Abstract
Type 2 diabetes mellitus is a metabolic disease associated with low quality of life and early death. The goal in diabetes treatment is to prevent these outcomes by tight glycemic control and minimizing vascular risk factors. So far, even intensified combination regimen with the traditional antidiabetes agents have failed to obtain these goals. Incretin mimetics are a new class of antidiabetes drugs which involve modulation of the incretin system. They bind to and activate glucagon-like peptide-1 (GLP-1) receptors on pancreatic beta-cells following which insulin secretion and synthesis are initiated. Since the compounds have no insulinotropic activity at lower glucose concentrations the risk of hypoglycemia – a well-known shortcoming of existing antidiabetes treatments – is low. Additionally, incretin mimetics have been shown to be associated with beneficial effects on cardiovascular risk factors such as weight loss, decrease in blood pressure and changes in lipid profile. Current clinical data on the two available incretin mimetics, exenatide and liraglutide, are evaluated in this review, focusing on pharmacology, efficacy, safety and tolerability. The review is built on a systematic PubMed and Medline search for publications with the key words GLP-1 receptor agonist, exenatide, liraglutide and type 2 diabetes mellitus up to January 2009.
Introduction
Type 2 diabetes is a metabolic disease characterized by high blood glucose caused by an insufficiency of the pancreas to produce insulin, hyperglucagonemia and impaired insulin sensitivity. The typical symptoms include thirst, polyuria, recurrent infections and weight loss.Citation1 However, the majority of patients do not experience symptoms and are diagnosed in a late stage of the disease. The etiology of type 2 diabetes is unknown; however, genetic and environmental factors have been linked to its development. It is a chronic progressive disease associated with micro- and macrovascular complications such as nephropathy, neuropathy, retinopathy and cardiovascular morbidity. These complications often result in low quality of life and early death. In 2000 the global mortality due to diabetes was estimated to be 5.2% or 2.9 million deaths.Citation2 The increased mortality is mainly due to cardiovascular events. Recent estimates indicate that 171 million people worldwide had diabetes in 2000 and this number is projected to increase to 366 million by 2030.Citation3 As a consequence, diabetes-related deaths are likely to increase by more than 50% in the next 10 years. In developed countries most people with diabetes are above 64 years of age while most people with diabetes in the developing countries are younger (45 to 64 years).Citation4 The disease is equally distributed among sexes.Citation3
The goal with diabetes treatment is to improve quality of life and prevent early death. It is well established that tight glycemic control reduces the risk of microvascular diseaseCitation5–Citation7 while recent randomized controlled trials have failed to show a substantial benefit on macrovascular outcomes.Citation7–Citation9 These results implicate that not only glycemic control but also minimizing cardiovascular risk factors (high blood pressure, hyperlipidemia, overweight, smoking, thrombosis risk) through medical intervention and life-style intervention should be addressed in the treatment of diabetes.
The optimal goal for glycemic control is a glycosylated hemoglobin A1c (HbA1c) below 7%.Citation10 In order to reach this target an intensified regimen with combinations of antidiabetes agents is often needed. Oral agents in monotherapy (thiazolidinediones [TZDs], metformin, repaglinide, α-glucosidase inhibitors and sulfonylurea [SU] compounds) improve glycemic control to almost the same degree (decrease in HbA1c of approximately 1%).Citation11 When combining two antidiabetes drugs another 1% HbA1c reduction can be obtained. However, with time, supplementation with subcutaneous (sc) injections of insulin or insulin analogues is often necessary in order to compensate for insulin deficiency and maintain an acceptable glycemic control. This is partly due to the fact that type 2 diabetes is a progressive disease with an almost linear decline in beta-cell function (probably combined with a decrease in beta-cell mass) over time. None of the mentioned antidiabetes drugs have been shown to preserve pancreatic beta-cell function over time and, notably, SUs have been shown to accelerate the apoptosis of human beta-cells.Citation12 Besides, the current available drugs are associated with a number of shortcomings: body weight increase (TZDs, SUs and insulin), hypoglycemia (SUs, repaglinides and insulin) and gastrointestinal side effects (metformin and α-glucosidase inhibitors).Citation11 The limitations of the pre-existing antidiabetes treatments, make new medical therapies that offer improved efficacy and/or durability, better convenience, and an improved safety and tolerability profile an absolutely imperative in order to get more patients to glycemic goal initially and to avoid or delay the need for additional treatment.
Incretin hormones
The incretin hormones glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP) are intestinal peptide hormones released in response to ingestion of meals.Citation13 The most important effect of GLP-1 and GIP is their ability to potentiate glucose-induced insulin secretion from the pancreas – the so-called incretin effect. In healthy subjects the incretin effect accounts for up to 70% of the insulin secreted in response to glucose ingestion.Citation14 GLP-1 is a 30-amino acid polypeptide processed from proglucagon in the endocrine L-cells distributed primarily in the mucosa of the distal part of the small intestine and colon. GIP is a 42-amino acid polypeptide released from endocrine K-cells found in the mucosa of the duodenum and upper jejunum.Citation15 While GLP-1 is rapidly degraded (by the ubiquitous enzyme dipeptidyl peptidase-4 (DPP-4)) in the circulation with an apparent half-life of 1 to 1.5 minutes,Citation16 GIP is degraded more slowly, with a half-life for the intact hormone of 7 minutes.Citation17 The hormones enhance insulin secretion from the beginning of a meal, but has no insulinotropic activity at lower glucose concentrations (less than 4 mM); thereby not promoting hypoglycemia. GLP-1 also enhances insulin biosynthesis and insulin gene expression. In addition, it exerts trophic and protective actions on the beta-cellsCitation18 and strongly inhibits pancreatic glucagon secretion in a glucose-dependent manner. Citation19 In contrast, GIP has been shown to stimulate glucagon secretion. The hormones exhibit their insulinotropic effect via G-protein coupled receptors on the pancreatic beta-cells.Citation20 Beside the effects on the endocrine pancreas, both hormones have several other functions. GLP-1 receptors are found in various regions of the brainCitation21 and when activated these are believed to promote feeling of satiety which in combination with GLP-1-induced inhibition of gastrointestinal motility (mediated through the vagus nerveCitation22) reduces food intake and body weight. GLP-1 receptors are also found in the heartCitation23 and most data suggest that GLP-1 exerts protective effects on the myocardium. GLP-1 has also been found to reduce the postprandial rise in triglycerides and lower the concentration of free fatty acids in humans.Citation24 Finally, animal as well as human studies indicate that GLP-1 has natriuretic and diuretic properties by modulation of renal Na+/H+ exchangeCitation25 – a mechanism that might serve to reduce blood pressure. GIP appears to have no physiological effect on the gastrointestinal tract, appetite or food intake, but may play a role in lipidCitation26 and bone metabolism.Citation27
Incretin hormones and type 2 diabetes pathophysiology
In patients with type 2 diabetes the incretin effect is severely reduced.Citation28,Citation29 This pathophysiological trait is likely to play a central role in the inability of these patients to secrete sufficient amount of insulin to prevent hyperglycemia following oral glucose.Citation30 Attenuated postprandial secretionCitation31 and decreased insulinotropic potency of GLP-1Citation32 in combination with abolished insulinotropic effect of GIPCitation33 seem to be responsible for the reduced incretin effect in patients with type 2 diabetes. Since the insulinotropic effect of only GLP-1 (and not GIP) is preserved in patients with type 2 diabetes, antidiabetes treatment modalities based on the effect of this peptide have been developed. Interestingly, intravenous (iv) infusion of native GLP-1 is capable of normalizing blood glucose in patients with type 2 diabetes, Citation34 but due to the short half-life of GLP-1, therapeutic administration of native GLP-1 is impractical. Therefore, in order to exploit the beneficial actions of GLP-1 in type 2 diabetes, long-acting stable receptor agonists of GLP-1 (incretin mimetics) have been developed. In the following section the current clinical data on the two available incretin mimetics, exenatide and liraglutide, will be described.
Incretin mimetics
Exenatide, the first in this new class of drugs, was introduced to the market in the United States in 2005 and in Europe in 2007 under the trade name Byetta® (Amylin Pharmaceuticals/Eli Lilly). Liraglutide has been introduced to the market in Europe July 2009 and in the United States and Japan in January 2010 under the trade name Victoza® (Novo Nordisk). The current review focuses on only these two incretin mimetics.
Pharmacology
Exenatide
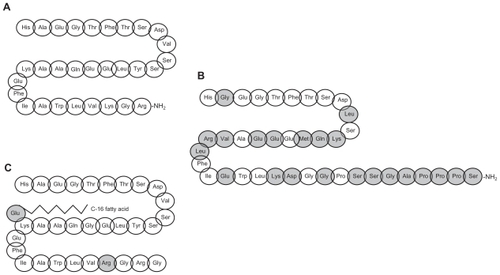
Exenatide was isolated from the saliva of the lizard Heloderma suspectum in a search for biologically active peptides. Citation35 Exenatide shares 53% homology with native GLP-1 () and binds to and activates GLP-1 receptors on pancreatic beta-cells following which insulin secretion and synthesis is initiated.Citation36 Following sc administration, exenatide is rapidly absorbed reaching peak concentrations in approximately 2 hours. The half-life of exenatide is approximately 2 hours, and after sc injection of the maximally tolerated dose, significant elevation of exenatide in plasma may be observed for 5 to 6 hours. Exposure is negligible after 12 hours post dose, explaining why twice-daily dosing is needed in order to obtain full effect on glycemic control. Citation37 Exenatide is, unlike native GLP-1, not substantially degraded by DPP-4 but is cleared primarily in the kidneys by glomerular filtrationCitation38 resulting in a plasma half-life for the peptide of approximately 30 minutes after iv administration.Citation37 Pharmacokinetics, safety and efficacy of exenatide have been tested in several subgroups of type 2 diabetes patients. In a rather small study of adolescent patients with type 2 diabetes, administration of exenatide appeared to be well tolerated;Citation39 in a study of Japanese patients with type 2 diabetes the pharmacokinetics seemed to be similar to that of Caucasian patientsCitation40 (no racial differences have been reported). Lastly, age does not seem to influence the pharmacokinetic properties of exenatide.Citation41
Liraglutide
Liraglutide is an acylated analogue of human GLP-1 and has 97% sequence homology to native GLP-1 (). The analogue is produced using the recombinant DNA technology in yeast.Citation42 It has a similar effect on the GLP-1 receptor as described for exenatide. A high degree of plasma protein binding causes decreased susceptibility to metabolism by DPP-4 and the half-life following sc administration of liraglutide is approximately 13 hours.Citation43 This protracted action profile makes liraglutide suitable for once-daily administration. There are no clinically significant differences in liraglutide pharmacokinetics between male and female subjects, subjects of different race, or elderly and younger subjects.Citation44
Efficacy
Exenatide
The clinical effects of exenatide treatment have been investigated in six published, randomized, controlled trials with a total of 2731 patients.Citation45 A summary of the trials is presented in . Exenatide as add-on therapy to metformin,Citation46 SUCitation47 or bothCitation48 showed statistically significant improvement in glycemic control (HbA1c reduction of 1.0% (baseline HbA1c: 8.2% to 8.6%) vs a minor increase of about 0.1% in the placebo groups) and reduction in fasting plasma glucose (0.5 mM in the exenatide groups vs an increase of about 1 mM in the placebo groups). In all three studies (the Three Amigos) exenatide was given twice daily in two different doses (of 5 and 10 μg, respectively). The changes in HbA1c for 10 μg exenatide are presented in . Patients receiving exenatide were more likely to achieve an HbA1c less than 7% compared with patients receiving placebo-with the best results in the high-dose (10 μg) exenatide groupsCitation49.
Table 1 Summary of exenatide clinical trials
The effect of exenatide has also been investigated with insulin as active control. In a 26-week study of patients inadequately controlled on metformin and SU in combination therapy, addition of exenatide induced similar reductions in HbA1c (1.1% from baseline HbA1c 8.2%) as addition of insulin glargine.Citation50 In a 52-week study comparing twice-daily biphasic insulin aspart and exenatide (both added to existing metformin and SU treatment), exenatide induced similar reductions in HbA1c as insulin aspart, and provided significantly better postprandial glucose control.Citation51
On average the weight loss in the three studies comparing exenatide to placebo amounted to 1.6 kg in the exenatide-treated patients (baseline body weight: 96 to 100 kg).Citation46–Citation48 Similar results was seen in a trial comparing exenatide with placebo in patients treated with TZDs.Citation52 The difference in body weight change was even bigger in the insulin trials. Body weight in the exenatide group decreased 2.3 kg; significantly different from an increase of 1.8 kg in the insulin glargine-treated group.Citation50 In the exenatide vs insulin aspart trial a significant between-group difference of 4.1 kg weight loss was found (baseline weight 86 kg (exenatide group)/83 kg (insulin aspart group).Citation51 outlines the weight loss for 10 μg exenatide groups in the different studies.
The beneficial effects of exenatide seem to last. In an open-label extension of the Three Amigos studies, 3 years’ sustained effects were demonstrated for glycemic control and body weight (decrease in HbA1c of 1% and body weight of 5.3 kg).Citation53 An important limitation of this study, in addition to its open-label design, was a high drop out rate of patients (due to adverse events, insufficient glycemic control, patient/investigator decision, and protocol violation), only 217 out of 517 randomized subjects completing the 3-year study period.
Only few studies have investigated the effect of exenatide on cardiovascular risk profile. The open-label studies with a 3-year follow-up found minor, but significant improvements in triglycerides (12% decrease compared to baseline), total cholesterol (5% decrease), low density lipoproteins (6% decrease) and high density lipoprotein (24% increase) in favor of a reduced cardiovascular risk.Citation54 A review looking at exenatide vs placebo or insulin on blood pressure measurements in pooled data from 6 exenatide trials, found that 6 months of exenatide treatment was associated with a significantly greater reduction in systolic blood pressure compared with placebo (difference of −2.8 mmHg) or insulin (difference of −3.7 mmHg).Citation55 Further studies are needed to elucidate the exact mechanisms behind the beneficial effects of exenatide on the cardiovascular risk profile.
Liraglutide
The clinical effects of liraglutide treatment have been investigated in the LEAD (Liraglutide Effect and Action in Diabetes) series of phase III studies including more than 4000 patients with type 2 diabetes. A summary of the trials is presented in with changes in HbA1c of 1.8 mg liraglutide. In average liraglutide reduced HbA1c by 1.2% from a baseline of 8.2% to 8.5%. Liraglutide in monotherapy (52 weeks of treatment) compared with the SU glimepiride was evaluated in the LEAD 3 studyCitation56 using two different doses of liraglutide (1.2 and 1.8 mg, respectively). The proportions of participants reaching the target HbA1c of 7.0% or less were 43%, 51% and 28% in the 1.2 mg liraglutide, 1.8 mg liraglutide and glimepiride groups, respectively. An open-label one-year extension of the LEAD 3 study showed a sustained beneficial effect of liraglutide compared to glimepiride on HbA1c.Citation57 Improved glycemic control has also been reported when liraglutide is given in combination with oral antidiabetes therapy. In the LEAD 2Citation58 different doses of liraglutide (0.6, 1.2 and 1.8 mg), glimepiride and placebo added to existing metformin treatment were evaluated, and in the LEAD 1 study,Citation59 liraglutide was added to glimepiride treatment and compared with rosiglitazone and placebo. In the LEAD 4 studyCitation60 liraglutide was added to metformin in combination with rosiglitazone. Liraglutide (1.8 mg) added to metformin in combination with glimepiride was compared to the active comparator insulin glargine (LEAD 5 study).Citation61 A direct comparison between liraglutide (1.8 mg once daily) and exenatide (10 μg twice daily) was reported from the open-labeled LEAD 6 study.Citation62 Mean HbA1c reduction was significantly greater with liraglutide treatment than with exenatide (1.1% vs 0.8%). Significant differences were also seen between the two agents with regard to fasting plasma glucose (1.6 mM reduction [liraglutide] vs 0.6 mM reduction [exenatide]). Liraglutide also reduced postprandial glucose across the LEAD trials. As for exenatide, liraglutide has a significant effect on body weight as shown by the data for 1.8 mg liraglutide in . Liraglutide reduced mean body weight or was weight neutral as monotherapyCitation56 and in combination with oneCitation58,Citation59 or twoCitation60–Citation62 oral antidiabetes agents compared to placebo or active comparators. The LEAD 6Citation62 study examined the lipid profile on exenatide and liraglutide. Significant greater reductions in triglycerides (−0.4 vs −0.2 mM) and free fatty acids (−0.17 vs −0.10 mM) in the liraglutide group were observed. Both compounds caused a significant decrease in blood pressure (systolic blood pressure −2.2 mmHg and diastolic pressure −1.5 mmHg) with no significant differences between the two compounds.
Table 2 Summary of liraglutide clinical trials
Safety and tolerability
The major side-effects of all compounds are mild to moderate and transient nausea and vomiting. These side effects are dose dependent and decline over time.Citation49 Other frequently reported side effects encompass headache and upper respiratory infection.Citation41,Citation63 The incidence of treatment-associated hypoglycemia is reported to be lowCitation41,Citation63 – apparently due to the glucose-dependent insulinotropic and glucagonostatic effects of GLP-1. However, in combination with SU the incidence increases, and is dependent on the dose of SU. In most exenatide trials minor hypoglycemic episodes are defined as plasma glucose <3.3 mM; in the LEAD studies it is defined as plasma glucose <3.1 mM. In studies using exenatide combined with SU a risk of minor hypoglycemic episodes is reported to be between 15% and 36%.Citation49 In studies combining liraglutide with SU the risk is reported to be 8% to 25%.Citation64 In the exenatide/insulin glargine study 1.5% of patients experienced severe hypoglycemia.Citation50 There was no difference between groups and the incidence was similar in the two groups. Only one severe hypoglycemic episode was reported with liraglutide and glimipiride.Citation58
Approximately 40% of exenatide-treated patients in long-term, placebo-controlled studies developed antibodies against exenatide during the initial 30 weeks of treatment.Citation49 Among liraglutide-treated patients up to approximately 8% exhibit antibodies.Citation56,Citation58–Citation61 The exact impact of antibodies in the longer term needs to be established. Neither exenatide nor liraglutide is recommended during pregnancy or lactation due to lack of sufficient data. Exenatide should not be used in patients with kidney failure since it is cleared primarily in the kidneys by glomerular filtrationCitation41 and reports of acute kidney failure have been filed. No data indicate inhibition or induction of cytochrome P450 drug metabolizing enzymes. Since 2005 the US Food and Drug Administration (FDA) has received 170 reports on pancreatitis in exenatide-treated patients, and recently the FDA received reports of acute pancreatitis, some of which were severe cases of hemorrhagic or necrotizing pancreatitis in patients taking exenatide. In the LEAD studies a total of 9 reports of pancreatitis were observed, with reports of pancreatitis in both in liraglutide-, placebo- and active comparator-treated patients. At this point it is not clear whether a causal relationship between pancreatitis and exenatide/liraglutide exists or the cases are incidental. However, it is recommended to discontinue incretin mimetic treatment if pancreatitis is suspected.Citation65 In carcinogenicity studies with liraglutide, C-cell tumors were observed in thyroid tissue of mice and rats. It has been suggested that the findings in rodents are caused by a non-genotoxic, specific GLP-1 receptor-mediated mechanism to which rodents are particularly sensitive. The relevance for humans is likely to be clinical insignificant, but additional studies are needed to clarify the potential mechanisms behind C-cell tumor development in GLP-1 analogue-treated patients and their possible clinical implications.Citation63
Patients
Both products are parenteral solutions intended for sc injection. They can be administered in the subcutis of the abdomen, arm or thigh. To improve gastrointestinal tolerability, exenatide therapy should be initiated at 5 μg per dose administered twice daily for at least a month. The dose of exenatide can then be increased to 10 μg twice daily.Citation41 Liraglutide should be initiated in a dose of 0.6 mg daily. After at least 1 week, the dose should be increased to 1.2 mg. The dose can be increased to 1.8 mg to further improve glycemic control. Daily doses higher than 1.8 mg are not recommended because of side effects. Patient satisfaction using exenatide has been evaluated in a couple of studies. The addition of exenatide to metformin and SU resulted in significant improvements in treatment satisfaction and patients’ health-related quality of life from baseline to 26 weeks.Citation66 The improvement was similar for patients treated with insulin glargine.
Liraglutide has been on the market for only a short time and few reports about compliance and satisfaction exist. In the LEAD 6 study,Citation62 379 patients completed a diabetes treatment satisfaction questionnaire at baseline and week 26, which showed a higher satisfaction among patients on liraglutide than on exenatide.
Conclusion
Incretin mimetics offer a new and interesting treatment modality in diabetes. Clinical studies have shown beneficial effects on glycemic control, body weight, lipid profile and blood pressure. This could imply substantial benefit on macrovascular outcomes. So far the safety profile of incretin mimetics is promising. The main side-effect is mild to moderate and transient (weeks) nausea. The frequency of hypoglycemia – a well-known side effect of several pre-existing antidiabetes treatment modalities – is low and occurs mainly when incretin mimetics are administered in combination with SU. Incretin mimetics are not yet recommended in combination with insulin. Animal studies indicate that administration of GLP-1 receptor agonists is associated with beta-cell proliferation and beta-cell protection, but these effects have not yet been established in clinical trials. Thus, future mechanistic studies and more long-term clinical studies are required to elucidate these promising outcomes. Treatment with incretin mimetics is also associated with unsolved problems: The reports about acute pancreatitis and rodent C-cell carcinomas have caused concerns about long-term effects, but there are as yet no indications of these effects in humans. These concerns, taken together with the relatively short overall clinical experience, resulted in the label “less validated” treatment in the recent consensus statement for the management of type 2 diabetes from the American Diabetes Association and the European Association for the Study of Diabetes. However, new incretin mimetics are in the pipeline of several pharmaceutical companies. They are all characterized by improved pharmacology and longer half-lives, implying fewer injections. The next couple of years will elucidate whether the incretin mimetics will become well established in the treatment of type 2 diabetes. The data so far are encouraging.
Disclosures
The authors have no conflict of interests in relation to the present paper.
References
- World Health Organization, Geneva Definition, Diagnosis and Classification of Diabetes Mellitus and its Complications Report of a WHO Consultation World Health Organization Department of Noncommunicable Disease Surveillance 1999
- Roglic G Unwin N Bennett PH The burden of mortality attributable to diabetes: realistic estimates for the year 2000 Diabetes Care 2005 28 2130 2135 16123478
- Wild S Roglic G Green A Sicree R King H Global prevalence of diabetes: estimates for the year 2000 and projections for 2030 Diabetes Care 2004 27 1047 1053 15111519
- King H Aubert RE Herman WH Global burden of diabetes, 1995–2025: prevalence, numerical estimates, and projections Diabetes Care 1998 21 1414 1431 9727886
- Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33) UK Prospective Diabetes Study (UKPDS) Group Lancet 1998 352 837 853 9742976
- Gaede P Lund-Andersen H Parving HH Pedersen O Effect of a multifactorial intervention on mortality in type 2 diabetes N Engl J Med 2008 358 580 591 18256393
- Patel A MacMahon S Chalmers J Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes N Engl J Med 2008 358 2560 2572 18539916
- Gerstein HC Miller ME Byington RP Effects of intensive glucose lowering in type 2 diabetes N Engl J Med 2008 358 2545 2559 18539917
- Duckworth W Abraira C Moritz T Glucose control and vascular complications in veterans with type 2 diabetes N Engl J Med 2009 360 129 139 19092145
- Nathan DM Buse JB Davidson MB Medical management of hyperglycaemia in type 2 diabetes mellitus: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement from the American Diabetes Association and the European Association for the Study of Diabetes Diabetologia 2009 52 17 30 18941734
- Bolen S Feldman L Vassy J Systematic review: comparative effectiveness and safety of oral medications for type 2 diabetes mellitus Ann Intern Med 2007 147 386 399 17638715
- Maedler K Carr RD Bosco D Zuellig RA Berney T Donath MY Sulfonylurea induced beta-cell apoptosis in cultured human islets J Clin Endocrinol Metab 2005 90 501 506 15483097
- Holst JJ The physiology of glucagon-like peptide 1 Physiol Rev 2007 87 1409 1439 17928588
- Nauck MA Homberger E Siegel EG Incretin effects of increasing glucose loads in man calculated from venous insulin and C-peptide responses J Clin Endocrinol Metab 1986 63 492 498 3522621
- Brown JC Mutt V Pederson RA Further purification of a polypeptide demonstrating enterogastrone activity J Physiol 1970 209 57 64 5499047
- Deacon CF Johnsen AH Holst JJ Degradation of glucagon-like peptide-1 by human plasma in vitro yields an N-terminally truncated peptide that is a major endogenous metabolite in vivo J Clin Endocrinol Metab 1995 80 952 957 7883856
- Vilsboll T Agerso H Lauritsen T The elimination rates of intact GIP as well as its primary metabolite, GIP 3–42, are similar in type 2 diabetic patients and healthy subjects Regul Pept 2006 137 168 172 16934887
- Drucker DJ The biology of incretin hormones Cell Metab 2006 3 153 165 16517403
- Orskov C Holst JJ Poulsen SS Kirkegaard P Pancreatic and intestinal processing of proglucagon in man Diabetologia 1987 30 874 881 3446554
- Orci L Bordi C Unger RH Perrelet A Glucagon- and glicentin-producing cells Lefebvre PJ Glucagon Berlin Springer Verlag 1983 57 79
- Goke R Larsen PJ Mikkelsen JD Sheikh SP Distribution of GLP-1 binding sites in the rat brain: evidence that exendin-4 is a ligand of brain GLP-1 binding sites Eur J Neurosci 1995 7 2294 2300 8563978
- Flint A Raben A Astrup A Holst JJ Glucagon-like peptide 1 promotes satiety and suppresses energy intake in humans J Clin Invest 1998 101 515 520 9449682
- Gros R You X Baggio LL Cardiac function in mice lacking the glucagon- like peptide-1 receptor Endocrinology 2003 144 2242 2252 12746281
- Meier JJ Gethmann A Gotze O Glucagon-like peptide 1 abolishes the postprandial rise in triglyceride concentrations and lowers levels of non-esterified fatty acids in humans Diabetologia 2006 49 452 458 16447057
- Carraro-Lacroix LR Malnic G Girardi AC Regulation of Na+/H+ exchanger NHE3 by glucagon-like peptide 1 receptor agonist exendin-4 in renal proximal tubule cells Am J Physiol Renal Physiol 2009 297 F1647 F1655 19776173
- Gault VA O’Harte FP Flatt PR Glucose-dependent insulinotropic polypeptide (GIP): anti-diabetic and anti-obesity potential? Neuropeptides 2003 37 253 263 14607102
- Tsukiyama K Yamada Y Yamada C Gastric inhibitory polypeptide as an endogenous factor promoting new bone formation after food ingestion Mol Endocrinol 2006 20 1644 1651 16469773
- Knop FK Vilsboll T Hojberg PV Reduced incretin effect in type 2 diabetes: cause or consequence of the diabetic state? Diabetes 2007 56 1951 1959 17513701
- Nauck M Stockmann F Ebert R Creutzfeldt W Reduced incretin effect in type 2 (non-insulin-dependent) diabetes Diabetologia 1986 29 46 52 3514343
- Bagger JO Knop FK Lund A Vestergaard H Holst JJ Vilsboll T Impaired Regulation of the Incretin Effect in Patients with Type 2 Diabetes Mellitus Diabetes 2010 58 Suppl 1 A369
- Vilsboll T Krarup T Deacon CF Madsbad S Holst JJ Reduced postprandial concentrations of intact biologically active glucagon-like peptide 1 in type 2 diabetic patients Diabetes 2001 50 609 613 11246881
- Kjems LL Holst JJ Volund A Madsbad S The influence of GLP-1 on glucose-stimulated insulin secretion: effects on beta-cell sensitivity in type 2 and nondiabetic subjects Diabetes 2003 52 380 386 12540611
- Nauck MA Heimesaat MM Orskov C Holst JJ Ebert R Creutzfeldt W Preserved incretin activity of glucagon-like peptide 1 [7–36 amide] but not of synthetic human gastric inhibitory polypeptide in patients with type-2 diabetes mellitus J Clin Invest 1993 91 301 307 8423228
- Nauck MA Kleine N Orskov C Holst JJ Willms B Creutzfeldt W Normalization of fasting hyperglycaemia by exogenous glucagon-like peptide 1 (7–36 amide) in type 2 (non-insulin-dependent) diabetic patients Diabetologia 1993 36 741 744 8405741
- Eng J Kleinman WA Singh L Singh G Raufman JP Isolation and characterization of exendin-4, an exendin-3 analogue, from Heloderma suspectum venom. Further evidence for an exendin receptor on dispersed acini from guinea pig pancreas J Biol Chem 1992 267 7402 7405 1313797
- Thorens B Porret A Buhler L Deng SP Morel P Widmann C Cloning and functional expression of the human islet GLP-1 receptor. Demonstration that exendin-4 is an agonist and exendin-(9–39) an antagonist of the receptor Diabetes 1993 42 1678 1682 8405712
- Edwards CM Stanley SA Davis R Exendin-4 reduces fasting and postprandial glucose and decreases energy intake in healthy volunteers Am J Physiol Endocrinol Metab 2001 281 E155 E161 11404233
- Simonsen L Holst JJ Deacon CF Exendin-4, but not glucagon-like peptide-1, is cleared exclusively by glomerular filtration in anaesthetised pigs Diabetologia 2006 49 706 712 16447056
- Malloy J Capparelli E Gottschalk M Guan X Kothare P Fineman M Pharmacology and tolerability of a single dose of exenatide in adolescent patients with type 2 diabetes mellitus being treated with metformin: a randomized, placebo-controlled, single-blind, dose-escalation, crossover study Clin Ther 2009 31 806 815 19446153
- Iwamoto K Nasu R Yamamura A Safety, tolerability, pharmacokinetics, and pharmacodynamics of exenatide once weekly in Japanese patients with type 2 diabetes Endocr J 2009 56 951 962 19706990
- European medicines Agency European Public Assessment Report Byetta EMEA/H/C/698 2006
- Knudsen LB Nielsen PF Huusfeldt PO Potent derivatives of glucagon-like peptide-1 with pharmacokinetic properties suitable for once daily administration J Med Chem 2000 43 1664 1669 10794683
- Elbrond B Jakobsen G Larsen S Pharmacokinetics, pharmacodynamics, safety, and tolerability of a single-dose of NN2211, a long-acting glucagon-like peptide 1 derivative, in healthy male subjects Diabetes Care 2002 25 1398 1404 12145241
- Damholt B Golor G Wierich W Pedersen P Ekblom M Zdravkovic M An open-label, parallel group study investigating the effects of age and gender on the pharmacokinetics of the once-daily glucagon-like peptide-1 analogue liraglutide J Clin Pharmacol 2006 46 635 641 16707410
- Bosi E Lucotti P Setola E Monti L Piatti PM Incretin-based therapies in type 2 diabetes: A review of clinical results Diabetes Res Clin Pract 2008 82 Suppl 2 S102 S107 19022515
- DeFronzo RA Ratner RE Han J Kim DD Fineman MS Baron AD Effects of exenatide (exendin-4) on glycemic control and weight over 30 weeks in metformin-treated patients with type 2 diabetes Diabetes Care 2005 28 1092 1100 15855572
- Buse JB Henry RR Han J Kim DD Fineman MS Baron AD Effects of exenatide (exendin-4) on glycemic control over 30 weeks in sulfonylurea-treated patients with type 2 diabetes Diabetes Care 2004 27 2628 2635 15504997
- Kendall DM Riddle MC Rosenstock J Effects of exenatide (exendin-4) on glycemic control over 30 weeks in patients with type 2 diabetes treated with metformin and a sulfonylurea Diabetes Care 2005 28 1083 1091 15855571
- Amori RE Lau J Pittas AG Efficacy and safety of incretin therapy in type 2 diabetes: systematic review and meta-analysis JAMA 2007 298 194 206 17622601
- Heine RJ Van Gaal LF Johns D Mihm MJ Widel MH Brodows RG Exenatide versus insulin glargine in patients with suboptimally controlled type 2 diabetes: a randomized trial Ann Intern Med 2005 143 559 569 16230722
- Nauck MA Duran S Kim D A comparison of twice-daily exenatide and biphasic insulin aspart in patients with type 2 diabetes who were suboptimally controlled with sulfonylurea and metformin: a non-inferiority study Diabetologia 2007 50 259 267 17160407
- Zinman B Hoogwerf BJ Duran GS The effect of adding exenatide to a thiazolidinedione in suboptimally controlled type 2 diabetes: a randomized trial Ann Intern Med 2007 146 477 485 17404349
- Klonoff DC Buse JB Nielsen LL Exenatide effects on diabetes, obesity, cardiovascular risk factors and hepatic biomarkers in patients with type 2 diabetes treated for at least 3 years Curr Med Res Opin 2008 24 275 286 18053320
- Klonoff DC Buse JB Nielsen LL Exenatide effects on diabetes, obesity, cardiovascular risk factors and hepatic biomarkers in patients with type 2 diabetes treated for at least 3 years Curr Med Res Opin 2008 24 275 286 18053320
- Okerson T Yan P Stonehouse A Brodows R Effects of exenatide on systolic blood pressure in subjects with type 2 diabetes Am J Hypertens 2010 23 334 339 20019672
- Garber A Henry R Ratner R Liraglutide versus glimepiride monotherapy for type 2 diabetes (LEAD-3 Mono): a randomised, 52-week, phase III, double-blind, parallel-treatment trial Lancet 2009 373 473 481 18819705
- Garber A Henry R Ratner R Hale PM Chang CT Bode B Liraglutide, a human GLP-1 analogue, maintains greater reductions in HbA1c, FPG and weight than glimepiride over 2 years in patients with type 2 diabetes: LEAD-3 extension study Diabetologia 2010 52 Suppl 1 S287
- Nauck MA Frid A Hermansen K for the LEAD-2 Metformin Study Group Efficacy and safety comparison of liraglutide, glimepiride and placebo, all in combination with metformin in type 2 diabetes mellitus Diabetes Care 2009 32 84 90 18931095
- Marre M Shaw J Brandle M Liraglutide, a once-daily human GLP-1 analogue, added to a sulphonylurea over 26 weeks produces greater improvements in glycaemic and weight control compared with adding rosiglitazone or placebo in subjects with Type 2 diabetes (LEAD-1 SU) Diabet Med 2009 26 268 278 19317822
- Zinman B Gerich J Buse JB Efficacy and safety of the human glucagon- like peptide-1 analog liraglutide in combination with metformin and thiazolidinedione in patients with type 2 diabetes (LEAD-4 Met + TZD) Diabetes Care 2009 32 1224 1230 19289857
- Russell-Jones D Vaag A Schmitz O Liraglutide vs insulin glargine and placebo in combination with metformin and sulfonylurea therapy in type 2 diabetes mellitus (LEAD-5 met + SU): a randomised controlled trial Diabetologia 2009 52 2046 2055 19688338
- Buse JB Rosenstock J Sesti G Liraglutide once a day versus exenatide twice a day for type 2 diabetes: a 26-week randomised, parallel-group, multinational, open-label trial (LEAD-6) Lancet 2009 374 39 47 19515413
- European medicines Agency European Public Assessment Report Victoza EMEA/H/C/1026 2009
- Blonde L Russell-Jones D The safety and efficacy of liraglutide with or without oral antidiabetic drug therapy in type 2 diabetes: an overview of the LEAD 1–5 studies Diabetes Obes Metab 2009 11 Suppl 3 26 34 19878259
- US Food and Drug Administration Information for Healthcare Professionals Exenatide (marketed as Byetta) [press release] 2009 http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm124713.htm
- Cvetkovic RS Plosker GL Exenatide: a review of its use in patients with type 2 diabetes mellitus (as an adjunct to metformin and/or a sulfonylurea) Drugs 2007 67 935 954 17428109