2.5–40 mg produced consistent and clinically significant HbA1c decreases of 0.72% to 0.90% compared with a 0.27% reduction in the placebo group (P < 0.007).Citation8 The placebo-adjusted change from baseline in HbA1c ranged from −0.45% to −0.63%. More patients with baseline HbA1c ≥7% achieved the glycemic target of HbA1c <7% with saxagliptin than with placebo (41%–53% versus 20%).Citation8 Comparable results were achieved in the high-dose cohort, in which saxagliptin 100 mg reduced HbA1c from baseline by 1.09% versus 0.36% for placebo, and 66% versus 22% of patients achieved the HbA1c target of <7%.Citation8 At each dose level, saxagliptin also reduced fasting plasma glucose (FPG) levels, which was first evident by the second week of treatment, as well as one-hour postprandial glucose (PPG) as part of an OGTT.Citation8 In general, the efficacy of saxagliptin in reducing HbA1c and FPG was greatest in subgroups with higher baseline values of these glycemic parameters.Citation8
Based on the results of the dose-ranging study, a multicenter, randomized, double-blind, placebo-controlled Phase III trial was conducted to compare saxagliptin 2.5, 5, and 10 mg once daily versus placebo.Citation6 A total of 401 treatment-naïve T2D patients with baseline HbA1c >7% to 10% were allocated to study treatment for 24 weeks; mean baseline HbA1c and FPG were 7.9% and 9.71 mmol/L, respectively.Citation6 Saxagliptin produced clinically significant improvements in HbA1c and FPG at each dose tested. The mean changes from baseline in HbA1c were −0.43%, −0.46%, and −0.54% with the 2.5, 5, and 10 mg doses, respectively, compared with an increase of 0.19% in the placebo group (all P < 0.0001). Similarly, the mean changes in FPG were −0.83, −0.50, and −0.94 mmol/L, respectively, compared with an increase of 0.33 mmol/L in the placebo group (P ≤ 0.0074).Citation6 The two higher saxagliptin doses allowed a significantly greater number of patients to achieve the HbA1c target of <7% compared with placebo (38% and 41% versus 24%; P ≤ 0.05), and each saxagliptin group had significantly greater reductions in PPG-AUC in the 75 g OGTT (P ≤ 0.0002). This study also enrolled an open-label cohort of 68 patients with a baseline HbA1c between 10% and 12% who received saxagliptin 10 mg once daily for 24 weeks.Citation6 Saxagliptin produced greater mean improvements in HbA1c (−1.9%), FPG (−1.83 mmol/L), and PPG-AUC (−615 mmol·min/L) in this cohort than in the main study, consistent with their poorer glycemic status at baseline.Citation6
Taken together, these two clinical trials demonstrated that saxagliptin monotherapy produced clinically significant improvements in glycemic control compared with placebo in treatment-naïve patients with T2D. The clinical benefit of saxagliptin was evident across a wide range of baseline HbA1c levels, and in general, greater HbA1c decreases were achieved in patients with poorer glycemic control at baseline.Citation6,Citation8
Efficacy of saxagliptin in combination therapy
Saxagliptin was evaluated in combination with metformin in two clinical trials, one evaluating add-on saxagliptin in patients with inadequate glycemic control on stable doses of metformin,Citation9 and the other evaluating saxagliptin in combination with metformin as initial therapy.Citation7
The saxagliptin add-on trial was a randomized, double-blind, placebo-controlled multicenter study that enrolled 743 patients with T2D and an HbA1c of 7% to 10% despite treatment with stable metformin doses from 1500 to 2550 mg/day.Citation9 Patients were randomly allocated to receive saxagliptin 2.5, 5, or 10 mg once daily or placebo for 24 weeks in addition to continuing their stable open-label metformin dose. From a mean baseline HbA1c of 8.0%, add-on saxagliptin 2.5, 5, and 10 mg significantly reduced HbA1c by −0.59%, −0.69%, and −0.58%, respectively, compared with an increase of 0.13% with add-on placebo (all P ≤ 0.0001).Citation9 Clinically relevant and statistically significant reductions from baseline to week 24 for patients taking saxagliptin 2.5, 5, and 10 mg in FPG (0.79, 1.22, and 1.14 mmol/L, respectively; all P < 0.0001) and PPG-AUC (−493, −532, and −452 mmol.min/L, respectively; all P < 0.0001), as well as in the proportion of patients achieving an HbA1c target <7% were achieved compared with placebo, with maximal benefit seen in the saxagliptin 5 mg dose group. Patients receiving add-on saxagliptin had significant increases in postprandial insulin compared with those receiving add-on placebo in the OGTT (all P < 0.0001), and in addition, β-cell function measured by the HOMA-2β method was improved in all saxagliptin treatment groups.Citation9 Patients who completed the 24-week study period with or without need for rescue medication were eligible to enter a 42-month long-term extension period,Citation34 during which saxagliptin added to metformin provided sustained glycemic improvement. At 102 weeks, the placebo-corrected decrease in HbA1c was −0.62%, −0.72%, and −0.52% with add-on saxagliptin 2.5, 5, and 10 mg, respectively.Citation34
The initial combination therapy trial was a multicenter, randomized, double-blind, 24-week study that enrolled 1306 treatment-naïve patients with T2D and HbA1c between 8% and 12% (mean baseline HbA1c = 9.5%).Citation7 Patients were randomly allocated to one of four treatment arms: saxagliptin 5 mg + metformin 500 mg, saxagliptin 10 mg + metformin 500 mg, saxagliptin 10 mg + placebo, or metformin 500 mg + placebo. The dose of metformin was increased to 1000 mg after one week, then titrated in 500 mg increments to a maximum of 2000 mg/day if FPG was >6.11 mmol/L. Saxagliptin was administered once daily, while metformin was given in two divided doses. Initial therapy with the saxagliptin–metformin combination significantly improved HbA1c, FPG, and PPG-AUC compared with treatment with either saxagliptin or metformin alone.Citation7 The mean decrease from baseline HbA1c was 2.5% in each combination group compared with reductions of 1.7% and 2.0% for saxagliptin or metformin alone, respectively (P < 0.0001 versus each monotherapy). The difference in HbA1c was evident by week 4—the first time point assessed—and was maintained throughout the remainder of the treatment period. Moreover, the combination of saxagliptin 5 or 10 mg + metformin allowed a significantly greater proportion of patients to reach HbA1c targets <7% than either saxagliptin or metformin alone (60.3% and 59.7% versus 32.2% and 41.1%, respectively; all P < 0.0001 for combination versus monotherapy).Citation7 A similar relationship favoring combination therapy was seen when achievement of HbA1c ≤6.5% was assessed.Citation7 Saxagliptin 5 mg + metformin significantly improved FPG (−3.33 mmol/L) compared with metformin (−2.61 mmol/L, P = 0.0002) and saxagliptin (−1.72 mmol/L; P < 0.0001) monotherapy, and similarly produced significant improvements in PPG-AUC compared with either agent alone (both P < 0.0001). In addition, saxagliptin–metformin therapy produced significant improvements in β-cell function (based on HOMA-2β assessment, compared with saxagliptin [P < 0.0001] or metformin [P ≤ 0.0004] alone), numerically greater increases in postprandial insulin compared with metformin alone, and in early insulin response measured by the insulinogenic index compared with either agent alone.Citation7
Add-on saxagliptin therapy was evaluated in two other multicenter, randomized, double-blind Phase III trials (of sulfonylurea [glyburide] or thiazolidinediones).Citation10,Citation11 In the glyburide add-on trial, 768 patients were enrolled who had inadequate glycemic control defined by HbA1c of 7.5% to 10% while receiving a sulfonylurea at less than the maximum approved dose (mean baseline HbA1c = 8.4%).Citation10 Patients were randomly allocated to one of three treatment groups using a double-dummy design, saxagliptin 2.5 mg/day, saxagliptin 5 mg/day, or glyburide 2.5 mg/day, each in addition to open-label glyburide 7.5 mg/day. Blinded titration in the glyburide monotherapy group to a maximum total daily dose of 15 mg was allowed at weeks 2 and 4. By the end of the 24-week study, the mean total daily dose of glyburide was 7.4 mg in the combination therapy group compared with 14.6 mg in the monotherapy group, with 92% of patients in the glyburide monotherapy group receiving the maximum allowed dose of 15 mg.Citation10 Saxagliptin + glyburide significantly improved HbA1c, FPG, and PPG compared with glyburide uptitration.Citation10 The adjusted mean change from baseline in HbA1c was −0.54% and −0.64% with add-on saxagliptin 2.5 and 5 mg, respectively, compared with an increase in HbA1c of 0.08% with glyburide monotherapy (both P < 0.0001). The corresponding changes in FPG were −0.40 and −0.50 mmol/L versus +0.04 mmol/L (P = 0.022 and P = 0.002, respectively) and in PPG-AUC −2 and −2 mmol·min/L versus +0.44 mmol·min/L (both P < 0.0001).Citation10 The proportion of patients reaching the HbA1c target of <7% was approximately 1.5 times higher with add-on saxagliptin compared with glyburide uptitration (22.4% and 22.8% versus 9.1%; P < 0.0001).Citation10 The proportion reaching the more aggressive HbA1c target of ≤6.5% was also higher with add-on saxagliptin 5 mg than with glyburide alone (10.4% versus 4.5%; P = 0.012).
Add-on saxagliptin was also evaluated in T2D patients with HbA1c of 7% to 10% despite treatment with stable doses of a thiazolidinedione—either pioglitazone 30 or 45 mg once daily or rosiglitazone 4 or 8 mg once daily or 4 mg twice daily.Citation11 In this study, a total of 565 patients were randomly allocated to receive saxagliptin 2.5 or 5 mg once daily or placebo for 24 weeks in addition to continuing on their stable dose of thiazolidinedione. Consistent with the other trials, add-on saxagliptin 2.5 and 5 mg significantly improved HbA1c, FPG, and PPG compared with thiazolidinedione alone.Citation11 For HbA1c, the mean changes from baseline were −0.66% and −0.94% with add-on saxagliptin 2.5 and 5 mg, respectively, compared with −0.30% with the thiazolidinedione alone (P = 0.0007 and P < 0.0001, respectively), and again, significantly more patients treated with add-on saxagliptin than with a thiazolidinedione alone were able to achieve the HbA1c targets of <7% (42.2% and 41.8% versus 25.6%, P = 0.001) and ≤6.5% (19.3% and 20.7% versus 9.4%). In addition, add-on saxagliptin, particularly at the 5 mg dose, improved β-cell function as reflected by increases in HOMA-2β, and increased postprandial insulin and reduced PPG in the OGTT, compared with thiazolidinedione alone.Citation11
In the latter study, each treatment group had a small increase in mean body weight (1.3 and 1.4 kg with add-on saxagliptin 2.5 or 5 mg compared with 0.9 kg with a thiazolidinedione alone).Citation11 Weight gain is a well-recognized side effect of thiazolidinedione therapy, presumably resulting from fluid retention and increased adipose tissue.Citation11 Similarly, in the trial with glyburide, small increases in mean body weight were seen in each treatment group (0.7 and 0.8 kg with add-on saxagliptin 2.5 or 5 mg compared with 0.3 kg with glyburide uptitration; P = 0.038 and P = 0.012, respectively).Citation10 In comparison, saxagliptin had weight neutral effects when used as monotherapy,Citation6,Citation8 or in combination with metformin.Citation7,Citation9
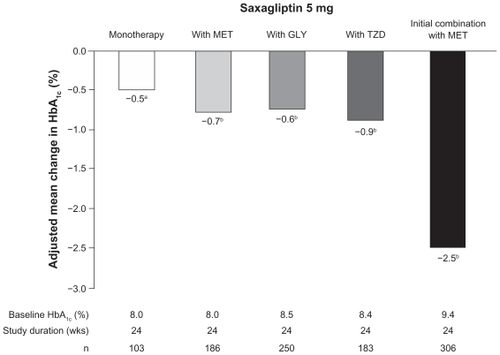
Taken together, these trials demonstrate that saxagliptin added to metformin, glyburide, or a thiazolidinedione significantly improves glycemic control in patients inadequately controlled by metformin, glyburide, or a thiazolidinedione alone ().Citation7,Citation9–Citation11
Figure 3 Efficacy of saxagliptin in monotherapy, add-on combination therapy, or initial combination therapy in patients with T2D. Shown are the adjusted mean changes in HbA1c with saxagliptin 5 mg once daily in the 24-week trials.Citation6,Citation7,Citation9–Citation11
Abbreviations: GLY, glyburide; HbA1c, glycated hemoglobin; MET, metformin; TZD, thiazolidinedione.
A pooled analysis of five 24-week Phase III clinical trials was conducted to explore the efficacy of saxagliptin in elderly patients.Citation35 In total, saxagliptin 5 mg was administered as monotherapy or add-on therapy to 882 adults in these trials. Saxagliptin 5 mg produced similar reductions in HbA1c in patients aged ≥65 years as in those aged <65 years. Saxagliptin produced mean changes from baseline HbA1c of −0.73% (compared with −0.17% for placebo) in the elderly cohort and −0.68% (compared with −0.01%) in the younger adult cohort. The tolerability of saxagliptin also did not differ between the younger and older patients.Citation35
Safety and tolerability of saxagliptin
Saxagliptin was generally well tolerated whether administered alone or in combination with metformin, glyburide, or a thiazolidinedione. Across clinical trials, the incidence of adverse events in the saxagliptin 5 mg treatment arms was comparable with the rates in the control arms ().Citation6,Citation7,Citation9–Citation11 In a placebo-controlled pooled safety analysis, the incidence of gastrointestinal adverse events was comparable in patients receiving saxagliptin 5 mg (18.4%) versus a comparator (19.1%). Furthermore, the percentage of patients receiving metformin as initial combination therapy with saxagliptin 5 mg had a similar frequency of gastrointestinal adverse events compared with those on metformin monotherapy (20.1%). Patients treated with saxagliptin 5 mg plus a thiazolidinedione had a higher rate of edema, most commonly peripheral edema, than those patients who received a thiazolidinedione alone (8.1% versus 4.3%).Citation11 The higher incidence of edema with a pedal distribution is well recognized to occur when a thiazolidinedione is used in combination with another glucose-lowering agent.Citation36
Table 2 Incidence of common adverse events and hypoglycemia in controlled 24-week clinical trials with saxagliptin 5 mg
Hypoglycemia—when defined as events consistent with signs or symptoms of hypoglycemia with or without a documented blood glucose level—was reported by up to 5% of patients who received saxagliptin 5 mg alone or in combination with metformin or a thiazolidinedione, and by nearly 15% when saxagliptin was given in combination with glyburide ().Citation6,Citation7,Citation9–Citation11 These rates, however, were comparable with the rates seen in the control groups. Treatment with combinations that include a sulfonylurea have historically been associated with increased risk of hypoglycemia. However, the incidence of reported hypoglycemic events (14.6% versus 10.1%; P = 0.14) and confirmed hypoglycemic events (0.8% versus 0.7%; P = 1.00) did not differ significantly between the saxagliptin 5 mg plus glyburide and glyburide uptitration groups.Citation10 In the authors’ opinion, the dose of the sulfonylurea should be reduced, rather than the dose of saxagliptin, in patients who experience hypoglycemia with the combination.Citation10
In a pooled analysis of the three Phase III trials evaluating add-on saxagliptin 5 mg, the incidence of reported hypoglycemia was 8.3% in the saxagliptin arms compared with 6.8% in the placebo arms.Citation37 Less than 2.5% of the reported hypoglycemia episodes across all studies were confirmed by a fingerstick glucose level ≤2.78 mmol/L in association with symptoms (). Even when higher doses of saxagliptin were included in the analysis, the incidence of confirmed hypoglycemia remained infrequent in both younger adults and elderly patients.Citation37 In the long-term extension study, adding saxagliptin 5 mg to metformin did not increase the incidence of reported hypoglycemia compared with adding placebo to metformin (8.9% versus 10.1%), and confirmed hypoglycemia was rare in both groups (≤1.1%).Citation34 These findings show that saxagliptin used as monotherapy or in combination therapy has a low propensity for causing hypoglycemia in patients with T2D.Citation37
In late 2008, the US Food and Drug Administration (FDA) issued guidance recommendations for demonstrating that an investigational antidiabetic agent for T2D is not associated with unacceptable increases in cardiovascular risk.Citation38 Specifically, a meta-analysis of important cardiovascular events should be conducted across Phase II and Phase III controlled clinical trials, and if possible, it should also explore similarities and differences among subgroups.Citation38 Saxagliptin met this FDA recommendation in a post hoc analysis.Citation38 The meta-analysis included 4607 patients, of whom 3356 were exposed to saxagliptin (including 1269 to the 5 mg dose) and 1251 to control treatment.Citation39 This dataset included 3758 patient-years of exposure to saxagliptin. Overall, the incidence of major cardiovascular events (MACE), consisting of cardiovascular death, nonfatal myocardial infarction, and nonfatal stroke, was 0.7% among patients treated with saxagliptin (0.5% for those who received the 5 mg dose) compared with 1.4% for patients who received control treatment (hazard ratio 0.44; 95% confidence interval 0.24–0.82). Moreover, the incidence of MACE was lower with saxagliptin than controls in most subgroups considered to be at increased cardiovascular risk, including patients with a history of cardiovascular disease, patients with at least one cardiovascular risk factor besides T2D, patients with a history of hypertension or hypercholesterolemia, and male patients. This meta-analysis showed no evidence of increased cardiovascular risk in patients with T2D exposed to saxagliptin for up to 2.5 years.Citation39 A prospective outcomes trial to evaluate whether or not saxagliptin may have a cardioprotective effect is currently underway.Citation40
Guideline recommendations for DPP-4 inhibitors
The American Association of Clinical Endocrinologists and American College of Endocrinology recommend a glycemic target (HbA1c) of 6.5%.Citation41 Similarly, the Asociación Latinoamericana de Diabetes defines an appropriate HbA1c level as <6.5%.Citation42 According to the American Association of Clinical Endocrinologists/American College of Endocrinology algorithm, the treatment approach should be stratified based on the current HbA1c.Citation41 Monotherapy may be used initially for patients with HbA1c ≤7.5%, and if not sufficient to achieve the glycemic target, then an additional medication (ie, add-on therapy) is recommended. For patients with HbA1c of 7.6% to 9.0%, combination therapy with two antidiabetic agents should be initiated, because no single agent is likely to achieve the target glycemic control level. Metformin is recommended as the cornerstone of therapy, or if contraindicated due to renal or hepatic disease, gastrointestinal intolerance, or risk of lactic acidosis, a thiazolidinedione may be used.Citation41 Because these agents serve as insulin sensitizers, DPP-4 inhibitors are an appropriate option for use as the second agent in dual-therapy regimens. For asymptomatic patients with HbA1c >9.0%, triple therapy may be initiated, such as metformin, a DPP-4 inhibitor, and either a sulfonylurea or thiazolidinedione.Citation41
The American Diabetes Association and European Association for the Study of Diabetes recommend an HbA1c target of <7%.Citation43 Metformin is also the cornerstone of treatment in the American Diabetes Association/European Association for the Study of Diabetes algorithm, but either a sulfonylurea or insulin is suggested for add-on therapy, with the latter recommended for patients with HbA1c >8.5%. DPP-4 inhibitors, like several other antidiabetic drug classes are not listed in the treatment algorithm, but are recognized to be an appropriate choice for selected patients.Citation43 The Asociación Latinoamericana de Diabetes has set a more aggressive target for glycemic control in patients with T2D. The Asociación Latinoamericana de Diabetes guidelines recommend an HbA1c of <6%, FPG <5.56 mmol/L, and a one- to two-hour PPG <7.78 mmol·min/L.Citation42
Conclusions
Multiple classes of oral antidiabetic agents are available for managing T2D. Although improved glycemic control may be achieved initially, the efficacy of these agents may diminish over time due in part to increasing β-cell dysfunction that occurs with disease progression.Citation13 Traditionally, T2D was managed with metformin, sulfonylureas, and insulin, but surveys indicated that many patients fail to achieve recommended HbA1c targets. Despite increased awareness about the importance of tight glycemic control, as well as the availability of several new antidiabetic agents, surveys continue to show that many patients are still not at recommended glycemic targets. In Brazil, a recent survey found that only 23% of patients with T2D had achieved an HbA1c <7%.Citation44 Higher glycemic control rates were reported in the National Health and Nutrition Examination Surveys conducted in 1999–2002 and 2003–2004 in the United States but still >40% of patients with T2D had not achieved an HbA1c <7%, even though many reported that they were taking either oral hypoglycemic drugs or insulin.Citation45,Citation46
With the evolution of T2D management, treatment guidelines issued by professional organizations are being updated to include the use of DPP-4 inhibitors (as discussed above).Citation41,Citation42 Like other DPP-4 inhibitors, saxagliptin is effective, well tolerated, and can be administered orally once daily with or without food.Citation1 As shown across multiple controlled clinical trials, saxagliptin, in particular, is effective in reducing HbA1c, FPG, and PPG, whether used as monotherapy or in combination with other oral agents, including metformin, sulfonylureas, and thiazolidinediones. Moreover, saxagliptin and other DPP-4 inhibitors offer an attractive safety and tolerability profile, with a low risk of hypoglycemia and gastrointestinal intolerance when added on to existing therapy, compared with a glinide or sulfonylurea.Citation7,Citation9–Citation11,Citation41,Citation43 Saxagliptin is weight neutral and also has a beneficial effect on β-cell function.Citation22,Citation43 Taken together, the efficacy, safety, and tolerability of saxagliptin combined with the convenience of once-daily dosing makes it an attractive agent for use in the management of T2D.
Disclosure
Dr Antonio R Chacra has received research grants from Eli Lilly, MSD, Novartis, Novo Nordisk, Bristol-Myers Squibb, AstraZeneca, and sanofi-aventis. He is a member of the Brazilian Bristol-Myers Squibb/AstraZeneca Saxagliptin Board and has received honorarium fees for lecturing on saxagliptin. Technical and editorial assistance was provided by Gina Coviello, MS, Quintiles Medical Communications, Parsippany, NJ, USA.
References
- Onglyza [package insert] Princeton, NJ/Wilmington, DE Bristol-Myers Squibb Company/AstraZeneca Pharmaceuticals LP 7 2009
- Januvia [package insert] Whitehouse Station, NJ Merck and Co., Inc. 2 2010
- Galvus [European Public Assessment Report] West Sussex, UK Novartis Europharm Limited Available from: http://www.ema.europa.eu/humandocs/Humans/EPAR/galvus/galvus.htm Accessed Jun 8, 2010
- Augeri DJ Robl JA Betebenner DA Discovery and preclinical profile of Saxagliptin (BMS-477118): A highly potent, long-acting, orally active dipeptidyl peptidase IV inhibitor for the treatment of type 2 diabetes J Med Chem 2005 48 15 5025 5037 16033281
- Fura A Khanna A Vyas V Pharmacokinetics of the dipeptidyl peptidase 4 inhibitor saxagliptin in rats, dogs, and monkeys and clinical projections Drug Metab Dispos 2009 37 6 1164 1171 19251818
- Rosenstock J Aguilar-Salinas C Klein E CV181-011 Study Investigators Effect of saxagliptin monotherapy in treatment-naive patients with type 2 diabetes Curr Med Res Opin 2009 25 10 2401 2411 19650754
- Jadzinsky M Pfützner A Paz-Pacheco E CV181-039 Investigators Saxagliptin given in combination with metformin as initial therapy improves glycemic control in patients with type 2 diabetes compared with either monotherapy: A randomized controlled trial Diabetes Obes Metab 2009 11 6 611 622 19515181
- Rosenstock J Sankoh S List JF Glucose-lowering activity of the dipeptidyl peptidase-4 inhibitor saxagliptin in drug-naive patients with type 2 diabetes Diabetes Obes Metab 2008 10 5 376 386 18355324
- DeFronzo RA Hissa M Garber AJ Saxagliptin 014 Study Group The efficacy and safety of saxagliptin when added to metformin therapy in patients with inadequately controlled type 2 diabetes on metformin alone Diabetes Care 2009 32 9 1649 1655 19478198
- Chacra AR Tan GH Apanovitch A Ravichandran S List J Chen R Saxagliptin added to a submaximal dose of sulphonylurea improves glycaemic control compared with uptitration of sulphonylurea in patients with type 2 diabetes: A randomised controlled trial Int J Clin Pract 2009 63 9 1395 1406 19614786
- Hollander P Li J Allen E Chen R CV181-013 Investigators Saxagliptin added to a thiazolidinedione improves glycemic control in patients with type 2 diabetes and inadequate control on thiazolidinedione alone J Clin Endocrinol Metab 2009 94 12 4810 4819 19864452
- Drucker DJ The biology of incretin hormones Cell Metab 2006 3 3 153 165 16517403
- Tahrani AA Piya MK Barnett AH Saxagliptin: A new DPP-4 inhibitor for the treatment of type 2 diabetes mellitus Adv Ther 2009 26 3 249 262 19330494
- Ahrén B Landin-Olsson M Jansson PA Svensson M Holmes D Schweizer A Inhibition of dipeptidyl peptidase-4 reduces glycemia, sustains insulin levels, and reduces glucagon levels in type 2 diabetes J Clin Endocrinol Metab 2004 89 5 2078 2084 15126524
- Barnett A DPP-4 inhibitors and their potential role in the management of type 2 diabetes Int J Clin Pract 2006 60 11 1454 1470 17073841
- Baggio LL Drucker DJ Biology of incretins: GLP-1 and GIP Gastroenterology 2007 132 6 2131 2157 17498508
- Drucker DJ The role of gut hormones in glucose homeostasis J Clin Invest 2007 117 1 24 32 17200703
- Kirby MS Dorso C Wang A In vitro enzymologic characteristics of saxagliptin, a highly potent and selective DPP4 inhibitor with ‘slow binding’ characteristics Abstract presented at: 3rd International Conference on Dipeptidyl Peptidase and Related Proteins 2008, Apr 23–25 Antwerp, Belgium
- Kirby M Yu DM O’Connor S Gorrell MD Inhibitor selectivity in the clinical application of dipeptidyl peptidase-4 inhibition Clin Sci (Lond) 2010 118 1 31 41 19780719
- Wang A Dorso C Kopcho L Marcinkeviciene J Kirby MS Implications of the prolonged dissociation rate of saxagliptin, a highly potent and selective DPP4 inhibitor, on plasma DPP measurements [ADA Abstr 2088-PO] Diabetes 2008 57 Suppl 1 A576 A577
- Boulton D Geraldes M Safety, tolerability, pharmacokinetics and pharmacodynamics of once-daily oral doses of saxagliptin for 2 weeks in type 2 diabetic and healthy subjects Diabetes 2009 56 Suppl 1 606P
- Henry R Smith S Schwartz S List JF Duan Y Chen R β-cell stimulation by saxagliptin in patients with type 2 diabetes Diabetes 2009 58 Suppl 1 A119
- Boulton D Goyal A Li L Kornhauser D Frevert E The effects of age and gender on the single-dose pharmacokinetics and safety of saxagliptin in healthy subjects [ADA Abstr 551-P] Diabetes 2008 57 Suppl 1 A165
- Patel C Castaneda L Frevert U Li L Kornhauser DM Boulton DW Single-dose pharmacokinetics and safety of saxagliptin in subjects with hepatic impairment compared with healthy subjects [ADA Abstr 537-P] Diabetes 2008 57 Suppl 1 A160
- Patel CG Zhang J Li L Effect of a high-fat meal on the pharmacokinetics of saxagliptin in healthy subjects J Clin Pharmacol 2010 Feb 11 [Epub ahead of print]
- Patel CG Boulton DW Brenner E Royzman K Li L Effect of ketoconazole on the pharmacokinetics of saxagliptin in healthy subjects J Clin Pharmacol 2007 47 Abstr 89
- Girgis S Patel C Li L Effect of diltiazem on the pharmacokinetics of saxagliptin in healthy subjects J Clin Pharmacol 2007 47 Abstr 72
- Girgis S You X Li L Maurer C Whigan D Boulton DW Effect of simvastatin on the pharmacokinetics of saxagliptin in healthy subjects J Clin Pharmacol 2007 47 Abstr 28
- Patel CG Wolf RA Komoroski B Li L Boulton DW No meaningful pharmacokinetic drug-drug interaction between saxagliptin and pioglitazone in healthy subjects Poster presented at: The American College of Clinical Pharmacy Annual Meeting 2007 Oct 14–17 Denver, CO Abstr 226
- Patel CG Komoroski B Li L Boulton DW No meaningful pharmacokinetic drug-drug interaction between saxagliptin and glyburide in healthy subjects Poster presented at: The American College of Clinical Pharmacy Annual Meeting 2007 Oct 14–17 Denver, CO Abstr 212
- Patel CG Komoroski B Brenner E Li L Boulton DW No meaningful pharmacokinetic drug-drug interactions between saxagliptin and metformin in healthy subjects Poster presented at: The American College of Clinical Pharmacy (ACCP) 2007 Oct 14–17 Denver, CO Abstr 213
- Boulton DW Li L Patel CG No pharmacokinetic interaction between saxagliptin and digoxin in healthy subjects [Abstr PIII-69] Clin Pharmacol Ther 2008 83 Suppl 1 S93
- Boulton DW Adams D Li L Maalox® Max, famotidine or omeprazole do not meaningfully affect the pharmacokinetics of saxagliptin in healthy subjects Clin Pharmacol Ther 2008 83 Suppl 1 S92
- DeFronzo R Hissa MN Garber AJ Duan RY Ravichandran S Chen R Once-daily saxagliptin added to metformin provides sustained glycemic control and is well tolerated over 102 weeks in patients with type 2 diabetes [ADA Abstr 547-P] Diabetes 2009 58 Suppl 1 A147
- Maheux P Doucet J Allen E Efficacy and safety of saxagliptin 5 mg once-daily therapy in elderly patients with type 2 diabetes mellitus [EASD Abstr 766-P] Diabetologia 2009 52 Suppl 1 S302
- Nesto RW Bell D Bonow RO Thiazolidinedione use, fluid retention, and congestive heart failure: A consensus statement from the American Heart Association and American Diabetes Association Diabetes Care 2004 27 1 256 263 14693998
- Chen R Donovan M Rusnak JM Saxagliptin used as monotherapy or in combination with other antihyperglycemic agents does not significantly increase risk of hypoglycemia [ADA Abstr 2082-PO] Diabetes 2009 58 Suppl 1 A536
- US Food and Drug Administration Guidance for industry: Diabetes mellitus –evaluating cardiovascular risk in new antidiabetic therapies to treat type 2 diabetes Silver Spring, MD Center for Drug Evaluation and Research 12 2008
- Frederich R Alexander TH Fiedorek FT A systematic assessment of cardiovascular outcomes in the saxagliptin drug development program for type 2 diabetes Postgrad Med 2010 122 3 16 27 20463410
- Bristol-Myers Squibb/AstraZeneca Does saxagliptin reduce the risk of cardiovascular events when used alone or added to other diabetes medications (SAVOR-TIMI 53) ClinicalTrialsgov [Internet] Bethesda (MD) National Library of Medicine (US) 2010 [cited 2010 May 4]. Available from: http://clinicaltrials.gov/ct2/show/NCT01107886. NLM Identifier: NCT01107886 Accessed on Jun 30, 2010
- Rodbard HW Jellinger PS Davidson JA Statement by an American Association of Clinical Endocrinologists/American College of Endocrinology consensus panel on type 2 diabetes mellitus: An algorithm for glycemic control Endocr Pract 2009 15 6 541 559
- Asociación Latinoamericana de Diabetes Guías ALAD de diagnóstico control y tratamiento de la diabetes mellitus tipo 2 Available from: http://www.sld.cu/galerias/pdf/sitios/diabetes/guias_alad_de_diagnostico_y_tratamiento_de_la_diadetes_tipo_2(2006.pdf) Accessed on Mar 25, 2010
- Nathan DM Buse JB Davidson MB Medical management of hyperglycemia in type 2 diabetes: A consensus algorithm for the initiation and adjustment of therapy: A consensus statement of the American Diabetes Association and the European Association for the Study of Diabetes Diabetes Care 2009 32 1 193 203 18945920
- Mendes AB Fittipaldi JA Neves RC Chacra AR Moreira EDJr Prevalence and correlates of inadequate glycaemic control: Results from a nationwide survey in 6,671 adults with diabetes in Brazil Acta Diabetol 2010 47 2 137 145 19655083
- Resnick HE Foster GL Bardsley J Ratner RE Achievement of American Diabetes Association clinical practice recommendations among US adults with diabetes, 1999–2002: The National Health and Nutrition Examination Survey Diabetes Care 2006 29 3 531 537 16505501
- Hoerger TJ Segel JE Gregg EW Saaddine JB Is glycemic control improving in US adults? Diabetes Care 2008 31 1 81 86 17934153