Abstract
Taiep (tremor, ataxia, immobility, epilepsy, paralysis) mutants show a significant increase in myelin thickness from 10 to 30 days of age but then demonstrate a decrease in myelin thickness from 1 to 6 months. The severity of the demyelination in the optic nerve suggests that visual deficits may exist in the taiep mutants. Animals were trained on a discrimination task, in which responses to a light stimulus (the SD period) were reinforced on a fixed ratio (FR)-1 schedule, and responses in the absence of the light stimulus (the SΔ period) were not reinforced. Following training, the light intensity presented during the SD period was gradually reduced between sessions until −6.0 candela/m2 was reached. Both groups of animals – taiep mutants and control Sprague Dawley rats – successfully recognized and responded in the presence of the stimulus near perfectly by the final day of training, suggesting that taiep mutants demonstrated normal learning, at least under this paradigm. Despite the severe demyelination of the taiep optic nerve, no visual deficits were detected as both groups of animals performed similarly as the light intensity decreased. Though the myelin loss of the optic nerve may have negatively affected signal transduction, this did not result in an increase in visual threshold.
Introduction
It has been demonstrated that demyelination of the central nervous system (CNS) produces a slowing and eventual blockage of conduction in the previously myelinated fibers.Citation1,Citation2 Saltatory conduction, the process by which myelin increases nerve conductance, is dependent on a number of factors that would be affected by myelin deterioration. A decrease in myelin thickness produces an increase in its capacitance as a result of a decrease in transverse resistance. An increase in current leakage through the remaining myelin results, which delays the excitation at the axonal nodes and, in turn, the rate of action potential propagation.Citation1,Citation2 Demyelination results in numerous morphological and behavioral abnormalities in human diseases of myelin, such as multiple sclerosis (MS) and phenylketonuria.
Animal models of myelin diseases have been used successfully for investigation of the anatomical and physiological effects of diseases of myelin.Citation3 Unfortunately, the short life span of many of the animal models of myelin disease has made intensive study of the behavioral effects of nervous system demyelination difficult; however, the taiep (tremor, ataxia, immobility, epilepsy, paralysis) rat is unique amongst the myelin mutants, living nearly to full term. This relatively long life span has made it a useful model for investigating behavioral manifestations of myelin disease.Citation4
An autosomal recessive disorder, the taiep mutation is characterized by a progressive accumulation of microtubules near the smooth endoplasmic reticulum of the oligodendrocytes.Citation5 This cytoskeletal abnormality appears to coincide with astrocytosis in the brain and interferes with the intracellular mechanisms necessary to maintain normal CNS myelin.Citation6 One of these intracellular mechanisms is likely dopamine activity, as one study has demonstrated that increased binding to D1-like receptors could cause some of the behavioral symptoms seen in the taiep mutant.Citation7 Taiep rats demonstrate near-normal levels of myelin at birth but, as the cytoskeletal disorder worsens, a progressive demyelination takes place throughout the CNS.Citation8 A number of behavioral effects of the taiep disease have been established, demonstrating a wide range of motor deficits, including both gait and splay abnormalities.Citation9
Sensory effects of myelin disease have been demonstrated in human diseases such as MS. Although there is biological evidence to suspect similar dysfunction in the taiep mutant,Citation10 no attempts to quantify sensory deficits have been undertaken to date.Citation11 Research indicates that the optic nerve of the taiep rat is affected by the developmental demyelination.Citation8 In control optic nerves, mean myelin thickness increased significantly with age beginning from 10 days postnatally to 6 months of age. The taiep condition, however, showed normal myelination in the optic nerve only for the first 20 days, after which myelin thickness began deteriorating and continued to do so until 12 months of age.Citation8 The importance of the optic nerve in vision is well established, making the visual modality a good candidate for investigation of taiep visual deficits.
While electrophysiology is often used to obtain estimates of visual sensitivity in animals, results have been surprisingly inconsistent.Citation12 Behavioral techniques have provided researchers with a second approach to assessing visual deficits in animals.Citation13 Many techniques, such as the visual water box method, require that animals recognize a visual cue to choose the proper pathway in a maze-like procedure.Citation14 Visual deficits are quantified as a function of the latency to reach the correct maze goal after having recognized the visual cue. Though an effective technique, methods like these are problematic in animals in which the visual deficit is accompanied by a motor deficit. Increased latency to the maze goal is equally likely to be due to the motor deficits as to any visual dysfunction. An alternative technique, centered on operant stimulus-discrimination training, assesses visual function by quantification of behavioral changes in the presence of a light stimulus of varying intensities. Earlier research using similar techniques has demonstrated visual thresholds that agree reasonably well with those derived via visual evoked potentials (VEPs).Citation15 This method requires only a single lever-press, a behavior that is easily performed, even by animals with neuromuscular dysfunction.
Because demyelination is known to produce a slowing and eventual blockage of signal transduction in the nervous system, and the importance of the optic nerve in vision is established, demyelination of the optic nerve in the taiep mutant likely results in a lower level of visual discrimination when compared to control rats. In the present study, we trained taiep and control rats on a simple discrimination procedure in which the operant behavior is reinforced only in the presence of a light stimulus. We gradually reduced the intensity of this light signal and compared the frequency of successful discrimination between the two groups. Animals with a visual deficit would be expected to fail to discriminate the light stimulus at a higher magnitude. It is proposed that this technique allows for an assessment of visual deficits that is less susceptible to interference from the motor deficits of taiep.
Methods
Subjects
Fourteen male rats – seven normal Sprague Dawley and seven taiep mutant albino rats – were used in this investigation. Taiep autosomal recessive mutant strain was obtained from the University of Wisconsin–Madison, Madison, WI, USA. The animals were self-mated in our laboratory, housed in pairs, and kept on a 12-hour light– dark cycle.
At 35 days of age, all animals were placed on a food deprivation schedule of 90% of their ad libitum weight. Initial training on the operant paradigm began at 40 days and continued until 70 days, when testing procedures started. Seventy days was determined to be an appropriate start date because the taiep optic nerve has significantly less myelin than in normal rats by this age.Citation8
Apparatus
Operant chamber
Training took place in a standard operant chamber with the light source 11.5 cm from the floor. The operandum was 6 cm above the grid floor and the food dispenser was 8.5 cm from each of the operant chamber’s inner walls.
Photometer
The intensity of the light stimulus was measured via a photometer (Tektronix, Inc, Beaverton, OR, USA) in foot-lamberts and later converted to candela (cd)/m2.
Procedure
Following food deprivation, animals were trained to press a response lever to receive food reinforcement (45 mg pellet). Initial training occurred in the presence of a light stimulus of −1.0 log cd/m2 – well above the rodent visual threshold and thus easily detected by both groups of animals.
Following successful lever training, animals were trained on a discrimination task. Daily training periods consisted of 50 signal-light-present (SD) and a varied number of signal-light-not-present (SΔ) trials presented randomly on a variable time (VT) 30-second schedule. SD trials consisted of the presentation of a 20-second light stimulus that signaled and was accompanied by reinforcement in the form of a fixed ratio (FR)-1 schedule delivering a 45 mg food pellet. Each trial ended immediately following reinforcement, at which point the stimulus light was removed. SΔ trials (20 seconds) occurred without the presentation of the light stimulus, and responses that occurred in the SΔ period were not reinforced. To prevent any accidental contiguity between an SΔ response and the onset of the following SD period, responses during the last 5 seconds of the SΔ period delayed onset of the following trial by an average of 5 seconds (VT-5). A day’s session concluded following the 50th SD presentation, typically resulting in a total duration of 90 minutes. Discrimination training continued in this way for 8 days, when the animal’s behavior reached asymptote.
Training was then adapted to allow for a behavioral measure of visual thresholds. Using the same procedure as in training, the light stimulus presented during the SD period was gradually reduced on each subsequent training session in 0.5 cd/m2 increments. This process continued for 10 days, at which point, performance during the light (SD) and dark (SΔ) trials was no longer statistically different.
Results
Training
The success of discrimination responding was assessed using two dependent measures: percentage of response during SD periods and percentage of response during SΔ periods. Discrimination was achieved when the percentage of SD response was significantly higher than that of SΔ.
A 2 × (8) mixed model analysis of variance (ANOVA) was used to assess training with disease condition acting as the between factor and day of training as the within factor.
Percentage of SD responding
Main effect testing for disease condition on the dependent measure of SD responses was found to be nonsignificant (F = 1.182, P > 0.28). When collapsing across the day of training variable, taiep and normal rats responded similarly during the SD periods.
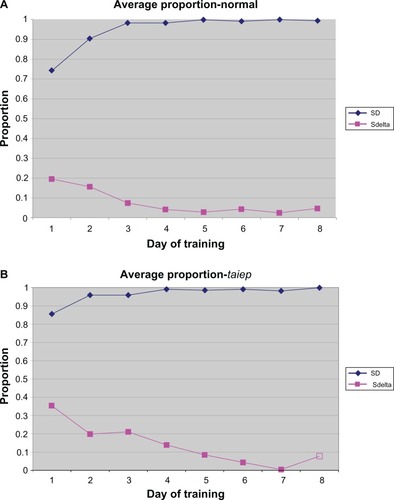
As shown in , main effect testing for the day of training was found to be significant (F = 9.609, P = 0.001), demonstrating an increase in SD response percentage over the course of training. Tests of the condition by day of training interaction were found to be nonsignificant (F = 1.141, P = 0.345), suggesting that no differences in response rate existed between taiep and normal rats.
Percentage of SΔ responding
Main effect testing for disease condition on the dependent measure of SΔ responses was found to be nonsignificant (F = 0.001, P > 0.98). When collapsing across the day of training variable, taiep and normal rats responded similarly during the SΔ periods.
As demonstrated in , main effect testing for the day of training was found to be significant (F = 28.128, P < 0.001), demonstrating a decrease in the SΔ response percentage over the course of training. Tests of the condition by day of training interaction were found to be nonsignificant (F = 2.215, P > 0.068), suggesting no differences in response rates between taiep and normal rats.
Visual deficits
For the purpose of assessing visual deficits, the dependent variable “error” was created. The error index was calculated by summing the numbers of trials without a response in the presence of SD (false negatives) and the number of responses during the SΔ (false positives) divided by the total number of opportunities to respond. This value represents the proportion of errors made, with a value of 0 representing no errors and 1 representing an error on every trial.
A 2 × (9) mixed-model ANOVA was used to assess the impact of disease condition on index at each of the nine levels of illumination. Post hoc analyses were completed where appropriate using Scheffe’s planned comparisons. Main effects tests for disease condition were not significant (F = 0.218, P = 0.641), suggesting that, when collapsing across levels of illumination, no differences existed between normal and taiep rats.
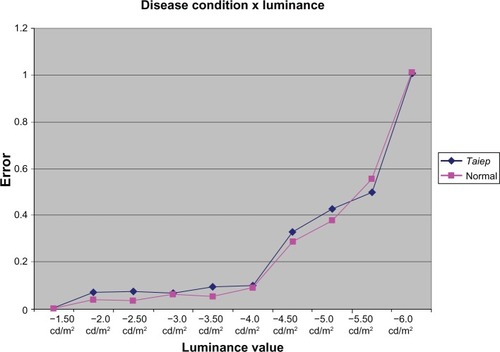
As shown in , main effect tests for illumination magnitude were found to be significant (F = 36.320, P = 0.001), demonstrating that, when ignoring disease condition, the magnitude of illumination had a statistically significant effect on index.
Figure 2 Successful responding in the presence of the stimulus light as a function of magnitude for both normal (A) and taiep (B) animals.
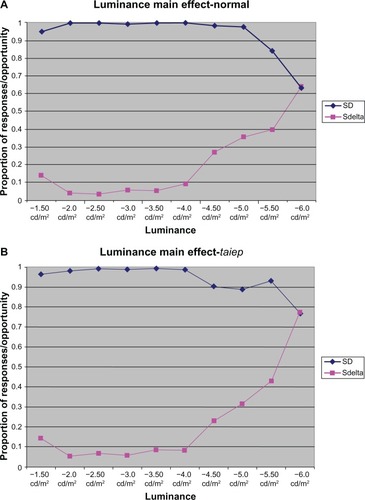
The interaction between disease condition and luminance magnitude was found to be nonsignificant (F = 0.123, P = 0.999), as is depicted in . This result suggests that differences in index were solely a function of luminance magnitude and not affected by disease condition.
Stimulus discrimination
Examination of error rates for both conditions suggests that visual discrimination for both taiep and normal rats failed between −5.5 and −6.0 log cd/m2, which is consistent with expected visual thresholds. These findings do not support visual deficits in the taiep myelin mutants, suggesting instead that visual sensitivity of the diseased and normal conditions are nearly identical.
Discussion
The taiep myelin mutant’s long life span makes it unique amongst animals with similar diseases of myelin, as its relative longevity makes it possible to carry out detailed investigations of the physiological, behavioral, and sensory manifestations of the disease.
The primary mechanism in the myelin aberrance of the taiep rat is linked to a malfunction in microtubule production.Citation3 Lunn et al found that this microtubule proliferation closely mirrored the developmental progression of demyelination in the optic nerve, ventral columns, and corticospinal tracts of the taiep mutant.Citation8 The severity of the damage to the optic nerve myelin suggests that visual deficits may be likely in the taiep mutant. Lunn et al also reported that, by 20 days of age, taiep mutants already demonstrate significantly less myelin than do similarly aged normal rats.Citation8 The schism between normal and taiep optic nerve myelin continues throughout the progression of the disease and, by 150 days, the optic tracts of taiep rats are nearly devoid of myelin.
The operant technique used in this study provided us with an opportunity to explore the possibility of visual deficits in the taiep visual system. Acquisition of the stimulus discrimination progressed at similar rates to normal rats, and at no time were any differences between the groups on the two dependent measures statistically significant. Both groups of animals successfully recognized and responded in the presence of the stimulus near perfectly by the final day of training.
Despite the severe demyelination of the taiep optic nerve, no visual deficits were detected by this procedure. Testing of the interaction between luminance value and disease condition was not significant, suggesting that both normal and taiep animals performed similarly as the level of the light stimulus was decreased. Index values increased from 0.5 to 1.0 at luminance values −5.5 cd/m2 and −6.0 cd/m2, indicating that it is between these two values that the light is no longer detectable by any of the rats. This result is consistent with VEP studies of albino and lends further support to the use of operant techniques for the investigation of visual thresholds in rodents;Citation14 the consistency of these data lends further support to the use of operant techniques for the investigation of visual thresholds in rodents. Collectively, this evidence suggests that taiep rat vision does not differ in light sensitivity from normal rats. Previous work by Benitez et al demonstrates a progressive reduction in amplitude and latency in VEP in the occipital cortex of taiep rats when compared to controls.Citation16 Our results suggest that the change in wave forms described by Benitez does not manifest in visual deficits, underscoring the role of behavioral techniques in identifying the practical manifestation of neurodegenerative disease in vivo.
It is important to note that the taiep animals were tested fairly early in the development of the disease. Though the operant technique utilized is less susceptible than other techniques to motor deficits contaminating the results, later symptoms of taiep (ataxia, paralysis, seizure) are very severe and would make successful testing under the operant paradigm difficult. To avoid this possibility, examination of the visual thresholds took place during a period in the disease in which the symptoms were at a reasonable level, characterized primarily by tremors. Lunn et al showed that, at the age investigated in this experiment, the optic nerve of taiep rats was significantly compromised, though much of the myelin was still intact and relatively healthy.Citation8 Despite the myelin deficits at the time of testing, failure to find visual deficits may still be related to the disorder’s progressive nature. It has recently been suggested that the symptoms of myelin diseases may largely be a function of axonal degeneration.Citation17 Oligodendrocytes, which serve to myelinate axons, may also play an axonotrophic role via other biochemical mechanisms. Wilkins et al report that, at the time of testing (between 2–3 months of age), taiep animals display a loss of only 25% of optic nerve axons, demonstrating a slower progression in axonal degeneration when compared to the loss of myelin.Citation17 It may be that visual deficits are better predicted as a result of axonal degeneration rather than demyelination.Citation1,Citation2,Citation16 Future work utilizing the technique described may allow for the tracking of visual deficits throughout the progression of the disease and provide evidence that deficits follow a time course more closely tied to axonal degeneration than to the demyelination itself.
Disclosure
The authors report no conflicts of interest in this work.
References
- McDonaldWISearsTAThe effects of experimental demyelination on conduction in the central nervous systemBrain19709335835984319185
- McDonaldWISearsTAFocal experimental demyelination in the central nervous systemBrain19709335755825507016
- DuncanIDLunnKFHolmgrenBUrba-HolmgrenRBrignolo-HolmesLThe taiep rat: A myelin mutant with an associated oligodendrocyte micro-tubular defectJ Neurocytol19922118708841469463
- AnchAMPowellEBloomCDycheJFaulknerKRichterRRLocomotor analysis of the taiep ratJ Gen Psychol2000127441242511110003
- LiFYSongJDuncanIDMapping of taiep rat phenotype to rat Chromosome 9Mamm Genome2003141070370514694906
- Leon ChavezBAGuevaraJGalindoSRegional and temporal progression of reactive astrocytosis in the brain of the myelin mutant taiep ratBrain Res2001900115215511325359
- FloresGFloresJMenaRValenciaJMutant taiep rats exhibit an increase in D1 binding in basal gangliaBrain Res20029561242912426042
- LunnKBaasPDuncanIDMicrotubule organization and stability in the oligodendrocyteJ Neurosci1997171492149329185530
- PowellEAnchAMDycheJBloomCRichterRRThe splay angle: a new measure of assessing neuromuscular dysfunction in ratsPhysiol Behav1999765819821
- BlackJADib-HajjSBakerDNewcombeJCuznerMLWaxmanSGSensory neuron-specific sodium channel SNS is abnormally expressed in the brains of mice with experimental allergic encephalomyelitis and humans with multiple sclerosisProc Natl Acad Sci U S A20009721115981160211027357
- HolmgrenBUrbá-HolmgrenRRiboniLVega-SaenzdeMieraECSprague Dawley rat mutant with tremor, ataxia, tonic immobility episodes, epilepsy and paralysisLab Anim Sci19893912262282724922
- Muñoz TedóCHerreros de TejadaPGreenDGBehavioral estimates of absolute threshold in ratVis Neurosci1994116107710827841117
- BloughDBloughPAnimal psychophysicsHandbook of Operant BehaviorNew York, NYAcademic Press1970
- PruskyGTWestPWDouglasRMBehavioral assessment of visual acuity in mice and ratsVision Res200040162201220910878281
- AccorneroNDe VitoGRotunnoAPeruginoUManfrediMCritical fusion frequency in MS during mild induced hyperthermiaActa Neurol Scand19897965105142782032
- BenitezJHolmgrenBEguibarJRoncaglioloMMultimodal sensory evoked potentials in a rat model of demyelinating diseasesElectroen Clin Neuro20071031189189 (1).
- WilkinsAKondoYSongJSlowly progressive axonal degeneration in a rat model of chronic, nonimmune-mediated demyelinationJ Neuropathol Exp Neurol201069121256126921107138