Abstract
Previous trials describe a decrease of vitamin D levels in patients with Parkinson’s disease and relationships to clinical disease severity. This case control study found higher but not significant 25-OH-vitamin D plasma levels in patients with Parkinson’s disease compared with age- and sex-matched controls and no associations to clinical parameters, such as rating scores of disease severity or assessments of cognitive function. A certain variability of vitamin D concentrations was observed in both cohorts, which were investigated during the same season. These outcomes put into perspective the emerging discussion on the importance of vitamin D in Parkinson’s disease. Our results warrant further confirmatory research with a strict matching design of patients and controls, which has not been done in previous investigations. We stress that this case control study does not allow any comment on the putative beneficial effects of vitamin D supplementation, ie, on bone mass or bone mineral density, in patients with Parkinson’s disease.
Keywords:
Introduction
Vitamin D is a fat-soluble secosteroid that exerts its effects by binding to the vitamin D receptor (VDR). Activation of vitamin D takes place by enzymatic hydroxylation in the liver and kidney with generation of 1.25-dihydroxycholecalciferol or 1.25-dihydroxyvitamin D3, (1,25-(OH)2D3).Citation1 Thus, vitamin D may directly and indirectly modulate the expression of hundreds to thousands of genes. A high prevalence of vitamin D deficiency was described in Parkinson’s disease (PD).Citation2,Citation3 The results and conclusions remain inconsistent.Citation3 The discovery that VDR and 1alpha-hydroxylase, the enzyme that converts vitamin D to its active form, are highly expressed in the substantia nigra led to the hypothesis that inadequate levels of circulating vitamin D may lead to dysfunction or cell death within the substantia nigra. Accordingly, reports exist that describe lower vitamin D levels in PD patients compared with healthy controls.Citation4 Moreover, a negative association between vitamin D levels with PD risk and severity was shown.Citation5–7 However, outdoor activity and thus exposure to sunlight are also important influencing and confounding factors.Citation8–10 Further discussed reasons are reduced mobility in combination with sunlight deprivation, gastrointestinal dysfunction with inadequate vitamin D intake.Citation11,Citation12 Generally vitamin D is also well known for its role in the regulation of calcium homeostasis and metabolism. Thus, vitamin D is an essential for the gastrointestinal absorption of calcium, magnesium, phophate, and zinc.Citation13,Citation14 Therefore, the common onset of reduced bone mineral density in PD was also discussed as a consequence of vitamin D insufficiency.Citation15,Citation16 Accordingly, vitamin D supplementation has even been suggested for prevention and delay of progression of PD.Citation17–19 One even postulated that higher vitamin D concentrations predispose for better cognitive function in PD patients.Citation20 However only one study showed the presence of only slight and not significant lower vitamin D levels.Citation21 One believes that this outcome may be explained with the harsh climate, frequent cloud cover, high latitude, and the low vitamin content in the common diet of the Faroe Islands, where this trial was undertaken.Citation2 Only a few foods contain vitamin D, which is biological inactive similar to the one from skin synthesis. Two prospective studies investigated the association between mid-life vitamin D levels and risk of PD. They produced conflicting results. One showed an increased risk for PD with lower mid-life vitamin D levels, and the other showed no association between vitamin D and PD risk.Citation3 Another more consistent finding is an inverse association between serum vitamin D level and motor symptom severity in cross-sectional trials.Citation3,Citation6,Citation8,Citation22 In view of the emerging aforementioned discussions on the role of vitamin D in PD, we performed a further case control study and determined vitamin D concentrations in PD patients and matched controls.
Materials and Methods
Subjects
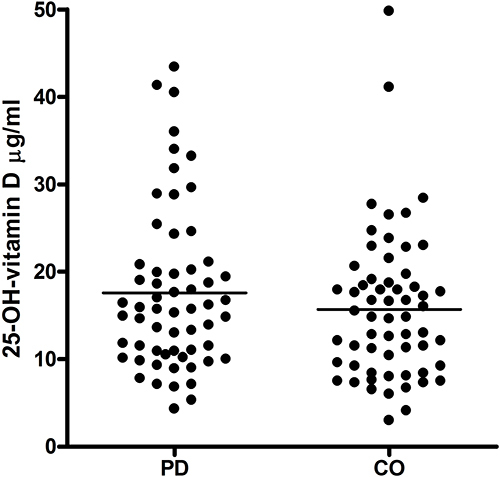
Sixty treated PD patients (19 female, 41 male; 72.28±2.98 years) () and 60 age- and sex-matched controls (19 female, 41 male; 72.58±3.33) participated.
Table 1 Scored Clinical Characteristics of PD Patients
Design
25-OH-vitamin D levels were determined. Intake of vitamin D, respectively vitamin K containing formulations was an exclusion criterion.Citation23 Blood was taken during an out-patient visit. Scoring of PD symptoms with the Unified Parkinson’s Disease rating scaleCitation24 and performance of the Montreal Cognitive Assessment (MoCA) was executed before blood sampling.Citation25 Blood sampling for both patients and controls was performed between March and May.
Methods
Blood samples were drawn in EDTA containing tubes. Vitamin D assessment was performed with LC-MS (company: Chromsystems®), which combines reversed-phase high performance liquid chromatography (HPLC [company: Shimadzu®] with mass spectrometry [AB-Sciex API5000®].
Statistics
The Mann Whitney U-test for independent samples was used for comparisons. Spearman rank correlation was employed for the correlation analysis. Data showed no normal distribution. A seasonal adjustment as a time dependent variable of vitamin D was not performed as collection and vitamin D determination for PD patients and controls was done in the same season.Citation26
Ethics
The study protocol and the patient informed consent form were reviewed and approved by the independent ethics committee of the Medical Faculty in the University of Wuerzburg, Germany (sign: 30/17 on 4–19-2017). It complies with the Declaration of Helsinki. Participants gave written informed consent after information on the study protocol.
Data Availability
The data sets generated or analyzed during the current study are available from the corresponding author on reasonable request.
Results
There was no significant difference of 25-OH-vitamin D between PD patients (17.59±9.28 [μg/L; mean±SD]) and controls (15.69±8.43) (). No significant associations between vitamin D levels and clinical parameters were found (). There was no impact of sex and age (results not shown).
Table 2 Correlation Analysis Between 25-(OH)-Vitamin D Blood Concentrations and Clinical Parameters
Discussion
We did not find significant higher 25-OH-vitamin D blood concentrations in treated PD patients in comparison with matched controls. No correlation to rating scores for PD and neuropsychological assessments of cognitive function appeared. Our outcomes put into perspective the emerging discussion on the importance of vitamin D in PD to a certain extent. Other investigations report vitamin D deficiency in PD and therefore hypothesize on the role vitamin D in the pathogenesis of PD. We show that a certain variability of vitamin D levels was observed in both cohorts. Our results warrant further confirmatory research with a strict matching design of patient and controls. We stress that the design of this case control study does not allow any comment on the putative beneficial effects of vitamin D supplementation, ie, on bone mass or bone mineral density in PD patients.Citation2,Citation17,Citation27,Citation28 It is known that vitamin D elevation shifts blood flow parameters in a manner that tissue microcirculation is improved.Citation1 As a consequence, oxygen transport and perfusion of tissue may increase and improve mitochondrial function and defence of oxidative stress, as shown in patients with multiple sclerosis.Citation29 Both mitochondrial impairment and reduction of free radical scavenging capacity also play an essential role in the pathophysiology of chronic neurodegenerative disorders.Citation30 Various trials demonstrated beneficial effects on body function following vitamin D supplementation in disease entities, such as cognitive dysfunction.Citation31 Therefore, one may hypothesize that higher vitamin D levels contribute to better coping with symptoms in PD, such as cognitive impairment. and age-related comorbidities, such as diabetes mellitus or cardiovascular disorders.Citation6,Citation8,Citation13,Citation22,Citation29,Citation32,Citation33
There are several limitations. We only assessed once and participants were not taken off PD medication. Therefore, the correlation analysis cannot provide a profound value on the assessments of functional deficits in relation to vitamin D measurement in our PD cohort.
In conclusion, vitamin D levels did not significantly vary between PD patients and matched controls and did not show any relationship to disease severity in contrast to other clinical investigations.
Disclosure
The authors have no competing interests in this work.
Additional information
Funding
References
- Müller T, Lohse L, Blodau A, Frommholz K. Vitamin D rise enhances blood perfusion in patients with multiple sclerosis. J Neural Transm. 2019;126:1631–1636. doi:10.1007/s00702-019-02093-x
- Lv Z, Qi H, Wang L, et al. Vitamin D status and Parkinson’s disease: a systematic review and meta-analysis. Neurol Sci. 2014;35:1723–1730. doi:10.1007/s10072-014-1821-6
- Fullard ME, Duda JE. A Review of the Relationship Between Vitamin D and Parkinson Disease Symptoms. Front Neurol. 2020;11:454. doi:10.3389/fneur.2020.00454
- Luo X, Ou R, Dutta R, Tian Y, Xiong H, Shang H. Association Between Serum Vitamin D Levels and Parkinson’s Disease: a Systematic Review and Meta-Analysis. Front Neurol. 2018;9:909. doi:10.3389/fneur.2018.00909
- Knekt P, Kilkkinen A, Rissanen H, Marniemi J, Saaksjarvi K, Heliovaara M. Serum vitamin D and the risk of Parkinson disease. Arch Neurol. 2010;67:808–811. doi:10.1001/archneurol.2010.120
- Barichella M, Cereda E, Iorio L, et al. Clinical correlates of serum 25-hydroxyvitamin D in Parkinson’s disease. Nutr Neurosci. 2020;1:1–9.
- Liu Y, Zhang BS. Serum 25-hydroxyvitamin D predicts severity in Parkinson’s disease patients. Neurol Sci. 2014;35:67–71. doi:10.1007/s10072-013-1539-x
- Tanaka K, Miyake Y, Fukushima W, et al. Vitamin D receptor gene polymorphisms, smoking, and risk of sporadic Parkinson’s disease in Japan. Neurosci Lett. 2017;643:97–102. doi:10.1016/j.neulet.2017.02.037
- Zhou Z, Zhou R, Zhang Z, Li K. The Association Between Vitamin D Status, Vitamin D Supplementation, Sunlight Exposure, and Parkinson’s Disease: a Systematic Review and Meta-Analysis. Med Sci Monit. 2019;25:666–674. doi:10.12659/MSM.912840
- Zhu D, Liu G-Y, Lv Z, Wen S-R, Bi S, Wang W-Z. Outdoor activity and vitamin D intake are inversely associated with Parkinson’s disease risk. J Zhejiang Univ Sci B. 2014;15(10):923–927. doi:10.1631/jzus.B1400005
- Wang J, Yang D, Yu Y, Shao G, Wang Q. Vitamin D and Sunlight Exposure in Newly-Diagnosed Parkinson’s Disease. Nutrients. 2016;8:142. doi:10.3390/nu8030142
- Wang L, Evatt ML, Maldonado LG, et al. Vitamin D from different sources is inversely associated with Parkinson disease. Mov Disord. 2015;30:560–566. doi:10.1002/mds.26117
- Kheiri B, Abdalla A, Osman M, Ahmed S, Hassan M, Bachuwa G. Vitamin D deficiency and risk of cardiovascular diseases: a narrative review. Clin Hypertens. 2018;24:9. doi:10.1186/s40885-018-0094-4
- Shoemaker TJ, Mowry EM. A review of vitamin D supplementation as disease-modifying therapy. Mult Scler. 2018;24:6–11. doi:10.1177/1352458517738131
- van Den BF, Speelman AD, Samson M, Munneke M, Bloem BR, Verhaar HJ. Parkinson’s disease and osteoporosis. Age Ageing. 2013;42:156–162. doi:10.1093/ageing/afs161
- van Den BF, Speelman AD, Van NM, et al. Bone mineral density and vitamin D status in Parkinson’s disease patients. J Neurol. 2013;260:754–760. doi:10.1007/s00415-012-6697-x
- Liu Y, Li YW, Tang YL, et al. Vitamin D: preventive and therapeutic potential in Parkinson’s disease. Curr Drug Metab. 2013;14:989–993. doi:10.2174/1389200211314090005
- Evatt ML. Beyond vitamin status: is there a role for vitamin d in Parkinson disease? Arch Neurol. 2010;67:795–797. doi:10.1001/archneurol.2010.123
- Evatt ML. Parkinson disease: low vitamin D and Parkinson disease--a causal conundrum. Nat Rev Neurol. 2014;10:8–9. doi:10.1038/nrneurol.2013.252
- Santangelo G, Raimo S, Erro R, et al. Vitamin D as a possible biomarker of mild cognitive impairment in parkinsonians. Aging Ment Health. 2021;25:1998–2002. doi:10.1080/13607863.2020.1839860
- Petersen MS, Bech S, Christiansen DH, Schmedes AV, Halling J. The role of vitamin D levels and vitamin D receptor polymorphism on Parkinson’s disease in the Faroe Islands. Neurosci Lett. 2014;561:74–79. doi:10.1016/j.neulet.2013.12.053
- Zhang HJ, Zhang JR, Mao CJ, et al. Relationship between 25-Hydroxyvitamin D, bone density, and Parkinson’s disease symptoms. Acta Neurol Scand. 2019;140:274–280. doi:10.1111/ane.13141
- Ferguson CC, Knol LL, Halli-Tierney A, Ellis AC. Dietary Supplement Use is High among Individuals with Parkinson Disease. South Med J. 2019;112:621–625. doi:10.14423/SMJ.0000000000001041
- Fahn S, Elton R. Members of the UPDRS Development Committee. Unified Parkinson’s Disease Rating Scale. In: Fahn S, Marsden CD, Goldstein M, editors. Recent Developments in Parkinson’s Disease II. New York: Macmillan; 1987:153–163.
- Nasreddine ZS, Phillips NA, Bedirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53:695–699. doi:10.1111/j.1532-5415.2005.53221.x
- Brola W, Sobolewski P, Szczuchniak W, et al. Association of seasonal serum 25-hydroxyvitamin D levels with disability and relapses in relapsing-remitting multiple sclerosis. Eur J Clin Nutr. 2016;70:995–999. doi:10.1038/ejcn.2016.51
- Ozturk EA, Gundogdu I, Tonuk B, et al. Bone mass and vitamin D levels in Parkinson’s disease: is there any difference between genders? J Phys Ther Sci. 2016;28:2204–2209. doi:10.1589/jpts.28.2204
- Ozturk EA, Gundogdu I, Tonuk B, Umay E, Kocer BG, Cakci A. Bone mineral density and serum vitamin D status in Parkinson’s disease: are the stage and clinical features of the disease important? Neurol India. 2020;68:394–400. doi:10.4103/0028-3886.283755
- Glueck CJ, Jetty V, Rothschild M, et al. Associations between Serum 25-hydroxyvitamin D and Lipids, Lipoprotein Cholesterols, and Homocysteine. N Am J Med Sci. 2016;8:284–290. doi:10.4103/1947-2714.187137
- Siotto M, Filippi MM, Simonelli I, et al. Oxidative Stress Related to Iron Metabolism in Relapsing Remitting Multiple Sclerosis Patients With Low Disability. Front Neurosci. 2019;13:86. doi:10.3389/fnins.2019.00086
- Wise J. Low vitamin D is linked to faster cognitive decline in older adults. BMJ. 2015;351:h4916. doi:10.1136/bmj.h4916
- Gatti D, Idolazzi L, Fassio A. Vitamin D: not just bone, but also immunity. Minerva Med. 2016;107:452–460.
- Iacopetta K, Collins-Praino LE, Buisman-Pijlman FTA, Liu J, Hutchinson AD, Hutchinson MR. Are the protective benefits of vitamin D in neurodegenerative disease dependent on route of administration? A systematic review. Nutr Neurosci. 2020;23:251–280. doi:10.1080/1028415X.2018.1493807