Abstract
A paradoxical metabolic imbalance in inorganic phosphate occurs from the early onset of diabetes and may lead to a reduction of high energy phosphates and tissue hypoxia. These changes take place in the cells and tissues in which the entry of glucose is not controlled by insulin, and particularly in poorly regulated diabetes patients in whom long-term vascular complications are more likely to occur. Several therapeutic intervention trials have been carried out, including assessment of optimal glucose regulation, the effect of dietary inclusion of calcium diphosphate and pharmaceutical intake of etidronate disodium (EHDP), but none of these modalities wholly overcome the problem. The potential therapeutic application of fructose-1, 6-diphosphate, however, which also acts as human bioenergy, holds a great deal of promise as an efficacious and well-tolerated therapeutic regimen.
Diabetes mellitus is the most common human metabolic disorder, and a major health concern is the severe morbidity and mortality of the late diabetic complications. In our efforts to elucidate the mechanisms leading to the early functional changes in the retina and kidneys of diabetes patients, we noted repeated evidence of a disturbance in the metabolism of inorganic phosphate (Pi).Citation1–Citation6 Phylogenetic, experimental and clinical research data in nondiabetic conditions strongly suggests that the plasma and/or intracellular concentration of Pi may be a determining factor in regulation of energy metabolism and rate of oxygen consumption.Citation7–Citation13 Diabetes mellitus, however, demonstrates the opposite behavior, in that the highest oxygen consumption is associated with the lowest content of inorganic phosphate, and lowering oxygen consumption is associated with higher concentration of inorganic phosphate. Since a reduction of high energy phosphates and tissue hypoxia may be important factors in the development of long-term diabetic complications (DLC), the effects of Pi on the metabolism and function of the erythrocyte and renal tubular cell, as well as local and systemic consequences of severe hypophosphatemia in and during recovery from diabetic ketoacidosis, have recently been reviewed (J Ditzel and HH Lervang, unpublished data). The results indicate, that most conventionally treated diabetic patients respond as if their tissues are in a state of chronic hypoxia and suggesting that erythropoietin (EPO) is stimulated at an early stage in diabetes. A major disturbance in phosphate handling occurs in the kidney tubules, where the excessive sodium-dependent glucose reabsorption in diabetics depolarizes the electrochemical sodium gradient. Since Pi use the same driving force, but have less binding to sodium than glucose and amino acids such as alanine, the Pi reabsorption, particularly in poorly regulated patients, becomes impaired. This paradoxical phosphate imbalance may lead to affinity hypoxia and impaired formation of high energy phosphates. The lack of intracellular phosphate complementary to the increased intracellular glucose takes place in the insulin-insensitive cells and tissues, resulting in the possibility of DLC.
Many of the obvious dietary and pharmacological routes explored thus far to overcome this problem are evaluated herein.
Effect of optimal blood glucose control
Many investigators have found decreased concentrations of Pi in poorly regulated diabetic patients and slightly elevated levels when optimally controlled.
In newly diagnosed, nonacidotic insulin-dependent diabetic patient’s plasma Pi concentration was normal at admission, showed lower range on the day after initial insulin administration and slightly above normal level on the day of best metabolic control. Red cell 2, 3-diphosphoglycerate (2, 3-DPG) exhibited the same fluctuating pattern, and Pi correlated closely to 2, 3-DPG (r =0.61; P < 0.001). Red cell 2, 3-DPG concentration correlated equally well with P50 (oxygen tension at 50% oxygen saturation) of the oxyhemoglobin dissociation curve (ODC).Citation14,Citation15
Gertner et alCitation16 studied mineral metabolism in 7 juvenile-onset diabetic patients before and after achieving near-normal glucose levels by 7 to 14 days treatment with a portable subcutaneous insulin infusion system. They found that as plasma glucose decreased from an average of 221 mg/dL to 95.9 mg/dL, serum Pi rose from 4.09 to 5.01 mg/dL (P < 0.001) due to a 25% rise in renal tubular threshold for phosphate. No change was noted in immunoreactive parathyroid hormone (PTH) and in 1, 25 hydroxy-vitamin D.
Ditzel et alCitation4 studied renal handling of Pi in 26 conventionally treated diabetic children vs 28 healthy children and found fasting urinary phosphate excretion 3 times higher in the former group despite a significantly lower fasting Pi. The maximal capacity of renal tubular reabsorption of phosphate per liter of filtrate (TmPO4/GFR) was significantly suppressed in the diabetic patients. The increased urinary phosphate excretion correlated positively with both urinary glucose excretion and blood glucose concentration (P < 0.01). This finding was unrelated to serum PTH or to plasma growth hormone.
Raskin and PakCitation17 studied 21 diabetic patients in whom treatment results ranged from “suboptimal” to “optimal” control and found that, as the mean plasma glucose decreased from 17.1 mmol/L to 5.2 mmol/L over 4 to 10 days, serum phosphate level rose from 1.12 to 1.26 mmol/L (P < 0.001).
The same significant increase in Pi was found in 28 patients with type 2 diabetes who were examined both at admission when their disease was poorly controlled and following several days of hospitalization and treatment had markedly improved their metabolic status. In these patients, serum Pi levels increased significantly from 1.12 mmol/L to 1.21 mmol/L (P < 0.01), while serum calcium remained unchanged and urinary calcium and phosphorous excretion both decreased. On admission urinary calcium and phosphorous excretions showed a positive correlation with glucose excretion. Serum PTH decreased from a mid-normal to a low-normal value.Citation18
Thus, in both type 1 and type 2 diabetes, there is a close correlation between the Pi concentration in plasma and an improvement in diabetes control and in intracellular phosphate with a stimulating influence on the rate of cell glycolysis.
Effect of dietary phosphate intake
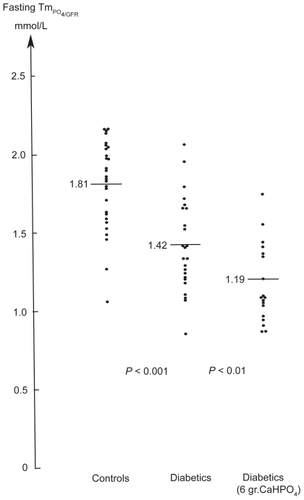
Pi concentration may also be increased by increasing dietary phosphate. Adding 2 g of calcium diphosphate to the three main daily meals over 4 weeks resulted in increased mean content of intraerythrocytic-2, 3-DPG and in the P50 of the ODC in both juvenile and adult diabetes patients.Citation19 To determine whether the immediate effect of increased phosphate intake persisted a subsequent double-masked, placebo-controlled study was undertaken for 1 year to assess renal processing of phosphate and bone mineral content in 43 juvenile type 1 diabetic patients. No increase was seen in the concentration of Pi (active n =19: 1.33 mmol/L vs placebo; n =24: 1.42 mmol/L, P =ns).Citation20 In the treatment group, the fasting urinary phosphate excretion increased as compared to the placebo group (median 1.28 vs 1.01 mmol/h; P < 0.001) and phosphate clearance/GFR was significantly enhanced (0.16 vs 0.10; P < 0.001). The threshold concentration of phosphate (TmPO4/GFR) was suppressed (1.19 vs 1.42 mmol/L; P < 0.01) in the treatment vs placebo group, and in contrast to the controls (1.81 vs 1.42 mmol/L; P < 0.001) (). The threshold concentration TmPO4/GFR was not related to the level of parathyroid hormone or to growth hormone level in serum, but inversely correlated with the degree of hyperglycemia. No difference in bone mineral content. was found between the treatment and placebo groups. These observations demonstrate that the dysfunction of the tubular handling of phosphate in diabetic children associated with hyperglycemia cannot be compensated for by daily dietary supplements of calcium diphosphate.
Figure 1 The phosphate threshold concentration, which is numerically equal to maximum tubular reabsorption rate for phosphate per unit volume of glomerular filtrate in 28 healthy children (1.81 mmol/L) vs 24 conventionally treated diabetic children without microvascular complications (1.42 mmol/L) and 19 diabetic children whom received 6 grams of calcium diphosphate daily for one year (1.19 mmol/L).
Effect of etidronate disodium (EHDP)
Intake of the diphosphonate, ethane-1, 1-diphosphonate (EHDP) is known to produce sustained hyperphosphatemia.Citation21–Citation23 This rise in Pi concentration occurs without a corresponding increase in urinary phosphate excretion, suggesting that EHDP partly elevates Pi by reducing its renal clearance. To study the effect of prolonged elevation of Pi on red cell metabolism and function, oxyhemoglobin dissociation curves (ODC) from zero to full saturation were performed on whole blood from 14 insulin-treated, nonacidotic diabetes patients and 5 healthy volunteers following oral intake of EHDP (20 mg kg−1day−1) or placebo for 28 days.Citation24 The mean Pi increased from 1.18 to 1.57 mmol/L (P < 0.001) in the diabetes patients and from 1.14 to 1.69 mmol/L (P < 0.005) in healthy controls. A significant rise in red cell 2, 3-DPG was seen only in the diabetic group from 15.2 to 16.3 μmol/g of hemoglobin (P < 0.005) and probably indicates a suppression of the 2, 3-DPG formation pathway in diabetes patients. However, there was a significant relationship between the concentration of Pi and the P50 of the ODC in both diabetics (r =0.58, P < 0.01) and healthy controls (r =0.69, P < 0.005). Mean P50 in diabetic patients was significantly lower than controls despite normal 2, 3-DPG. The study emphasizes the importance of Pi on red cell function and indicates that elevated Pi tends to counteract the defect in oxygen-release capacity of the erythrocytes in diabetes patients. A subsequent randomized, 6-month, double-blind study of 26 type 1 diabetic patients with nonproliferative retinopathy confirmed the effect of EHDP on Pi, red cell 2, 3-DPG and P50 of ODC.Citation25 Furthermore, the lack of changes in mean glucose and HbA1c indicated an interesting association between improvement in retinopathy as evaluated by retinal fluorescein angiography and increased Pi (P < 0.05).
Potential therapeutic application of fructose-1, 6-diphosphate
Fructose-1, 6-diphosphate (FDP) is a key intermediate in anaerobic glycolysis and is the product of the major regulatory enzyme in the pathway (phosphofructokinase). Natelson et alCitation26 showed that orally administrated fructose diphosphate calcium salt was absorbed directly by the intestinal tract without splitting the phosphate linkage, and that 6 g led to an increase of serum inorganic phosphate averaging 15%, citric acid 10.7%, and nonprotein organic phosphate as much as 173%. Further preclinical and clinical data indicate that FDP can enter cells and serve as a metabolizable substrate of glycolysis.Citation27–Citation29 FDP acts as human bioenergy and can transport phosphorous intracellularly as well as deliver 4 moles of ATP per mole of FDP. Thus FDP has substantial cytoprotective effects on a variety of ischemia-induced tissue damage.Citation30,Citation31 Recent studies also show that FDP can modulate nitric oxide production in a variety of cellular injury events, and indicate that FDP can influence red cell rheology and can increase red cell ATP and 2, 3-DPG levels.Citation32–Citation34 FDP can be given orally and intravenously to humans and it is well tolerated at pharmacological doses. Although FDP appears to be highly efficacious, no previous controlled study has been reported in diabetic patients.
Discussion
This review demonstrates a close correlation between plasma concentration of Pi and the degree of metabolic control of diabetes. The relationship may involve Pi handling in the kidney tubules; the major regulator of Pi homeostasis. Glucose and Pi (eg, alanine, myoinositol) reabsorption are all examples of secondary active transport processes with sodium (Na+) as the driving force. The sodium entry results from the active extrusion of Na+ across the basolateral segment of the proximal tubular cells, energized by adenosine triphosphate (ATP) hydrolysis, and catalyzed by the Na/K-ATPase. As glucose is more potent than inorganic phosphate in stimulating the uptake of Na+ in renal microvillus vesicles,Citation35 the elevated glucose concentrations depolarize the transmembrane electrochemical Na+ gradient of the brush border membrane for inorganic phosphate entry into the tubular cells and decrease intracellular phosphate leading to hyperphosphaturia. Therefore normalization of blood glucose levels leads to an improved capacity of the kidney tubules to reabsorb Pi and a subsequent increase in plasma Pi concentration. Over time this improved glucose regulation will positively influence or even prevent the long-term diabetic complications as has been proven in DCCT and UKPDS studies.Citation36,Citation37 Although effective, however, this method achieves only an approximation of normalcy given the chronic and fluctuating nature of diabetes and the nonphysiologic administration of daily injections vs immediate insulin secretion into the portal circulation in response to a rise in blood glucose in healthy organisms.
Increasing the dietary intake of Pi with 6 g of calcium diphosphate has been shown to be of no benefit since the maximal reabsorptive capacity for Pi in the kidney tubules does not increase, but actually decreases leading to a significant increase in Pi clearance ().Citation20 This effect was not found to be related to the serum level of parathyroid hormone or growth hormone, but inversely correlated with the degree of hyperglycemia.
The study of the effect of etidronate disodium (EHDP) superficially appears to be more promising since EHDP increased both plasma Pi and red cell 2, 3-diphophoglycerate levels and thereby the oxygen release capacity. Studies of nonproliferative retinopathy suggested that a 6-month course of EHDP administration may improve retinopathy.Citation25 EHDP in large doses may also suppress bone turnover, inhibits bone mineralization and affect tooth development, deleterious effects that discourage general use.Citation38,Citation39
The therapeutic use of fructose-1, 6 diphosphate (FDP) in diabetes is much more attractive since it is a natural intermediate in glycolysis and also acts as human bioenergy, delivering intracellular phosphorus and 4 moles ATP per mole of FDP. The substance is well tolerated at pharmacological doses and can be given orally. Future controlled studies may lead to a prophylactic therapy to prevent vascular complications and may also counteract the metabolic disturbances leading to type 2 diabetes and the metabolic syndrome.Citation40
Disclosures
The authors declare no conflicts of interest.
References
- DitzelJEffect of plasma inorganic phosphate on tissue oxygenation during recovery from diabetic ketoacidosisAdv Exp Med Biol197337A1631724220244
- DitzelJStandlEThe problem of tissue oxygenation in diabetes mellitus. II Evidence of disordered oxygen release from the erythrocytes of diabetics in various conditions of metabolic controlActa Med Scand1975Suppl 578S5968
- DitzelJBrøchner-MortensenJRødbroPElevated glomerular filtration rate in early diabetes may be explained by increased sodium reabsorption secundary to impairment in renal tubular handling of phosphateHormone Metab Res1981Suppl 11S8789
- DitzelJBrøchner-MortensenJKawaharaRDysfunction of tubular phosphate reabsorption related to glomerular filtration and blood glucose control in diabetic childrenDiabetologia19822354064107173517
- DitzelJBrøchner-MortensenJTubular reabsorption rates as related to elevated glomerular filtration in diabetic childrenDiabetes198332Suppl 2S28S32
- MathiassenBNielsenSJohansenJSLong-term bone loss in insulin-dependent diabetic patients with microvascular complicationsJ Diabet Complications1990441451492151224
- HardyHAWellmanMOxidative phosphorylation. Role of inorganic phosphate and acceptor systems in control of metabolic ratesJ Biol Chem1952185215224
- SestoftLRegulation of fructose metabolism in the perfused rat liver. Interrelation with inorganic phosphate, glucose, ketone body and ethanol metabolismBiochem Biophys Acta197434311164364128
- BrazyPCMandelIJDoes availability of inorganic phosphate regulate cellular oxidative metabolism?News Physiol Sci19861100103
- BoseSFrenchSEvansFJBalabanRSMetabolic network control of oxidative phosphorylation: Multiple roles of inorganic phosphateJ Biol Chem200327840391553916512871940
- SestoftLIs the relationship between plasma concentration of inorganic phosphate and the rate of oxygen consumption of significance in regulating energy metabolism in mammals?Scand J Clin Invest1979393191197523968
- FirmatGPrunierJRawsonRWRobertsKESchwartzMKEffect of phosphate enhancing the action of triiodothyronineEndocrinology195659556557013375578
- MosekildeLChristensenMSDecreased parathyroid function in hyperthyroidism: interrelationshipp between parathyroid hormone, calcium-phosporous metabolism and thyroid functionActa Endocrinol (Copenh)1977843566575576531
- DitzelJStandlEPlasma Pi and erythrocyte 2, 3DPG concentrations of non-acidotic diabetics in various degree of metabolic controlClin Chem19762245505511253438
- DitzelJJægerPStandlEAn adverse effect of insulin on the oxygen-release capacity of red blood cells in nonacidotic diabeticsMetabolism197827892793427696
- GertnerJMTamborlaneWVHorstRLSherwinRSFeligPGenelMMineral metabolism in diabetes mellitus. Changes accompanying treatment with portable subcutaneous insulin infusionJ Clin Endocrinol Metab19805058628666246134
- RaskinPPakCYCThe effect of chronic insulin therapy on phosphate metabolism in diabetes mellitusDiabetologia198121150537024029
- NagasakaSMurakamiTUchikawaTIshikawaSESatoTEffect of glycemic control on calcium and phosphorous handling and parathyroid level in patients with non-insulin-depemdemt diabetes mellitusEndocr J19954233773837670567
- DitzelJThe problem of tissue oxygenation in diabetes mellitus IIIActa Med Scand1975Suppl 578S6983
- DitzelJLervangHHBrøchner-MortensenJRødbroPThe influence of a dietary supplement of calcium and phosphate on bone mineral content and mineral homeostasis of diabetic childrenDiabetologia1993Suppl 1A58
- AltmanRDJohnsonCCKhairiMRAWellmanMSerafiniANSankeyRRInfluence of disodium etidronate (EHDPTM) on clinical and laboratory manifestastion of Paget’s disease of bone (osteitis deformans)N Engl J Med197328926137913844201876
- ReckerRRHassingGSLauJRSavillePDThe hyperphosphatemic effect of disodium-1-hydroxy-1, 1 diphophonate (EHDPTM): Renal handling of phosphorus and the renal response to parathyroid hormoneJ Lab Clin Med19738122582664345764
- RussellRGGSmithRPrestonCWaltonRJWoodsCGDiphosphonates in Paget’s diseaseLancet1974178638948984133419
- DitzelJHauCDaugaardNEffect of diphosphonate ethane-1-hydroxy-1, 1-diphosphonate (EHDP) on hemoglobin oxygen affinity of diabetic and healthy subjectsMicrovasc Res197713435536117812
- NielsenNVDitzelJJensenSKjærgaardJJThe effect of etidronate disodium (EHDP) on retinopathy in insulin-dependent diabetic patientsGraefe’s Arch Clin Exp Ophthalmol198221926063
- NatelsonSKleinMKramerBThe effect of oral administration of calcium fructose diphosphate on the serum organic phosphate, inorganic phosphate, calcium protein, and citric acid levelsJ Clin Invest1951130505414803556
- HardinCLazzarinoGTavazziBDi PierroDRobertsTMGiardinoBMyocardial metabolism of exogenous FDP is consistent with transport by a dicarboxylate transporterAm J Physiol20012816H2654H2660
- MarkowAKNeelyWADidiokiRHTerryIIIJCauseyALehanPHMetabolic responses of Fructose-1, 6-diphosphate in healthy subjectsMetabolism200049669870310877192
- EhringerWDNiuWChiangBWangOLGordonLChienSMembrane permeability of fructose.1, 6.diphosphate in lipid vesicles and endothelial cellsMol Cell Biochem20002101–2354510976756
- TakeuchiKCao-DanhHFrichsIGlynnPDÀgostinoDSimplaceanuEAdministration of fructose 1,6-diphosphate during early reperfusion significantly improves recovery of contractile function in the postischemic heartJ Thorac Cardiovasc Surg199811623353399699588
- AntunesNMartinussoCATakiyaCMde SilvaAJRde OmellasJFREliasPRFructose-1, 6 diphosphate as a protective agent for experimental ischemic acute renal failureKidney Int2006691687216374425
- RaoMROlindeKDMarkovAKIn vitro induction of nitric oxide by fructose-1, 6-diphosphate in the cardiovascular system of ratsMol Cell Biochem19981851–21711759746223
- CacioliDClivateAPelosiPMegevandJGaleoneMHaemorheological effects of fructose-1, 6-diphosphate in patients with lower extremity ischemiaCurr Med Res Opin198810106686743371082
- UrsoLBrillanteCOrlandiMRotundoMBallatiSEvaluation of fructose-1, 6-diphosphate on erythrocyte 2, 3 diphosphoglycerate and ATP in surgical orthopedic patientsAggressologie1982233115117
- BarrettPQAronsonPSGlucose and alanine inhibition of phosphate transport in renal microvillus membrane vesiclesAm J Physiol19822422F126F1317065130
- The Diabetes Control and Complication Trial Research GroupThe effect of intensive treatment of diabetes in insulin-dependent diabetes mellitusN Engl J Med1993329149779868366922
- UK Prospective Diabetes Study (UKPDS) GroupIntensive blood glucose control with sulfonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes. (UKPDS 33)Lancet199835291318378539742976
- ChristoffersenJChristoffersenNRRubenJArendaJThe effect of EHDP concentration on enamel demineralization in vitroJ Dent Res19917021231261846886
- HiranoTTurnerCHForwoodHRJohnsonCCBurrDRDoes suppression bone turnover impair mechanical properties by allowing microdamage accumulation?Bone2000271132010865204
- HåglinLLindbladABygrenLOHypophosphataemia in the metabolic syndrome. Gender differences in body weight and blood glucoseEur J Clin Nutr200155649349811423926