Abstract
Multifocal motor neuropathy (MMN) is a debilitating and rare disease causing profound weakness with minimal to no sensory symptoms. Conduction block is frequently seen on electrodiagnostic testing. An immune-mediated pathology is suspected though the exact underlying pathophysiology has yet to be elucidated. The presence of anti-GM1 ganglioside IgM antibodies coupled with favorable response to intravenous and subcutaneous immunoglobulins supports a complement-mediated mechanism which leads to destruction of nerve tissue with probable predilection to the nodes of Ranvier. High-dose immunoglobulin currently is the only treatment with proven efficacy for MMN patients. Unfortunately, many patients experience decreased responsiveness to immunoglobulins over time, requiring higher and more frequent dosing. In this review, we will focus on the pharmacology, efficacy, safety, and tolerability of intravenous and subcutaneous immune globulin infusion for treatment of MMN.
Introduction
Multifocal motor neuropathy (MMN) is an immune-mediated peripheral neuropathy affecting only motor nerves. MMN is rare; one study in the Netherlands estimated the prevalence to be approximately 0.6 per 100,000 individuals. The disease is more common in men than women by a ratio of 2.7:1.Citation1 Though there have been case reports of MMN in patients as young as 6 years of age,Citation2 onset typically occurs between 20 and 70 years of age with approximately 80% of patients reporting onset between 20 and 50 years of age.Citation3 Classic presenting symptoms include progressive painless, distal, asymmetric weakness in the upper extremities.Citation4–Citation6 The ulnar, radial, median, and tibial nerves frequently are affected.Citation4 Though exceedingly rare, involvement of cranial nerves has been reported.Citation7 Autonomic dysfunction is absent.Citation3 Muscle cramps, fasciculations, and exacerbation of weakness in cold weather may occur.Citation4,Citation8 In early stages of disease, there is weakness without significant muscle atrophy since the disease is caused by conduction block. With disease progression, however, muscle atrophy can occur.Citation3,Citation9
Prominent sensory symptoms are not typical and this may help differentiate MMN from compression neuropathies.Citation4,Citation5 Electrophysiological studies demonstrating conduction block in motor nerves not exposed to compression or entrapment and sparing of sensory nerves are characteristics of MMN.Citation4,Citation10 Notably, some patients with MMN will not have detectable conduction block.Citation11–Citation13 Routine nerve conduction studies may miss conduction block if present proximally.Citation14 Activity-dependent conduction block, defined as a temporary conduction block induced by exercise, can also go undetected on routine nerve conduction studies.Citation12,Citation13 Conduction block may also be difficult to demonstrate in cases of advanced disease with severe, confluent denervation.Citation11 Though serum anti-GM1 ganglioside IgM antibodies may be increased in MMN, these antibodies are not sensitive markers for the disease since they may be detected in as few as 25% of patients.Citation15
MMN occasionally may be misdiagnosed as motor neuron disease, progressive muscular atrophy, or lower motor neuron predominant amyotrophic lateral sclerosis (ALS).Citation11 However, clinical findings as summarized in and electrophysiology can help differentiate these.Citation9,Citation16 It is important to distinguish among these diseases as each has a dramatically different course. Most forms of ALS are rapidly progressive and the disease is incurable with little more than supportive treatments available currently. In contrast, MMN frequently responds well to treatment with IVIG, giving patients the potential for a normal lifespan.Citation16 IVIG may also be used in a diagnostic trialCitation11 for confirmation if a diagnosis of MMN cannot be made on the basis of physical examination and electrodiagnostic studies.Citation11
Table 1 Diagnostic clinical criteria for multifocal motor neuropathy
MMN typically follows a chronic progressive course. However, some patients may present with an acute form of the disease. The goal of treatment is to improve motor deficits by reducing conduction block, slowing axonal degeneration, and promoting reinnervation. Most patients require treatment for many years though some do achieve prolonged remission.Citation16 The mainstay of treatment is intravenous immunoglobulin (IVIG) or subcutaneous immunoglobulin (SCIG). Other treatments which have been investigated include corticosteroids and plasma exchange. These currently are not recommended due to potential to worsen weakness. Cyclophosphamide, mycophenolate mofetil (MMF), rituximab, and eculizumab have been explored as well though all have worse side effect profiles and are not as effective as IVIG or SCIG which remain the gold standard treatments for MMN.Citation6 In some patients, IVIG may lose its efficacy. In those patients, IV cyclophosphamide may reset the immune system and provide prolonged recovery.Citation11 In this review, we will focus on the pharmacology, efficacy, safety, and tolerability of IVIG and SCIG infusion for treatment of MMN.
Overview of IVIG pharmacology in MMN
The pathophysiology behind MMN is not entirely clear. The few available studies investigating tissue pathology of motor nerves have shown differing findings,Citation11,Citation17,Citation18 though demyelination typically is absent as MMN likely is not a demyelinating neuropathy.Citation17 The presence of anti-GM1 ganglioside IgM antibodies, efficacy of IVIG treatment, and similar clinical features to the pure motor axonal variant of Guillain–Barre syndrome (acute motor axonal neuropathy) all are suggestive of an autoimmune pathophysiology.Citation16,Citation17,Citation19
A proposed mechanism of injury from anti-GM1 ganglioside IgM antibodies in MMN involves complement-mediated damage to the sodium channels in the nodes of RanvierCitation11 which has been observed in the rabbit model of acute motor axonal neuropathy. This model demonstrates how IgM GM1 activates the complement cascade, causing production of a membrane attack complex which weakens membrane integrity by disrupting sodium channels and facilitating antibody binding to the axolemma.Citation20 In this model, complement inhibitors prevent further antibody-mediated damage, supporting a complement-mediated mechanism of injury.Citation20 By extension, a 2015 case-control study demonstrated that increased activity of the classical complement pathway and anti-GM1 ganglioside IgM antibodies determined disease severity in MMN patients, providing further evidence in support of a complement-mediated disease mechanism.Citation21 Since not all patients with MMN have detectable anti-GM1 ganglioside IgM antibodies, T-cells and cytokines are thought to play a significant role as well.Citation22
As with MMN, the exact mechanism by which IVIG affects immunomodulation in the treatment of MMN and other inflammatory neuropathies is not fully understood, though several mechanisms have been proposed. IVIG contains antibodies against components of the classical complement pathway to prevent membrane attack complex formation leading to tissue degradation at the axolemma.Citation23,Citation24 The presence of anti-GM1 ganglioside IgM antibodies and possible complement-mediated mechanism described suggests the effect of IVIG on the complement pathway may play a significant role in its efficacy for treating MMN as shown in .
Figure 1 Proposed mechanism of IVIG within the classical complement pathway for treatment of multifocal motor neuropathy.
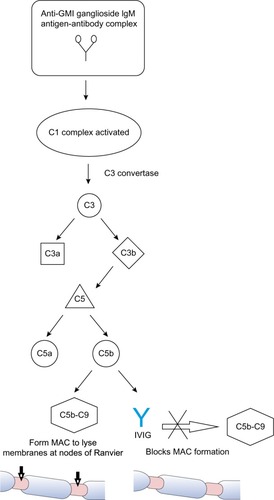
Other mechanisms may be at work. IVIG affects B-cells and antibodies. The anti-idiotype antibodies to different gangliosides present in IVIG may prevent binding of pathogenic autoantibodies to target epitopes in MMN, helping to regulate immune function.Citation25 IVIG also has been shown to inhibit antibody productionCitation26 and B-cell differentiationCitation27 and to downregulate certain autoreactive B-cellsCitation28,Citation29 all of which may have an effect. Another proposed mechanism of IVIG involves T-cell regulation. In 2008, several regulatory T-cell epitopes (Tregitopes) were discovered in the heavy and light chains of IgG, and administration of these Tregitopes in vivo for several animal models of autoimmune disease suppressed immune responses to antigen.Citation30 Similarly, in humans IVIG treatment selectively activated Tregitopes, enhancing suppressive function and potentially explaining how IVIG restores balanced immune function.Citation22,Citation31 Other proposed mechanisms include IVIG’s effect on cytokines,Citation32 mediation of Fc receptor blockade on macrophages,Citation33 and cell migration.Citation33,Citation34 None of these proposed mechanisms has been proven as the dominant pathway and several mechanisms may work synergistically in treatment of MMN and other autoimmune diseases for which IVIG is effective.Citation22
MMN typically responds well to treatment with IVIG; however, the degree of recovery is variable, and optimal dosing and treatment intervals for IVIG maintenance therapy have not conclusively been established.Citation35 In our experience, patients treated early in their disease, ie, prior to significant muscle atrophy, tend to do better than patients treated later when atrophy clearly is present. Increased IgG concentration (ΔIgG) after IVIG infusion recently was identified as a major factor in determining outcomes of IVIG therapy for GBSCitation36 and some posit ΔIgG could be a useful indicator for optimizing outcomes of IVIG therapy and dosing for treatment of MMN.Citation37 A recent small studyCitation37 examined the variability of IVIG pharmacokinetics among MMN patients relative to treatment response. ΔIgG was greater in patients who responded favorably to IVIG treatment. Researchers also examined if variability in pharmacokinetics was associated with genetic polymorphisms in the endothelial IgG receptor (FcRn), a determinant of IgG half-life. IVIG likely saturates these receptors, thereby accelerating the degradation of endogenous IgG and potentially balancing immune function.Citation38 Researchers found no association of ΔIgG levels and responsiveness to treatment with genetic variation in the FcRn gene. However, there was a high degree of variability in ΔIgG levels among patients who received identical dosing protocols as the underlying mechanisms behind variable IVIG efficacy and metabolism have yet to be elucidated. This poses a challenge for establishing standardized treatment dosages and interval protocols.
Efficacy studies
IVIG remains the first-line treatment for patients with MMN and has been investigated thoroughly with studies evaluating efficacy, dosing, and routes of administration.Citation6,Citation39,Citation40 Meta-analysis of multiple randomized, placebo-controlled, double-blind trialsCitation41,Citation42 showed that nearly 80% of all subjects had a significant improvement in strength short term after treatment with IVIG as compared to less than 5% after treatment with placebo.Citation43
A 2008 retrospective study (Study A, ) examined dosing in 40 patients with MMN, 22 of whom were IVIG naïve.Citation44 A cumulative dose of 2 g/kg IVIG was effective in 70% of patients. Though not statistically significant, researchers found that positive predictors for favorable response to IVIG were female and lower Medical Research Council (MRC) muscle strength scores. No correlation was found between electrophysiological findings of conduction block and clinical improvements in this study, though correlation has been found in other trials. A recent randomized, double-blind, controlled trialCitation45 from 2013 (Study B, ) assessed efficacy, safety, and tolerability of 10% liquid IVIG. Forty-four patients were randomized to 12 weeks of treatment with IVIG followed by 12 weeks of placebo or vice versa. To ensure stability, all patients received IVIG for 12 weeks at the beginning and end of the study. To prevent carry-over effects, IVIG also was given between the two double-blind 12-week periods. While on IVIG, patients’ mean maximal grip strength increased by nearly 4% and while on placebo it declined by approximately 31% (P=0.005). Using Guy’s Neurological Disability Scale to assess disability related to upper limb function, researchers found a significant number of subjects worsened while on placebo with over two-thirds of participants needing to stop placebo prematurely and resume IVIG therapy. These findings supported researchers’ conclusions that IVIG significantly improves muscle strength and function in MMN patients.
Table 2 Summary of studies evaluating efficacy of IVIG/SCIG for treatment of MMN
Though IVIG therapy is helpful for nearly all MMN patients to some degree, the treatment does not always prevent loss of muscle strength and function, and patients often require increased doses and frequency of treatments over time. MMN follows a chronic progressive course and most patients require maintenance therapy with IVIG for years. Prolonged remission is achieved for a minority, some remain stable, and many experience a gradual decline in strength despite being on maintenance therapy. Long-term therapy has been investigated in several studies. A 2002 studyCitation46 (Study C, ), over a period of 4–8 years, followed eleven patients with MMN initially treated with one full course of IVIG (2 g/kg) followed by 0.4 g/kg every week and then with maintenance therapy of one infusion every 1–7 weeks. Patients’ strength was evaluated using the MRC score summated for 20 muscle groups, handheld dynamometry, electrodiagnostic studies, and Guy’s Neurological Disability Scale. Muscle strength was reevaluated within 3 weeks after starting IVIG and significant gains were noted for all patients as compared to baseline evaluations performed prior to starting treatment. Slight but significant decreases in strength were noted during subsequent follow-ups over the 4–8-year period. Electrodiagnostic evaluations showed improvement in 13 nerves and decline in 14 nerves. Researchers concluded that maintenance treatment with IVIG improved overall muscle strength and function over time but did not prevent minimal but statistically significant decreases in muscle strength. These findings correlated with electrophysiological data showing a link between IVIG treatments and diminished ongoing axon loss. A 2004 studyCitation47 (Study D, ) of ten MMN patients revealed similar findings clinically and electrophysiologically with ongoing maintenance therapy. Patients responded well to initial treatment with IVIG, but by the time of the final follow-up, after anywhere from 5 to 12 years of maintenance treatments, only two maintained the maximum gains, while eight showed a decline after 3–7 years despite ongoing treatments. Decreased responsiveness to IVIG leading to clinical decline correlated with electrodiagnostic findings showing reduced compound motor action potential amplitudes. Diminished efficacy of IVIG with decreasing compound motor action potential supports the theory that loss of efficacy of IVIG over time occurs due to worsening axonal loss with more advanced stages of disease.Citation46,Citation48,Citation49 As compared to the previous two studies, another study from 2004Citation50 (Study E, ) examined MMN patients on significantly higher IVIG dosing regimens of 2 g/kg over 5 days monthly for 3 consecutive months, followed by monthly maintenance therapy over 3.5–12 years. Patients on higher doses had improved outcomes, with ongoing statistically significant gains in strength and function and lower disability scores based on the modified Rankin Disability Scale.Citation50 Clinical improvements for these patients correlated with improvements on electrodiagnostic testing including resolution of conduction blocks, decreased evidence of axonal degeneration, and ongoing reinnervation. A 2008 retrospective studyCitation44 (Study A, ) of 40 patients with MMN found that only eight participants from the original cohort remained in remission, defined as clinical improvement without further treatment lasting for at least 6 months. Twenty-five participants required IVIG maintenance therapy to preserve motor function. Of these, eight were selected at various times to try adjunct immunosuppressive agents but with limited success. A retrospective study from 2010Citation1 (Study F, ) examined increases in strength with IVIG therapy as well as dosage increases required for maintenance therapy over time. Of 88 patients, 94% responded favorably to IVIG. The 6% who did not respond had been diagnosed much later in their disease course and had far more advanced MMN symptoms. Delayed initiation of treatment with IVIG was a statistically significant independent determinant for greater weakness and disability. Seventy-six percent of patients evaluated were given IVIG maintenance therapy with a median duration of 6 years. Median dosage increased from 12 to 17 g/week for these patients. Thirty-five patients tried immunosuppressive agents other than immunoglobulin without improvement. In light of these findings, guidelines established in 2010 by the European Federation of Neurological Societies/Peripheral Nerve SocietyCitation6 recommends initial dosing of 2 g/kg infused over 2–5 consecutive days. If clinical improvement occurs but is not sustained, then maintenance therapy of 1 g/kg infused every 2–4 weeks or 2 g/kg every 1–2 months is recommended. Some clinicians recommend holding initiation of maintenance therapy until symptoms plateau or begin to decline.Citation51 Other clinicians advocate starting a specific regimen every 14 days and making adjustments gradually based upon clinical response.Citation52 Typical maintenance therapy dosages are 0.4 g/kg weekly or 1–2 g/kg every 2–6 weeks depending on responsiveness and ability to tolerate therapy.Citation9,Citation53 Adjunctive or alternative immunosuppressive treatments can be considered if IVIG alone is not sufficient.Citation6
While effective, IVIG is not without associated risks, which include potentially life threatening complications such as thromboembolic events and anaphylaxis. Intravenous administration is also expensive and burdensome, requiring patients to be monitored closely in a hospital or clinic setting while undergoing infusion. Because of these risks, expenses, and logistical burdens, a popular alternative is subcutaneous administration of immunoglobulins which can be self-administered at home.
A 2009 randomized, controlled, single-blinded studyCitation54 (Study G, ) of nine IVIG responsive patients with MMN evaluated dynamometric strength of muscles weakened by disease and quality of life as measured by the SF-36 quality of life questionnaire. Patients treated with IVIG and SCIG saw statistically equivalent mean improvements in muscle strength and no significant difference was noted for SF-36 scores between the two groups. One study participant had irritation, erythema, and swelling at injection sites for a few weeks but all other adverse reactions to SCIG were mild and short-lived. A majority, five of nine participants, decided to continue with SCIG upon completion of the study. After 2 years, researchers followed up with the participants who continued maintenance therapy with SCIG. Dosages varied between 12.8 and 24.8 g or 80 and 155 mL infused two to three times per week. There were no serious adverse side effects reported and any reactions occurring at injection sites were mild and short-lived. Strength and SF-36 scores were stable over the 2-year period. Another 2009 trialCitation55 (Study H, ) examined SCIG dosing. Ten patients were randomized to SCIG dosing equivalent to 50% of prior IVIG maintenance dosing or SCIG dosing equivalent to 100% of prior IVIG maintenance dosing. Of patients receiving SCIG dosing at 50% of prior IVIG dosing, one withdrew and four had a significant decline in MRC scores. Of patients receiving equivalent SCIG doses, four of five maintained equal MRC scores. This study demonstrated SCIG therapy to be safe and as effective as IVIG in maintaining motor function when used at equivalent but not lesser doses to treat MMN. To date, weekly dosing of SCIG has been shown to have a similar efficacy to IVIG.Citation16
SCIG may even reduce fluctuations in strength related to troughs in IgG serum concentration between IVIG dosage intervals known as “end of dose” weakness.Citation56 Though a direct link between serum IgG levels and degree of strength and functionality has yet to be established conclusively, two small studies examining SCIG and IVIG dosing show results supporting the hypothesis that strength is related directly to serum IgG concentration. One studyCitation57 (Study I, ) examined a patient with MMN who suffered cyclical fluctuations in strength while on IVIG therapy dosed every 3–4 weeks with trough serum IgG serum levels at 1,500 mg/dL. The patient showed improvement in strength with fewer fluctuations after switching to weekly SCIG infusions with total monthly dosing increased by 25% resulting in a steady-state concentration of IgG serum levels at 2,100 mg/dL. A small, open-label, multicenter, Phase II studyCitation58 (Study J, ) examined transitioning from equivalent monthly IVIG to weekly SCIG in eight MMN patients and showed similar dose-dependent effects. Seven of eight patients were able to maintain trough IgG serum levels of 1,680 mg/dL (±5.0) on SCIG, comparable to trough IgG serum levels of 1,750 mg/dL (±4.9) measured before the last IVIG dose. SCIG preserved strength as measured by MRC sum scores for over 6 months. One patient, despite receiving equivalent doses of SCIG and subsequent dosage increases by 25%, had deterioration in strength and correspondingly low IgG serum trough at 935 mg/dL. This was significantly lower than the IgG serum trough of 1,890 mg/dL while on IVIG. Once this patient resumed IVIG therapy, IgG serum troughs rose to previous levels and strength improved to baseline. These studies support the idea that higher steady-state concentrations of serum IgG are beneficial for maintaining strength. Since all studies to date have been small, larger trials are needed comparing IVIG and SCIG to determine if one form of delivery is more effective than the other. Since a minority of patients with MMN are non-responders to IVIG and SCIG and since a majority of patients who do respond favorably to immunoglobulins require progressively more aggressive regimens to remain in remission, alternate or adjunctive immunosuppressive treatments are necessary. Unfortunately, no other immunosuppressive therapies used in place of or in addition to IVIG or SCIG conclusively have demonstrated benefit in clinical trials. Corticosteroids and plasma exchanges can lead to worsening of motor function and are not recommended for treatment of MMN.Citation6 A randomized controlled trialCitation59 of 28 patients at a single center examined MMF as an adjunctive treatment for MMN. Researchers used 1 g MMF twice daily in addition to pre-established IVIG maintenance therapy over the course of a year. Patients did not have significant increases in muscle strength, functional scores, or a reduction in maintenance IVIG dosing. Since no adverse side effects occurred, researchers determined that adjunctive treatment with MMF was safe but ineffective.Citation59,Citation60
Eculizumab, a monoclonal antibody successfully used to treat complement-mediated disorders, has shown some promise as an adjunctive therapy. Eculizumab binds to and inactivates complement factor C5, thereby blocking terminal complement activation and subsequent lysis of membranes via membrane attack complexes in a manner similar to the complement-mediated mechanism of IVIG outlined in . A single small clinical trialCitation61 of 14 patients with aggressive neuromyelitis optica, a complement-mediated disease, demonstrated favorable responses including reduced frequency of attacks and disability scores following treatment with eculizumab. Further studies including a Phase III randomized, double-blind, open-label clinical trial have yet to be completed.Citation62 Similarly, studies of eculizumab’s efficacy in treating myasthenia gravis (MG), another complement-mediated disease, are limited. A single pilot Phase II trialCitation63 of eculizumab in 14 patients with severe MG refractory to other treatments demonstrated significant clinical benefits of increased strength and functionality for patients receiving eculizumab as compared to those receiving placebo. As discussed previously, a proposed mechanism of MMN involves anti-GM1 ganglioside IgM antibodies activating complement-mediated destruction of axolemmal membranes. Inhibition of the complement cascade could protect motor nerves.Citation16 A 2011 open-label clinical trial was conducted testing eculizumab over a period of 14 weeks on 13 MMN patients. Of the patients included in the study, ten were concurrently receiving maintenance therapy with IVIG.Citation64 Improvements were noted in patient-rated subjective scores, myometric measurements of muscle strength, and prevalence of nerves with conduction block on electrodiagnostic testing. Researchers noted a small treatment effect in patients with higher baseline motor function whether eculizumab was used in conjunction with or independent of IVIG. Given limited available studies of eculizumab for treatment of MMN, MG, and neuromyelitis optica, it is difficult to compare efficacy for the drug among these disorders though the preliminary data suggest that eculizumab may be a promising treatment for all of these conditions.
Treatment with rituximab, a monoclonal antibody against CD20 surface antigen for B-cell apoptosis, has also demonstrated some clinical improvement in patients with diminishing or insufficient responsiveness to IVIG. However, reports of efficacy are inconsistent and based on a small number of patients. Established dosing recommendations call for 375 mg/m2 weekly for 2–4 weeks for B-cell depletion with single booster doses of 375 mg/m2 given for maintenance if patients develop worsening weakness. The largest studyCitation65 with 14 patients showed strength improved by 13% in patients taking rituximab as compared to 3% in controls over 1 year. Strength improved 23% over 2 years. However, the next largest studyCitation66 with six IVIG responsive patients showed no significant improvement in strength and patients could not have their IVIG dosage reduced while on adjuvant therapy.
Though cyclophosphamide carries a much greater risk of toxicity compared to rituximab, there is evidence of its efficacy for treating MMN even in patients with no response to IVIG.Citation9 High-dose but not low-dose IV cyclophosphamide has been shown to be effective in up to half of patients in small uncontrolled trials.Citation67,Citation68 Unfortunately, use of high-dose IV cyclophosphamide is limited due to its toxicity, which include bone marrow suppression, hemorrhagic cystitis, bladder cancer, risk of infection, teratogenic effects, and infertility. In the studies listed earlier, several patients involved experienced severe side effects.
Safety/tolerability of IVIG
IVIG carries risks of adverse side effects with initial dosing and maintenance therapy. There is always potential for anaphylaxis, though risks are increased for patients with anti-IgA antibodies and selective IgA deficiency.Citation69,Citation70 More commonly, patients can develop flu-like symptomsCitation9 and should be offered symptomatic therapy.
One of the most common and serious adverse side effects is thromboembolic events including deep venous thrombosis, stroke, pulmonary embolism, and arterial ischemia leading to myocardial infarction,Citation71 with average incidence reported anywhere from 3% to 13%.Citation72 Thrombotic events are more likely to occur in patients receiving higher doses of IVIG.Citation73 Males and patients over 60 years of age are at higher risk for IVIG-induced thrombosis, as are those with diabetes, hyperlipidemia, coronary and peripheral vascular disease, renal insufficiency, hypertension, immobility, and coronary disease.Citation70 Patients predisposed to form blood clots due to atrial fibrillation, pregnancy, and prolonged immobility as well as those with a family history of thromboembolic disease are also at increased risk. A retrospective review demonstrated that arterial ischemic events such as stroke and myocardial infarction are more likely to occur within 12 hours following infusion, with over half occurring within this time frame, whereas venous thromboses are far more likely to occur later, with three-quarters occurring over 24 hours after infusion. No correlation between total number of infusions and arterial or venous thrombotic events was seen.Citation74 Assessment of patient risk factors and careful monitoring for thrombosis through serial physical examinations and, if indicated, Doppler ultrasound could be helpful in preventing adverse events.Citation70
Mechanisms underlying IVIG-associated thrombosis have yet to be elucidated. Theories proposed include a hypercoaguable state due to increased blood viscosityCitation75 and the passive transfer of anticardiolipin antibodiesCitation76 or high-molecular-weight proteinsCitation77 via IVIG. Passive transfer of factor XIa and other clotting factors could also occur due to insufficient anticoagulation of donated blood/plasma due to neglecting safety protocols or altering established manufacturing processes.Citation70
Other less common serious side effects include renal tubular necrosis, hemolytic anemia, and aseptic meningitis. Patients with pre-existing renal dysfunction are at greater risk of developing renal tubular necrosis on IVIG and must have renal function monitored regularly and receive proper hydration prior to infusion.Citation9 Hemolytic anemia typically occurs only with high-dose IVIG (over 100 g of IVIG in 2–4 days) with a 5.8% incidence rate.Citation78 Low-dose IgG replacement therapy rarely causes hemolysis with only a few cases reported.Citation79,Citation80 Females with blood group type A, B, or AB are at greater risk for IVIG-induced hemolysis. Basic lab work including complete blood counts before starting therapy with IVIG coupled with close follow-up is helpful for early detection of hemolytic anemia.Citation9 Aseptic meningitis is a rare complication and is usually self-limited, though systemic steroids sometimes are needed for severe cases. Infusing at a slower rate coupled with proper hydration prior to infusion and antihistamines when indicated can be helpful for prevention.Citation9,Citation81
Most patients do not develop severe complications on IVIG. Subcutaneous formulations of immunoglobulins may further reduce the risk of serious systemic complications as compared to intravenous formulations. This could be due to limitations of patients tolerating large volume doses of immunoglobulin subcutaneously. A 2009 studyCitation54 of SCIG as compared to IVIG showed good results for SCIG without severe adverse events and reported only minimal and transient side effects, primarily local irritation at injection sites. Review of long-term treatment with SCIG also showed good outcomes without severe complications.Citation82
Patient-focused perspectives
MMN profoundly affects patients’ ability to function and definitive diagnosis can be difficult. Due to focal distribution, patients frequently are misdiagnosed with entrapment mononeuropathies despite lack of sensory symptoms and may undergo unnecessary surgeries before being correctly diagnosed.Citation5 Progressive weakness coupled with fasciculations and cramping can lead to misdiagnosis of ALS. Many patients labor under an ALS diagnosis for years prior to receiving the correct determination of MMN.Citation83 Median time from symptom onset to diagnosis is approximately 4 years.Citation5 Though IVIG is highly effective in reversing symptoms, the longer the treatment is delayed, typically, the less effective IVIG will be.Citation1 Univariate analysis from a 2010 studyCitation1 suggested a correlation with greater severity of disability and greater length of time untreated; thus, earlier diagnosis is crucial and could prevent patients from acquiring debilitating impairments. Nearly one-fifth of patients in this study were found to have severe disability on the Overall Disability Sum Score due to profound upper extremity weakness. Another studyCitation84 from 2010 evaluated how patients’ weakness impacted their daily functioning. This cross-sectional study examined 47 patients with MMN and found that, in addition to muscle weakness, overall functionality was affected by multiple factors including fatigue, impairments in dexterity, and impaired ambulation. Fatigue, a factor often overlooked, had a significant impact. Some electrodiagnostic studies have demonstrated activity-dependent conduction blockCitation12 which may relate to fatigue.Citation9 Though fatigue was an ever-present issue, use of gait and hand aids significantly improved scores for autonomy and 94% of these patients were employed.
MMN clearly impacts strength and functionality, but there continues to be no consensus on how to evaluate degree of disability and quality of life for patients. Progress has been made however and in 2013 at the 196th ENMC International workshop, a panel of experts recommended that the primary outcome of patient activity and participation levels be measured by the disease-specific Rasch-built Overall Disability Scale (R-ODS).Citation85 These researchers anticipate creating a Rasch-transformed quality of life scale based on findings of future studies to help standardize assessments of quality of life, a parameter which is essential but difficult to quantify.Citation9,Citation85 Using these standardized measurements hopefully will provide a more reliable method with which to assess patients’ functioning, thereby making it easier to tailor interventions such as walking and hand aids, physical and occupational therapies, and immunoglobulin dosing protocols to better meet their needs.
Conclusion/place in therapy
MMN is a rare but treatable neuropathy. Early diagnosis and treatment is a crucial factor for preserving strength and functionality long term. It is critical to recognize MMN’s unique symptoms and to differentiate MMN from mimicking conditions such as compression neuropathies and ALS for which immunomodulatory therapies are not effective. The only proven treatments available at present are IVIG and SCIG. Further studies are needed to determine optimum steady-state IgG serum concentrations for therapy as well as the best individual doses, dosing intervals, and routes of administration to achieve these concentrations.
Though effective, IVIG and SCIG are costly to produce and administer. Unfortunately, patients still typically experience progressive motor decline due to diminished responsiveness to treatment over time. Patients often require higher and more frequent doses, thereby increasing the risk of adverse and potentially fatal outcomes including myocardial infarction and stroke. Research to elucidate the underlying pathophysiology behind MMN is ongoing. Hopefully, a more complete understanding of disease mechanisms will lead to development of more targeted and economical treatments with lasting effectiveness. For now, IVIG and SCIG remain the first-line agents for treatment of MMN.
Disclosure
The authors report no conflicts of interest in this work.
References
- CatsEAvan der PolWLPiepersSCorrelates of outcome and response to IVIg in 88 patients with multifocal motor neuropathyNeurology201075981882520805527
- MoroniIBugianiMCianoCBonoRPareysonDChildhood-onset multifocal motor neuropathy with conduction blocksNeurology200666692292416567714
- NguyenTPChaudhryVMultifocal motor neuropathyNeurol India201159570070622019654
- VlamLvan der PolWLCatsEAMultifocal motor neuropathy: diagnosis, pathogenesis and treatment strategies. Nat Rev NeurolNovember 222011814858
- TaylorBVWrightRAHarperCMDyckPJNatural history of 46 patients with multifocal motor neuropathy with conduction blockMuscle Nerve200023690090810842266
- Joint Task Force of the EFNS and the PNSEuropean federation of neurological societies/peripheral nerve society guideline on management of multifocal motor neuropathy. Report of a joint task force of the european federation of neurological societies and the peripheral nerve society – first revisionJ Peripher Nerv Syst201015429530121199100
- PringleCEBeldenJVeitchJEBrownWFMultifocal motor neuropathy presenting as ophthalmoplegiaMuscle Nerve19972033473519052814
- StraverDCvan AsseldonkJTNotermansNCWokkeJHvan den BergLHFranssenHCold paresis in multifocal motor neuropathyJ Neurol2011258221221720803025
- LawsonVHArnoldWDMultifocal motor neuropathy: a review of pathogenesis, diagnosis, and treatmentNeuropsychiatr Dis Treat20141056757624741315
- GalassiGGirolamiFAriattiAMonelliMSolaPFulminant multifocal motor neuropathy: a report of two casesInt J Neurosci2012122739540022332970
- KaramCDyckPJEngelstadJKMacgowanDJClinical reasoning: a 34-year-old man with recurrent limb weaknessNeurology20117712e68e7221931104
- KajiRBostockHKoharaNMuraseNKimuraJShibasakiHActivity-dependent conduction block in multifocal motor neuropathyBrain2000123Pt 81602161110908190
- StraverDCvan den BergLHvan den Berg-VosRMFranssenHActivity-dependent conduction block in multifocal motor neuropathyMuscle Nerve2011431313621171095
- DelmontEAzulayJPGiorgiRMultifocal motor neuropathy with and without conduction block: a single entity?Neurology200667459259616924010
- SleeMSelvanADonaghyMMultifocal motor neuropathy: the diagnostic spectrum and response to treatmentNeurology200769171680168717954783
- LegerJMGuimaraes-CostaRIancu FerfogliaRThe pathogenesis of multifocal motor neuropathy and an update on current management optionsTher Adv Neurol Disord20158310912225941538
- TaylorBVDyckPJEngelstadJGruenerGGrantIDyckPJMultifocal motor neuropathy: pathologic alterations at the site of conduction blockJ Neuropathol Exp Neurol200463212913714989599
- AuerRNBellRBLeeMANeuropathy with onion bulb formations and pure motor manifestationsCan J Neurol Sci19891621941972543490
- TakigawaTYasudaHTeradaMIncreases in K+ conductance and Ca2+ influx under high glucose with suppressed Na+/K+-pump activity in rat myelinated nerve fibersNeuroreport200011112547255110943720
- O’HanlonGMHumphreysPDGoldmanRSCalpain inhibitors protect against axonal degeneration in a model of anti-ganglioside antibody-mediated motor nerve terminal injuryBrain2003126Pt 112497250912937083
- VlamLCatsEAHarschnitzOComplement activity is associated with disease severity in multifocal motor neuropathyNeurol Neuroimmunol Neuroinflamm201524e11926161430
- JacobSRajaballyYACurrent proposed mechanisms of action of intravenous immunoglobulins in inflammatory neuropathiesCurr Neuropharmacol20097433734220514213
- HalsteadSKZitmanFMHumphreysPDEculizumab prevents anti-ganglioside antibody-mediated neuropathy in a murine modelBrain2008131Pt 51197120818184663
- YukiNWatanabeHNakajimaTSpathPJIVIG blocks complement deposition mediated by anti-GM1 antibodies in multifocal motor neuropathyJ Neurol Neurosurg Psychiatry2011821879120667861
- MalikUOleksowiczLLatovNCardoLJIntravenous gamma-globulin inhibits binding of anti-GM1 to its target antigenAnn Neurol19963911361398572660
- KondoNKasaharaKKameyamaTIntravenous immunoglobulins suppress immunoglobulin productions by suppressing Ca(2+)-dependent signal transduction through fc gamma receptors in B lymphocytesScand J Immunol199440137428029641
- StohlWElliotJEIn vitro inhibition by intravenous immunoglobulin of human T cell-dependent B cell differentiation induced by staphylococcal superantigensClin Immunol Immunopathol19967921221338620618
- VassilevTYamamotoMAissaouiANormal human immunoglobulin suppresses experimental myasthenia gravis in SCID miceEur J Immunol19992982436244210458757
- Korporal-KuhnkeMHaasJSchwarzAJariusSWildemannBPlasmacytosis is a common immune signature in patients with MMN and CIDP and responds to treatment with IVIgJ Neuroimmunol2015278606825595253
- De GrootASCousensLMingozziFMartinWTregitope peptides: the active pharmaceutical ingredient of IVIG?Clin Dev Immunol2013201349313824454476
- TjonASTha-InTMetselaarHJPatients treated with high-dose intravenous immunoglobulin show selective activation of regulatory T cellsClin Exp Immunol2013173225926723607448
- CrowARSongSSempleJWFreedmanJLazarusAHA role for IL-1 receptor antagonist or other cytokines in the acute therapeutic effects of IVIg?Blood2007109115515816954498
- CreangeAGregsonNAHughesRAIntravenous immunoglobulin modulates lymphocyte CD54 and monocyte FcgammaRII expression in patients with chronic inflammatory neuropathiesJ Neuroimmunol20031351–2919512576228
- VassilevTLKazatchkineMDDuong Van HuyenJPInhibition of cell adhesion by antibodies to arg-gly-asp (RGD) in normal immunoglobulin for therapeutic use (intravenous immunoglobulin, IVIg)Blood199993113624363110339467
- KerrJQuintiIEiblMIs dosing of therapeutic immunoglobulins optimal? A review of a three-decade long debate in EuropeFront Immunol2014562925566244
- KuitwaardKde GelderJTio-GillenAPPharmacokinetics of intravenous immunoglobulin and outcome in Guillain-Barre syndromeAnn Neurol200966559760319938102
- VlamLCatsEAWillemseEPharmacokinetics of intravenous immunoglobulin in multifocal motor neuropathyJ Neurol Neurosurg Psychiatry201485101145114824336791
- YuZLennonVAMechanism of intravenous immune globulin therapy in antibody-mediated autoimmune diseasesN Engl J Med199934032272289895405
- LegerJMVialaKMaisonobeTBouchePMultifocal motor neuropathy: a retrospective study of the response to high-dose intravenous immunoglobulin (IVIg) and current perspectives for diagnosis and treatmentBull Acad Natl Med2007191713951407 discussion 1407–918447061
- DonofrioPDBergerABrannaganTH3rdConsensus statement: the use of intravenous immunoglobulin in the treatment of neuromuscular conditions report of the AANEM ad hoc committeeMuscle Nerve200940589090019768755
- LegerJMChassandeBMussetLMeiningerVBouchePBaumannNIntravenous immunoglobulin therapy in multifocal motor neuropathy: a double-blind, placebo-controlled studyBrain2001124Pt 114515311133794
- FedericoPZochodneDWHahnAFBrownWFFeasbyTEMultifocal motor neuropathy improved by IVIg: randomized, double-blind, placebo-controlled studyNeurology20005591256126211087764
- van SchaikINvan den BergLHde HaanRVermeulenMIntravenous immunoglobulin for multifocal motor neuropathyCochrane Database Syst Rev200522CD004429
- LegerJMVialaKCancalonFIntravenous immunoglobulin as short- and long-term therapy of multifocal motor neuropathy: a retrospective study of response to IVIg and of its predictive criteria in 40 patientsJ Neurol Neurosurg Psychiatry2008791939618079302
- HahnAFBeydounSRLawsonVA controlled trial of intravenous immunoglobulin in multifocal motor neuropathyJ Peripher Nerv Syst201318432133024725024
- Van den Berg-VosRMFranssenHWokkeJHVan den BergLHMultifocal motor neuropathy: long-term clinical and electrophysiological assessment of intravenous immunoglobulin maintenance treatmentBrain2002125Pt 81875188612135977
- TerenghiFCappellariABersanoACarpoMBarbieriSNobile-OrazioEHow long is IVIg effective in multifocal motor neu-ropathy?Neurology200462466666814981195
- Van AsseldonkJTVan den BergLHKalmijnSAxon loss is an important determinant of weakness in multifocal motor neuropathyJ Neurol Neurosurg Psychiatry200677674374716705197
- BaumannAHessCWSturzeneggerMIVIg dose increase in multi-focal motor neuropathy: a prospective six month follow-upJ Neurol2009256460861419367358
- VucicSBlackKRChongPSCrosDMultifocal motor neuropathy: decrease in conduction blocks and reinnervation with long-term IVIgNeurology20046371264126915477549
- VlamLvan den BergLHCatsEAPiepersSvan der PolWLImmune pathogenesis and treatment of multifocal motor neuropathyJ Clin Immunol201333Suppl 1S38S4222941513
- NowacekDGTeenerJWMultifocal motor neuropathySemin Neurol201232550050523677657
- Nobile-OrazioEGalliaFMultifocal motor neuropathy: current therapies and novel strategiesDrugs201373539740623516024
- HarboTAndersenHHessAHansenKSindrupSHJakobsenJSubcutaneous versus intravenous immunoglobulin in multifocal motor neuropathy: a randomized, single-blinded cross-over trialEur J Neurol200916563163819236457
- EftimovFVermeulenMde HaanRJvan den BergLHvan SchaikINSubcutaneous immunoglobulin therapy for multifocal motor neuropathyJ Peripher Nerv Syst20091429310019691531
- DimbergELTreatment of multifocal motor neuropathy with immunoglobulin: does route of administration matter?Eur J Neurol200916555355419405192
- DacciPRivaNScarlatoMSubcutaneous immunoglobulin therapy for the treatment of multifocal motor neuropathy: a case reportNeurol Sci201031682983120574753
- MisbahSABaumannAFazioRA smooth transition protocol for patients with multifocal motor neuropathy going from intravenous to subcutaneous immunoglobulin therapy: an open-label proof-of-concept studyJ Peripher Nerv Syst2011162929721692906
- PiepersSVan den Berg-VosRVan der PolWLFranssenHWokkeJVan den BergLMycophenolate mofetil as adjunctive therapy for MMN patients: a randomized, controlled trialBrain2007130Pt 82004201017626040
- UmapathiTHughesRANobile-OrazioELegerJMImmunosuppressant and immunomodulatory treatments for multifocal motor neuropathyCochrane Database Syst Rev20153CD003217
- PittockSJLennonVAMcKeonAEculizumab in AQP4-IgG-positive relapsing neuromyelitis optica spectrum disorders: an open-label pilot studyLancet Neurol201312655456223623397
- ShermanEHanMHAcute and chronic management of neuromyelitis optica spectrum disorderCurr Treat Options Neurol2015171148,0150378-x26433388
- HowardJFJrBarohnRJCutterGRA randomized, double-blind, placebo-controlled phase II study of eculizumab in patients with refractory generalized myasthenia gravisMuscle Nerve2013481768423512355
- FitzpatrickAMMannCABarrySBrennanKOverellJRWillisonHJAn open label clinical trial of complement inhibition in multifocal motor neuropathyJ Peripher Nerv Syst2011162849121692905
- PestronkAFlorenceJMillerTChoksiRAl-LoziMTLevineTDTreatment of IgM antibody associated polyneuropathies using rituximabJ Neurol Neurosurg Psychiatry200374448548912640069
- ChaudhryVCornblathDRAn open-label trial of rituximab (rituxan(R)) in multifocal motor neuropathyJ Peripher Nerv Syst201015319620121040141
- FeldmanELBrombergMBAlbersJWPestronkAImmunosuppressive treatment in multifocal motor neuropathyAnn Neurol19913033974011952828
- PestronkACornblathDRIlyasAAA treatable multifocal motor neuropathy with antibodies to GM1 gangliosideAnn Neurol198824173782843079
- RachidRBonillaFAThe role of anti-IgA antibodies in causing adverse reactions to gamma globulin infusion in immunodeficient patients: a comprehensive review of the literatureJ Allergy Clin Immunol2012129362863421835445
- SpathPJGranataGLa MarraFKuijpersTWQuintiIOn the dark side of therapies with immunoglobulin concentrates: the adverse eventsFront Immunol201561125699039
- OrbachHKatzUShererYShoenfeldYIntravenous immunoglobulin: adverse effects and safe administrationClin Rev Allergy Immunol200529317318416391392
- RajaballyYAKearneyDAThromboembolic complications of intravenous immunoglobulin therapy in patients with neuropathy: a two-year studyJ Neurol Sci20113081–212412721679973
- RamirezERomero-GarridoJALopez-GranadosESymptomatic thromboembolic events in patients treated with intravenous-immunoglobulins: results from a retrospective cohort studyThromb Res201413361045105124731561
- MarieIMaureyGHerveFHellotMFLevesqueHIntravenous immunoglobulin-associated arterial and venous thrombosis: report of a series and review of the literatureBr J Dermatol2006155471472116965420
- DalakasMCHigh-dose intravenous immunoglobulin and serum viscosity: risk of precipitating thromboembolic eventsNeurology19944422232268309562
- SakemBMatozanKNydeggerUEWeigelGGriesmacherARischLAnti-red blood cell antibodies, free light chains, and antiphospholipid antibodies in intravenous immunoglobulin preparationsIsr Med Assoc J2013151061762124266088
- Grosse-WildeHBlasczykRWesthoffUSoluble HLA class I and class II concentrations in commercial immunoglobulin preparationsTissue Antigens199239274771574801
- KahwajiJBarkerEPepkowitzSAcute hemolysis after high-dose intravenous immunoglobulin therapy in highly HLA sensitized patientsClin J Am Soc Nephrol20094121993199719833910
- QuintiIPulvirentiFMilitoCHemolysis in patients with antibody deficiencies on immunoglobulin replacement treatmentTransfusion20155551067107425532440
- WilsonJRBhoopalamHFisherMHemolytic anemia associated with intravenous immunoglobulinMuscle Nerve1997209114211459270670
- JainRSKumarSAggarwalRKooknaJCAcute aseptic meningitis due to intravenous immunoglobulin therapy in guillainbarre syndromeOxf Med Case Reports20142014713213425988056
- HarboTAndersenHJakobsenJLong-term therapy with high doses of subcutaneous immunoglobulin in multifocal motor neuropathyNeurology201075151377138020938030
- DimachkieMMBarohnRJKatzJMultifocal motor neuropathy, multifocal acquired demyelinating sensory and motor neuropathy, and other chronic acquired demyelinating polyneuropathy variantsNeurol Clin201331253355523642723
- ErdmannPGLindemanECatsEAvan den BergLHFunctioning of patients with multifocal motor neuropathyJ Peripher Nerv Syst201015211311920626774
- VanhoutteEKFaberCGMerkiesISPeriNomS Study Group196th ENMC international workshop: outcome measures in inflammatory peripheral neuropathies, 8–10 February 2013, Naarden, the NetherlandsNeuromuscul Disord2013231192493323835324
