Abstract
Optic nerve sheath meningiomas are rare benign neoplasms of the meninges surrounding the optic nerve. They are a significant cause of morbidity. While the mortality rate is practically zero, these tumors can blind or disfigure patients. Given that the clinical course can be variable, and treatment has the capacity to cause morbidity itself, the management of these patients can be difficult. We review the literature to discuss the prevalence of optic nerve sheath meningiomas, the association with neurofibromatosis type 2, natural history, and management options and strategies.
Video abstract
Point your SmartPhone at the code above. If you have a QR code reader the video abstract will appear. Or use:
Introduction
Optic nerve sheath meningiomas (ONSMs) are usually benign neoplasms of the meninges surrounding the optic nerve. Patients classically present in the fourth decade with a triad of vision loss, optic atrophy, and optociliary shunt vessels on fundoscopy.Citation1 While optic nerve tumors are rare, ONSMs account for approximately one-third of all intrinsic tumors of the optic nerve.Citation2 Importantly, they can be associated with neurofibromatosis type 2. While there is no mortality and little nonvisual morbidity associated with them, ONSMs often lead to vision loss in the affected eye. Treatments carry a risk of vision loss, so management of these patients becomes the careful task of balancing risk of progression and vision loss with the risk of treatment.
This review will discuss the prevalence of ONSMs and the association with neu-rofibromatosis type 2, natural history, and management options.
Search strategies
We searched MEDLINE for articles with subject headings *Optic Nerve Neoplasms/ and *Meningioma/. Review articles previously publishedCitation3–Citation13 were examined and relevant references were obtained and included in this review. Only English language documents were eligible for inclusion in this review.
Epidemiology
Orbital tumors are rare, and ONSMs represent a small subset of these tumors. DuttonCitation3 conducted a comprehensive review, showing that ONSMs represent approximately 2% of all orbital tumors. ONSMs show a female predominance (61%–39% for males), and the mean age of patients at diagnosis is approximately 40 years. Males tend to present slightly earlier (36 years vs 42 years for females). Bilateral tumors occur in 5% of patients. Of meningiomas that involve the orbit, only 10% are of primary orbital origin, while the remainders are of intracranial origin. Tumors that arise from other intracranial or intraorbital locations and secondarily affect the optic nerve sheath are termed “secondary ONSMs”. Optic canal ONSMs, important due to the ease of compression of the optic nerve in this tight anatomical space, represent 8% of ONSMs.
Pediatric ONSMs are even more rare, comprising approximately 2%–4% of ONSMsCitation3,Citation14 and having an overall prevalence of between 1:95,000 and 1:525,000.Citation15 Almost a third of pediatric patients with ONSMs are diagnosed with neurofibromatosis type 2 (NF2), and almost a third of children with NF2 are subsequently diagnosed with an ONSM.Citation16
NF2
NF2 is strongly associated with the development of neural tumors, including ONSMs. It results from mutations in the NF2 tumor suppressor gene located on chromosome 22q12.Citation17 It is inherited in an autosomal-dominant fashion, although there is a high spontaneous mutation rate.Citation18 In addition, there is a high frequency of mosaicism in patients with sporadic mutations, which makes genetic diagnosis more difficult.Citation17 The incidence of NF2 is approximately 1:25,000 to 1:40,000.Citation17–Citation19 Diagnosis can be challenging, with many children receiving a delayed diagnosis.Citation18 Patients present with a variety of neural tumors, most commonly vestibular schwannomas and meningiomas, as well as cutaneous lesions and ophthalmic manifestations including cataract, retinal abnormalities (including retinal hamartomas), strabismus, and amblyopia.Citation17,Citation18
ONSMs are much more common in patients with NF2 than the general population. One study of patients with NF2 features described an incidence of 6.8% of ONSMs in patients with NF2.Citation20 The authors make clear that this incidence is subjected to selection bias. Evans reports the incidence of ONSM in NF2 patients to be between 4.1% and 4.8%.Citation19 Children who have meningiomas (not just ONSMs) are diagnosed with NF2 in 28% of cases.Citation16,Citation18,Citation20 There may be a difference in the natural history of ONSMs in patients with and without NF2; however, the rarity of these disorders makes accurate assessment of this difficult (discussed below).
Clinical features
ONSMs classically present with a triad of visual loss, optic atrophy, and optociliary shunt vessels;Citation1 however, this triad presenting in its entirety is rare.Citation13 Vision loss is extremely common (97%) at presentation in the affected eye, but the degree is variable, with 45% of patients having acuity of 20/40 or better, and 24% having acuity of counting fingers or worse.Citation3 Wright described a presenting acuity of “no perception of light” (NLP) in 24% of patients.Citation21 Visual field defects are extremely common (83%) but again variable, most commonly manifesting as peripheral constriction (35%), central, centrocecal, and paracentral scotomas (together 29%), an enlarged blind spot (13%), and altitudinal defects (16%).Citation3 Proptosis (2–5 mm) is common (59%), as is strabismus (47%), usually in attempted upgaze.Citation3 An orbital compartment syndrome has been reported to develop as a result of an ONSM.Citation22 The true incidence of pain and headache is difficult to quantify. The optic disc is almost always abnormal at presentation (98%) but may present as disc swelling and optic atrophy as well as the presence of optociliary shunt vessels, which are themselves less common (30%).
Optociliary shunt vessels are dilated normal anasto-moses between the retinal venous system at the optic disc, and the choroidal venous circulation. It is hypothesized that they occur due to compression of the central retinal vein by tumor as it passes through the optic nerve.Citation1 Direct evidence of altered central retinal venous flow has been obtained with Doppler imaging.Citation23 Further supporting this hypothesis, shrinkage of optociliary shunt vessels has been observed when a tumor has been surgically removedCitation24 and after radiotherapy treatment.Citation25 Such vessels can be imaged in detail using indocyanine-green angiography.Citation26 The shrinkage of optociliary shunt vessels suggests a reduction in central retinal venous pressure, which may be part of the mechanism behind vision improvement even when no macroscopic change in tumor volume is apparent.Citation27
ONSMs can also coexist with other orbital disease. A case has been reported of simultaneous ONSM and an optic nerve glioma in the same optic nerve in a patient with neurofibro-matosis type 1.Citation28 A case of concomitant thyroid orbitopathy and bilateral ONSM has been reported.Citation29
Diagnosis
The diagnosis of ONSM can be challenging due to their slow-growing nature and insidious vision loss. Optic nerve appearances can be variable, ranging from atrophy to normal to swollen in appearance, and the patients can be easily misdiagnosed.Citation30 The clinical presentation of ONSMs can also be variable – in one case the presence of bilateral ONSMs was a surprise to clinicians treating a patient for presumed bilateral optic neuritis who had pain on eye movement.Citation31 Careful follow-up and a high index of suspicion for retrobulbar pathology are, therefore, required to ensure that neuroimaging is appropriately ordered to enable the diagnosis to be made.
Imaging
Magnetic resonance imaging (MRI)
Diagnosis of ONSMs is best made with orbital and cranial imaging studies. The standard investigation for investigation and diagnosis of ONSM is MRI. ONSMs are best demonstrated on T1-weighted, gadolinium-enhanced, fat-suppressed sequences.Citation32 Some examples are included in –. Certain subtypes of ONSMs, such as those that arise within the optic canal, are more challenging to diagnose, and for these, high-resolution MRI is essential.Citation33 The superior soft tissue contrast of MRI, compared to computed tomography (CT), enables more effective differentiation of meningiomas from other optic nerve enlargements such as optic nerve gliomas, inflammatory conditions such as sarcoidosis, or other orbital lesions that do not arise from the optic nerve. Such differentiation is not perfect, and lesions can prove on biopsy to be of different histologic composition to that expected from imaging.Citation34
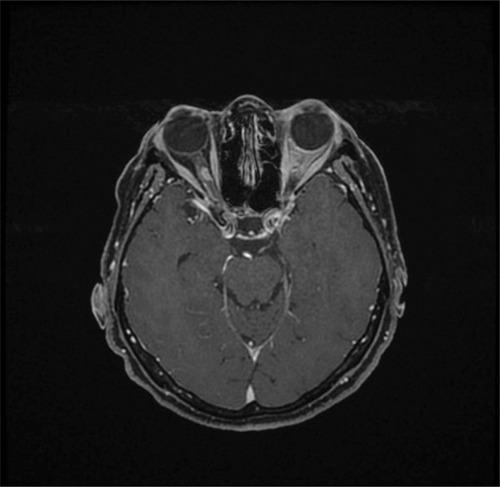
Figure 1 A typical appearance of a tubular optic nerve sheath meningioma on magnetic resonance imaging (gadolinium contrast-enhanced T1-weighted sequence with fat suppression). The tumor surrounds the nerve and shows the typical “tram track” appearance on the sagittal image with the hypointense optic nerve tissue lying between the enhancing tumor.
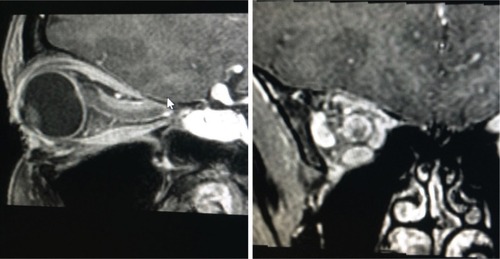
Figure 2 A large exophytic globular optic nerve sheath meningioma demonstrated on gadolinium contrast-enhanced T1-weighted fat suppressed magnetic resonance imaging.
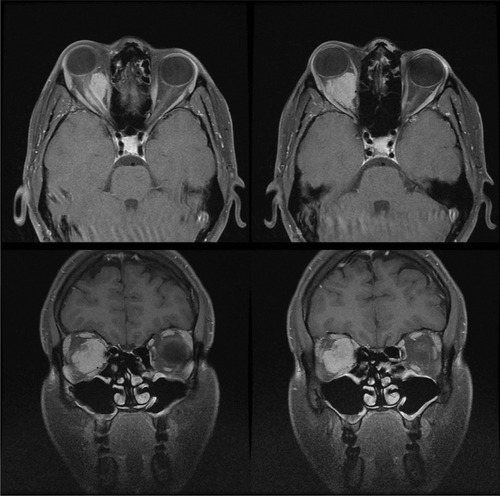
Figure 3 Gadolinium contrast-enhanced T1-weighted magnetic resonance imaging with fat suppression demonstrating a small right optic nerve sheath meningioma postradiotherapy that has remained clinically and radiologically stable. Incomplete fat suppression artifact is present in the left orbit.
Typical appearances of ONSMs on imagingCitation32 are tubular expansion of the meninges surrounding the optic nerve (most common, 62%), globular (23%), fusiform (11%), and focal enlargement of the optic nerve (4%). “Tram tracking”, with the meningioma hyperdense (or hyperintense) on either side, relative to the optic nerve in the center is a classic sign and is demonstrated in 24% of tumors. Most tumors have smooth margins (80%). Some tumors, however, demonstrate alternative growth patterns to these.Citation35 The presence of peri-optic cysts associated with ONSMs is also well demonstrated on MRI.Citation36
MRI is particularly superior to other modalities in the assessment of lesions near the orbital apex, as the soft tissue effacement, as a marker of the degree of compressive optic neuropathy, is well demonstrated. MRI is also superior to other modalities at demonstrating soft tissue involvement in the remainder of the orbit and intracranially.Citation32
CT
Contrast-enhanced CT scans can also demonstrate the classic tram track sign,Citation30,Citation37 in contrast to an intrinsic optic nerve tumor such as an optic nerve glioma, which will demonstrate relatively uniform enhancement of the optic nerve itself. The calcification of ONSMs can give a tram track appearance on CT in the absence of the administration of intravenous contrast. While soft tissue contrast of CT scanning is inferior to MRI, CT remains superior for the assessment of calcification and of bony anatomy. Because bony anatomy is well demonstrated, CT is better to assess overall lesion configuration and relations.Citation32
Ultrasound
Ultrasound can be used to demonstrate the tumor if it is located anteriorly,Citation38 and blood flow within it can also be demonstrated with Doppler scanning.Citation23 This imaging modality is noninvasive and does not require the prolonged immobilization of an MRI scan, and thus may be more appropriate for monitoring known lesions in children. Because posterior orbital tumors and intra-cranial ONSMs are not visible with ultrasound, this modality is not appropriate for primary screening and diagnosis.
Multifocal visual-evoked potential
A newer technique to monitor objective optic nerve function in patients is multifocal visual-evoked potential (mfVEP). In patients with known ONSMs, mfVEP can be used to monitor for functional compromise and progression of visual decline without the need for MRI.Citation39 This has particular applicability in a pediatric population, where MRI scanning can require general anesthetic,Citation40 with its associated risks.Citation41 Although this modality will not replace MRI, evidence of progressive optic neuropathy, even the absence of visible MRI changes, could result in earlier treatment for patients who will eventually require treatment.
Biopsy
Biopsy of an optic nerve neoplasm is indicated when there is uncertainty regarding the diagnosis and careful observation is not clinically reasonable. Reasons for this may include a malignant or aggressive disease course, progressive vision loss, or a clinically and radiologically atypical lesion. Biopsy may also be useful if there are medicolegal concerns.Citation42 It is worth considering that although the most common optic nerve tumors are gliomas and ONSMs, other neoplastic and inflammatory conditions can have a similar appearance on imaging, yet the management of these conditions is often entirely different from gliomas or ONSMs. Several techniques have been described for obtaining incisional biopsy specimens, including medial transconjunctival approachCitation42 conceptually similar to an optic nerve sheath fenestration, and fine-needle aspiration biopsy under CT guidance.Citation43 Biopsy carries similar risks to surgery (discussed below under “treatment options, surgery”; page 7) with a high risk of vision loss, and given modern neuroimaging is typically diagnostic, biopsy is no longer commonly indicated.
Natural history
The natural history of ONSM, concisely reviewed by Shapey,Citation13 is of slow, progressive visual loss in the affected eye. The clinical course and effect on vision are variable. Some patients demonstrate long-term clinical stability despite tumor growth, and some patients have rapid vision loss even without measurable increase in tumor size. Good acuity at presentation is an indicator that the clinical course will be more benign; however, 85% of patients will lose vision over time without treatment.Citation3 As a benign neoplasm, there is no significant systemic morbidity and no mortality associated with ONSMs.Citation3,Citation13 However, some ONSMs can exhibit aggressive behaviorCitation44 and cause profound orbital disfigurement. This is particularly true of tumors diagnosed in pediatric patients. Spontaneous improvement in vision is uncommon (18%) in one study,Citation45 but possible.
A direct comparison of the natural history of ONSMs in patients with and without NF2 has to our knowledge not been made. Observations can be made about the difference in clinical features between the eight patients with NF2 reported by BoschCitation20 and Dutton’s review of 380 patients both with and without NF2. Bosch reported bilateral ONSMs in two (25%) of the reported NF2 patients, vs the Dutton’s reported rate of 5% for the population (accepting selection bias). Presenting vision in NF2 patients was NLP in three of ten eyes (30%), and in five of ten eyes (50%) visual acuity was “count fingers” (CF) or poorer. This is worse than Dutton’s population rate of 24% for CF or worse. Bosch reported in NF2 patients seven eyes with impaired ductions, vs Dutton’s population rate of 47%. Proptosis was only mentioned specifically for one patient in Bosch’s review, which is below Dutton’s reported population rate of 59%; however, data were not specifically tabulated for proptosis and thus may be incomplete. It should be empha-sized that the low number of patients in this comparison makes drawing wider conclusions impossible. Meningiomas in other locations have been shown to be more aggressive in patients with NF2 compared to sporadic meningiomas.Citation17
Treatment options
Observation
Observation of ONSMs historically has resulted in poor patient outcomes. DuttonCitation3 reported that 86% of patients demonstrated decline in vision, with the remaining 14% demonstrating stable vision. No patients in this review demonstrated spontaneous improvement. TurbinCitation46 reported on 13 patients in whom the most significant visual decline was in the observation group. Interestingly, patients with good vision at presentation seem to have better long-term stabilityCitation32 than those with poor vision, perhaps reflecting that small amounts of tumor growth can have severe effects if the nerve is already compromised, whereas a healthy nerve can tolerate a similar insult without vision loss.
Despite clear evidence ONSMs overwhelmingly lead to progressive vision loss, certain cases can demonstrate remarkable stability. One patient with bilateral ONSMs (but not NF2) was observed for 27 years, from age 48 to 75, without progression.Citation47 As previously mentioned, spontaneous improvement is possible but uncommon, with one study of 42 patients observed showing a spontaneous improvement rate of 7%.Citation45 It is this variability in presentation that introduces the difficulty with when and how to commence treatment for these lesions, if at all.
Observation can, therefore, be advocated when the vision in the affected eye is normal. Once demonstrated visual decline is observed, vision loss becomes expected with observation and the case for treatment becomes much stronger.
Radiotherapy
The difficulty with management of ONSMs that confronted clinicians approximately 20 years ago has been elegantly summarized previously:Citation48–Citation50 observation usually leads to visual deterioration, medical therapy is generally inadequate, and surgical therapy usually leads to vision loss, which, in a tumor that rarely causes local disfigurement, rarely causes bilateral vision loss, and never causes mortality, is precisely the outcome that treatment is aiming to prevent.
From a historical perspective, Turbin’s review of long-term outcomes of patients with ONSM in 2002Citation46 proved influential. Visual outcomes for 64 patients were reported comparing surgery, observation, radiotherapy, and combination surgery and radiotherapy. The visual outcomes in the radiotherapy-only group were superior to all others, with this group only showing no significant decline in visual acuity from diagnosis to last follow-up. Furthermore, the radiation group demonstrated a favorable complication rate of 33.3% (including radiation retinopathy, vascular occlusion, persistent iritis, or temporal lobe atrophy), compared to the complication rate of surgery of 66.7%. Similar results have been demonstrated in other series.Citation32,Citation51,Citation52 Thus, radiotherapy, with superior outcomes and a favorable side effect profile, has emerged as a better treatment option for ONSMs.
Various modalities of radiotherapy have been used to treat ONSMs.Citation53 Fractionated external beam radiotherapy delivers radiation to the target area over a number of sessions, whereas radiosurgery delivers the radiation in a single session. Three-dimensional conformal radiotherapy (3D-CRT) uses beam-forming technology and specialized software to accurately model the target tissue, and then administer radiation conforming to the target tissue volume, minimizing the amount of radiation delivered to nontarget tissues. Intensity-modulated radiotherapy (IMRT) is a specialized kind of 3D-CRT that further modifies the dose distribution within the target volume. Stereotactic methods use fixed markers, usually invasively fixed to the patient, for registration of treatment machines with images, providing highly accurate delivery of radiation where critical structures lie close to target tissues. Image-guided radiation therapy reacquires images at the time of therapy to ensure precise alignment of target and treated tissue volumes. This is mainly useful when there may be interval change in the target tissue between image acquisition and treatment delivery. This is useful with rapidly changing tumor volumes, or indeed with mobile organs such as the eye.
A summary of recent studies is provided in for 3D-CRT,Citation54–Citation61 stereotactic 3D-CRT,Citation32,Citation51,Citation55,Citation58,Citation62–Citation76 IMRT,Citation58,Citation77,Citation78 and stereotactic radiosurgery.Citation79–Citation81 There appears to be no major differences between the type of radiotherapy used to treat ONSM. Stability or improvement of visual function is obtained in more than 80% of patients.Citation10,Citation12 A total radiation dose of less than 54 Gy over 30 fractions is typically used, as this is the ceiling for optic nerve tolerance.Citation66,Citation82 There is a concern that the larger dose fractions used in radiosurgery, compared to fractionated radiotherapy, may exceed the dose limits of surrounding tissues leading to more visual complications. As such, radiosurgery is generally used in patients with very poor vision.
Table 1 Summary of radiotherapy treatment for ONSM
Toxicity
Choice of the optimal radiation dose is currently based on maximum safe levels for each structure involved (optic nerve, retina). A significant predictor of toxicity is the presence of other risk factors, such as age, smoking, diabetes, hypertension, and hypercholesterolemia.Citation83 Overall, radiation-associated complications following radiotherapy for ONSMs are mostly mild and self-limited,Citation66 and those that are not are uncommon.Citation46 Acute toxicities include local effects such as erythema, alopecia, or orbital pain, inflammation of the anterior segment of the eye (blepharitis, conjunctivitis, kera-titis, or iritis), orbital edema, and systemic effects including nausea and vomiting, headache, and fatigue; their incidence is summarized in . Of note, one case of obstructive hydrocephalus was reported, presumably due to tumor swelling.Citation66 Chronic toxicities include dry eye from lacrimal gland irradiation, cataract formation, pituitary dysfunction, radiation retinopathy, and radiation optic neuropathy. These are rare at currently accepted dose ranges.
Table 2 Incidence of radiotherapy side effects
Abouaf et al observed that patients with decreased visual acuity at follow-up had higher mean eye dose levels (64 Gy vs 57.6 Gy).Citation59 As expected, complication rates increase with higher delivered doses.Citation83
Radiation retinopathy is more common when tumors are more anterior, and when patients have other predisposing microvascular risk factors as mentioned above. An eye dose of >50 Gy predisposes to retinal injury.Citation84 If identified, radiation retinopathy can be treated with intravitreal antivascular endothelial growth factor antibodies.Citation74,Citation85,Citation86
Radiation optic neuropathy is a late toxicity, developing between 3 months and 8 years after radiation therapy. Radiation doses of >50 Gy to the anterior visual pathway, or fractions >10 Gy, are generally required for radiation optic neuropathy to develop.Citation82 Treatment is with corticosteroids, hyperbaric oxygen, and possibly anticoagulation.Citation82 Unfortunately, the success of these measures is generally limited and highly time-critical.Citation82 Radiation optic neu-ropathy has also been reported 27 months after proton beam therapy,Citation87 which was successfully stabilized with intravenous methylprednisone.
Surgery
Surgery was the historical treatment of choice for ONSMs.Citation3 Surgical treatment is typically associated with loss of vision in the affected eye, as the tumor shares a blood supply from the pial vessels with the optic nerve. Therefore, complete removal of the tumor also strips the blood supply of the nerve, leading to vision loss.Citation3 The alternative, incision of the optic nerve sheath to provide decompression, was associated with seeding of the tumor into the orbital tissues.Citation21,Citation88 Sacrifice of the tumor and nerve en bloc has also been reported, usually in eyes with no vision,Citation21,Citation88 but also unfortunately in eyes with useful remaining vision.Citation89
Dutton’s reviewCitation3 showed progression to no perception of light with surgical treatment in 78% of cases, with less severe visual decline in a further 16%. WrightCitation88 reported that all 27 patients with surgery performed via lateral orbitotomy lost vision within 18 months of surgery. Wright later reviewed a series of 50 patients and noted that younger patients harbored more aggressive disease with more frequent intracranial involvement, and recommended sacrifice of the optic nerve in these cases.Citation21 With reference to 57 primary and secondary ONSMs, Cristante investigated visual outcomes following surgery and concluded that surgical treatment of primary ONSMs was unlikely to be of benefit.Citation90 DelfiniCitation91 reported that eleven of 13 patients with ONSM managed surgically suffered loss of vision; however, they were still recommended surgery when vision loss became apparent, as, in their opinion at the time, radiotherapy was ineffective. Saaed reported poor outcomes in patients with both partial resections and decom-pressions.Citation32 Roser reported that 50% of patients with rapid visual decline, treated surgically, had improved or stabilized vision, advocating surgical intervention in cases of intracranial extension, disfiguring orbitopathy, or rapid progression of visual decline.Citation92 Surgical intervention was also recommended in patients with no useful vision to enable en bloc resection of the tumor and optic nerve, with complete histologic clearance. Indeed, transection of the optic nerve and meninges from globe to pre-chiasm in unilaterally blind patients with ONSM has been proposed as a strategy for preventing contralateral spread.Citation93 Other summaries have been more conservative in their approach given the benefit of surgery is only clear when there is evidence of posterior tumor growth, which may threaten vision in the contralateral eye.Citation7,Citation94,Citation95
In light of these poor results, with the demonstration of the efficacy of radiotherapy and tumor control, and with its more favorable visual outcomes, surgical therapy is no longer recommended for patients with intraorbital ONSM who still have vision. Surgical therapy can be considered in patients who require tissue diagnosis, with posterior extension of the tumor, in patients with complete vision loss in whom en bloc resection is possible and desired, or in patients who have significant orbital disfigurement. These conditions preclude the majority of patients with ONSM. Surgical therapy is generally of limited use and careful consideration should be given to alternatives before proceeding.
Local chemotherapy
In some limited cases, surgery combined with local chemotherapeutic agents can be effective. A cystic ONSM, present in a patient with good vision in the affected eye, refractory to initial cyst excision and subsequent radiotherapy, was successfully treated with further surgical drainage of the cyst and application of mitomycin C 0.04% for 5 minutes directly to the cyst opening. No complications were observed either at the time of surgery or on follow-up over 19 months, and the patient’s vision stabilized in the affected eye.Citation96
Systemic chemotherapy
Prior to the routine application of radiotherapy for ONSMs, there were no intermediate treatments between observation and surgery. Several reports, therefore, were made of attempts to treat these tumors with systemic medical therapy. Nevertheless, despite occasional reported successes, systemic medical therapy has not been demonstrated to adequately treat ONSMs.Citation50
Hydroxyurea
A single case report of treatment with hydroxyurea has been published with encouraging results (visual acuity improvement from 20/400 to 20/25),Citation97 with the authors commenting that hydroxyurea has been used for treatment of untreatable meningiomas in other locations.
Hormonal therapy
Meningiomas express a wide range of hormone and growth factor receptors. Of particular note, progesterone receptors are frequently expressed by these tumors. One study reported 64% of tumors examined to be positive for progesterone receptors.Citation98 This may explain the higher incidence of these tumors in females, and their progression during pregnancy.Citation99 On this basis, therapy with hormonal antagonists has been trialled in cases of unresectable meningioma. One study of 14 patients used mifepristone, an antiprogesterone medication, with five patients showing tumor shrinkage and three further patients showing visual improvement.Citation100 Another case has been reported of unresectable bilateral ONSMs (not associated with NF2) that was successfully stabilized by treatment with a progesterone antagonist medication (gestrinone) after biopsy confirmation that this particular tumor expressed high levels of progesterone receptors.Citation101 Such therapy is usually inappropriate for adolescent patientsCitation14 given the possible effects on growth, and in patients contemplating pregnancy or who are currently pregnant.
Initiation of treatment
The critical issue in the management of ONSM is when to initiate treatment. On the basis of the evidence to date, we concur that once vision is demonstrated to decline, treatment should be commenced.
Patients with poor initial vision may also be appropriate for initial treatment, without waiting for demonstration of progression. Several observations support this hypothesis. Multiple studies support that poorer vision at the commencement of treatment results in a poorer outcome.Citation71,Citation77,Citation90 Other sensory tumors, for example acoustic neuromas, also have better outcomes when treated earlier.Citation62 The duration of symptoms has been negatively correlated with final visual acuity in a surgical treatment cohort of one study,Citation92 and in another radiotherapy study.Citation59 Patients who demonstrate stable or improved vision seem to be those who are treated earlier.Citation59,Citation60 Unfortunately, without a direct comparison of early vs delayed treatment it is difficult to draw accurate conclusions about causality from these observations.
Treatment of blind eyes
It remains controversial whether or not to treat eyes that have extremely poor vision, or no vision remaining. There are reports of patients with light perception vision recovering to CF vision following radiotherapy.Citation46,Citation73 In contrast, once patients have reached no light perception vision, visual recovery is very unlikely. Multiple reports of such patients having radiotherapy have shown no improvement.Citation57,Citation69,Citation73,Citation74,Citation79 In such patients, en bloc surgical resection has been reportedCitation92 and can be appropriate to prevent growth and recurrence, particularly if the tumor demonstrates posterior spread or if there is significant orbital disfigurement.
Responses to therapy
Location of ONSM, response to therapy, and outcomes
Historically ONSMs with intracranial extension were favored for surgical management to decrease the risk of extension to the optic chiasm and the risk of bilateral visual loss.Citation3 With the increased use of radiotherapy, this approach is now in need of reconsideration. The location of the meningioma has not been shown to alter its response to radiotherapy,Citation77 but meningiomas spread across multiple regions have lower rates of local control after radiotherapy.Citation102 Skull base meningiomas respond well to radiotherapy.Citation103 Opinion is divided on whether primary or secondary ONSMs show different visual acuity improvements with radiotherapy, with someCitation71 reporting improved outcomes for secondary ONSMs, and othersCitation66 reporting no difference.
Optical coherence tomography (OCT) as a prognostic indicator
A newer predictor of response to therapy is the retinal nerve fiber layer thickness, as measured by OCT. It has been demonstrated that low nerve fiber layer thickness on OCT predicts poor outcomes, along with duration of symptoms.Citation104 Poor nerve fiber layer thickness is a quantifiable marker of optic atrophy, and clearly once atrophy has occurred from compression from an ONSM the chance of recovery is low. These findings reflect similar findings in pituitary lesions causing optic chiasm compression.
Disc swelling
Another novel predictor of outcomes is the presence of optic disc edema at diagnosis. With increasing atrophy of the optic nerve head, disc swelling becomes less pronounced, while vision typically deteriorates. The presence of optic disc edema could, therefore, be expected to indicate a threatened, rather than atrophied, nerve. The presence of disc edema at the time of treatment has been strongly associated with a significantly improved visual response to primary radiotherapy.Citation55
Visual improvement despite stable radiographic appearance
Another peculiarity regarding treated ONSMs is demonstrated in patients who have improved visual function despite stable tumor size on MRI scanning. In most cases, tumors demon strate stabilization of size, rather than macroscopic shrinkage (). With the purported mechanism of optic neuropathy being compression, due to tumor volume, or ischemic, due to interposition of the tumor between the optic nerve and its pial blood supply, it would be reasonable to expect that patients with visual improvement should show reduction in tumor volume. Nevertheless, patients with tumors that demonstrate no change in size can show improvements in vision.Citation63,Citation73 Tumors in these cases demonstrated reduced metabolic activity when measured by 111In-octreotide scintigraphy. This may suggest that active tumor, consuming available blood supply, contributes to optic neuropathy, rather than simply direct compression effects. 111In-octreotide scintigraphy can therefore be useful in determining if failure to improve vision after therapy is due to failure of the tumor to respond, or due to treatment-related radiation-induced optic neuropathy.Citation73
Discordance between improvement in visual acuity and visual fields
It is also worth mentioning that visual acuity may not be the only factor that can improve with therapy. Visual fields also show change with treatment. More marked visual field improvement than visual acuity improvement with radiotherapy treatment has been demonstrated,Citation67 and other cases have shown decreased central acuity but with improved visual fields.Citation59 The improvement in visual fields with treatment may be due to the retinotopic organization of fibers within the optic nerve, with macular fibers in the nerve located centrally within the posterior nerve and less susceptible to extrinsic compression.Citation105 Irrespective of visual acuity outcomes, peripheral field improvement remains important from a functional perspective.
Conclusion
ONSMs continue to present a management challenge to clinicians. Until a therapy is developed that directly targets tumor cells and leaves normal surrounding structures undamaged, treatment-induced morbidity remains a real constraint on the feasibility of delivering early therapy to prevent vision loss. Given the variable natural history of these lesions, there will always be uncertainty around when and how to initiate treatment. Nevertheless, with earlier detection with improved imaging technology, more accurate radiotherapy delivery, and improved case selection for surgical management, it is hoped that outcomes will continue to improve for these patients.
Acknowledgement
The authors would like to acknowledge Dr Yael Barnett and Dr Geoffrey Parker for providing the images used in this review.
Disclosure
The authors report no conflicts of interest in this work.
References
- FrisènLRoytWFTengrothBMOptociliary veins, disc pallor and visual loss. A triad of signs indicating spheno-orbital meningiomaActa Ophthalmol19735122412494801582
- CantoreWANeural orbital tumorsCurr Opin Ophthalmol200011536737111148705
- DuttonJJOptic nerve sheath meningiomasSurv Ophthalmol19923731671831475751
- TurbinREPokornyKDiagnosis and treatment of orbital optic nerve sheath meningiomaCancer Control200411533434115377993
- CarrascoJRPenneRBOptic nerve sheath meningiomas and advanced treatment optionsCurr Opin Ophthalmol200415540641015625901
- MelianEJayWPrimary radiotherapy for optic nerve sheath menin-giomaSemin Ophthalmol2004193–413014015590556
- KimJWRizzoJFLessellSControversies in the management of optic nerve sheath meningiomasInt Ophthalmol Clin20054541523
- BermanDMillerNRNew concepts in the management of optic nerve sheath meningiomasAnn Acad Med Singapore200635316817416625265
- EddlemanCSLiuJKOptic nerve sheath meningioma: current diagnosis and treatmentNeurosurg Focus2007235E4
- JeremicBPitzSPrimary optic nerve sheath meningioma: stereotactic fractionated radiation therapy as an emerging treatment of choiceCancer2007110471472217582618
- GondiVTomeWAMehtaMPFractionated radiotherapy for intra-cranial meningiomasJ Neurooncol201099334935620809249
- BlochOSunMKaurGBaraniIJParsaATFractionated radiotherapy for optic nerve sheath meningiomasJ Clin Neurosci20121991210121522727747
- ShapeyJSabinHIDanesh-MeyerHVKayeAHDiagnosis and management of optic nerve sheath meningiomasJ Clin Neurosci20132081045105623809100
- GrobSRJakobiecFARashidAMacintoshPKellyHFayAPedi-atric optic nerve meningioma: diagnostic and therapeutic challengesOphthalmic Plast Reconstr Surg2016326e160e16425585303
- LevinLAJakobiecFAOptic nerve tumors of childhood: a decision-analytical approach to their diagnosisInt Ophthalmol Clin19923212232401537660
- Harold LeeHBGarrityJACameronJDStrianeseDBonavolontàGPatrinelyJRPrimary optic nerve sheath meningioma in childrenSurv Ophthalmol200853654355819026318
- AsthagiriARParryDMButmanJANeurofibromatosis type 2Lancet200937396791974198619476995
- Ardern-HolmesSFisherGNorthKNeurofibromatosis Type 2J Child Neurol201732192227655473
- EvansDGNeurofibromatosis type 2Handb Clin Neurol2015132879626564072
- BoschMMWichmannWWBoltshauserELandauKOptic nerve sheath meningiomas in patients with neurofibromatosis type 2Arch Ophthal2006124337938516534058
- WrightJEMcnabAAMcdonaldWIPrimary optic nerve sheath meningiomaBr J Ophthalmol198973129609662611192
- JaggiGPMironovAHuberARKillerHEOptic nerve compartment syndrome in a patient with optic nerve sheath meningiomaEur J Ophthalmol200717345445817534836
- JacqueminCBosleyTMullaneyPOrbital color Doppler imaging of optic nerve tumorsInt Ophthalmol1999231111511008893
- NicholsCPincusDWBhattiMTOptociliary shunt vesselsNeurology2005648144315851740
- MashayekhiAShieldsJAShieldsCLInvolution of retinochoroidal shunt vessel after radiotherapy for optic nerve sheath meningiomaEur J Ophthalmol2004141616415005588
- Muci-MendozaRArevaloJFRamellaMOptociliary veins in optic nerve sheath meningioma. Indocyanine green videoangiography findingsOphthalmology199910623113189951483
- de Alba CampomanesAGLarsonDAHortonJCImmediate shrinkage of optociliary shunt vessels after fractionated external beam radiation for meningioma of the optic nerve sheathAJNR Am J Neuroradiol20082971360136218403557
- Büyükkapu-BaySAkçaAKaradoğanMÇorapçioğluFAnikYConcomitant meningioma and glioma within the same optic nerve in neurofibromatosis type 1J Child Neurol201429338538823420652
- GargAPatelPLignelliABaronEKazimMCoincidental optic nerve meningioma and thyroid eye diseaseOphthal Plast Reconstr Surg2015314e94e95
- BaehringJMTram track signJ Neurooncol20078517517622487
- SawayaRASidaniCFarahNHourani-RiskRPresumed bilateral optic nerve sheath meningiomas presenting as optic neuritisJ Neur-oophthalmol20082815557
- SaeedPRootmanJNugentRAWhiteVAMackenzieIRKoornneefLOptic nerve sheath meningiomasOphthalmology2003110102019203014522782
- JacksonAPatankarTLaittRDIntracanalicular optic nerve meningioma: a serious diagnostic pitfallAJNR Am J Neuroradiol20032461167117012812948
- SavignacALeclerAOptic nerve meningioma mimicking cavernous hemangiomaWorld Neurosurg201811030130229191527
- SamarawickramaCFrydenbergEWellsMSteelTGhabrialRAn unusual radiological presentation of optic nerve sheath meningiomaSaudi J Ophthalmol201630213713927330394
- KoolenMLambrechtsIOptic nerve sheath meningioma with peri-optic cystJBR-BTR200992422819803105
- KanamallaUSThe optic nerve tram-track signRadiology2003227371871912773677
- GarciaJPSFingerPTKurliMHollidayRA3D ultrasound coronal C-scan imaging for optic nerve sheath meningiomaBr J Ophthalmol200589224424515665365
- JayanettiVKlistornerAIGrahamSLMonitoring of optic nerve function in neurofibromatosis 2 children with optic nerve sheath meningiomas using multifocal visual evoked potentialsJ Clin Neurosci20185026226729398196
- CahoonGDDavisonTEPrediction of compliance with MRI procedures among children of ages 3 years to 12 yearsPediatr Radiol201444101302130924859264
- Mir GhassemiANeiraVUfholzL-AA systematic review and meta-analysis of acute severe complications of pediatric anesthesiaPaediatr Anaesth201525111093110226392306
- GündüzKCatakEErdenEOptic nerve biopsy via a medial trans-conjunctival orbitotomy approach in the diagnosis of optic nerve and sheath tumorsOrbit201029419019320812834
- BanYKusabaKMiyaoAAkimotoKMakiKYoshidaTBiopsy of orbital meningioma by computed tomography-guided fine-needle aspirationJpn J Ophthalmol200549433633816075342
- AmoliFAMehrabaniPMTariASAggressive orbital optic nerve meningioma with benign microscopic features: a case reportOrbit200726427127418097966
- EganRALessellSA contribution to the natural history of optic nerve sheath meningiomasArch Ophthal2002120111505150812427064
- TurbinREThompsonCRKennerdellJSCockerhamKPKupersmithMJA long-term visual outcome comparison in patients with optic nerve sheath meningioma managed with observation, surgery, radiotherapy, or surgery and radiotherapyOphthalmology2002109589090011986093
- KothariNAKulkarniKMLamBLUntreated bilateral optic nerve sheath meningiomas observed for 27 yearsJ Neuroophthalmol2013331454723001155
- MillerNRThe evolving management of optic nerve sheath menin-giomasBr J Ophthalmol20028611119812386066
- MillerNRRadiation for optic nerve meningiomas: is this the answer?Ophthalmology2002109583383411986083
- MillerNRNew concepts in the diagnosis and management of optic nerve sheath meningiomaJ Neuroophthalmol200626320020816966942
- AdebergSWelzelTRiekenSDebusJCombsSEPrior surgical intervention and tumor size impact clinical outcome after precision radiotherapy for the treatment of optic nerve sheath meningiomas (ONSM)Radiat Oncol20116111721923947
- SmeeRISchneiderMWilliamsJROptic nerve sheath meningiomas — non-surgical treatmentClin Oncol2009211813
- StieberVWRadiation therapy for visual pathway tumorsJ Neurooph-thalmol2008283222230
- MoyerPDGolnikKCBrenemanJTreatment of optic nerve sheath meningioma with three-dimensional conformal radiationAm J Oph-thalmol20001295694696
- SaeedPBlankLSelvaDPrimary radiotherapy in progressive optic nerve sheath meningiomas: a long-term follow-up studyBr J Ophthalmol201094556456820447964
- NarayanSCornblathWTSandlerHMElnerVHaymanJAPreliminary visual outcomes after three-dimensional conformal radiation therapy for optic nerve sheath meningiomaInt J Radiat Oncol Biol Phys200356253754312738331
- MetellusPKapoorSKharkarSFractionated conformal radiotherapy for management of optic nerve sheath meningiomas: long-term outcomes of tumor control and visual function at a single institutionInt J Radiat Oncol Biol Phys201180118519220400241
- LesserRLKniselyJPSWangSLYuJBKupersmithMJLong-term response to fractionated radiotherapy of presumed optic nerve sheath meningiomaBr J Ophthalmol201094555956319965820
- AbouafLGirardNLefortTStandard-fractionated radiotherapy for optic nerve sheath meningioma: visual outcome is predicted by mean eye doseInt J Radiat Oncol Biol Phys20128231268127721640493
- AdamsGRoosDECromptonJLRadiotherapy for optic nerve sheath meningioma: a case for earlier intervention?Clin Oncol2013256356361
- LeeAGWooSYMillerNRSafranABGrantWHButlerEBImprovement in visual function in an eye with a presumed optic nerve sheath meningioma after treatment with three-dimensional conformal radiation therapyJ Neuroophthalmol19961642472518956159
- RichardsJCRodenDHarperCSManagement of sight-threatening optic nerve sheath meningioma with fractionated stereotactic radiotherapyClin Experiment Ophthalmol200533213714115807820
- PacelliRCellaLConsonMFractionated stereotactic radiation therapy for orbital optic nerve sheath meningioma – a single institution experience and a short review of the literatureJ Radiat Res2011521828721293073
- ArvoldNDLessellSBussiereMVisual outcome and tumor control after conformal radiotherapy for patients with optic nerve sheath meningiomaInt J Radiat Oncol Biol Phys20097541166117219406585
- ParidaensADAvan RuyvenRLJEijkenboomWMHMooyCMvan den BoschWAStereotactic irradiation of biopsy proved optic nerve sheath meningiomaBr J Ophthalmol200387224624712543765
- PaulsenFDoerrSWilhelmHBeckerGBambergMClaßenJFractionated stereotactic radiotherapy in patients with optic nerve sheath meningiomaInt J Radiat Oncol Biol Phys201282277377821300458
- PitzSBeckerGSchieferUStereotactic fractionated irradiation of optic nerve sheath meningioma: a new treatment alternativeBr J Ophthalmol200286111265126812386086
- LiuJKFormanSHersheweGLMoorthyCRBenzilDLOptic nerve sheath meningiomas: visual improvement after stereotactic radiotherapyNeurosurgery200250595095711950397
- Milker-ZabelSHuberPSchlegelWDebusJZabel-du BoisAFractionated stereotactic radiation therapy in the management of primary optic nerve sheath meningiomasJ Neurooncol200994341942419337693
- LandertMBaumertBGBoschMMLutolfUMLandauKThe visual impact of fractionated stereotactic conformal radiotherapy on seven eyes with optic nerve sheath meningiomasJ Neuroophthalmol2005252869115937428
- BeckerGJeremicBPitzSStereotactic fractionated radiotherapy in patients with optic nerve sheath meningiomaInt J Radiat Oncol Biol Phys20025451422142912459365
- BaumertBGVillàSStuderGEarly improvements in vision after fractionated stereotactic radiotherapy for primary optic nerve sheath meningiomaRadiother Oncol200472216917415297135
- AndrewsDWFaroozanRYangBPFractionated stereotactic radiotherapy for the treatment of optic nerve sheath meningiomas: preliminary observations of 33 optic nerves in 30 patients with historical comparison to observation with or without prior surgeryNeurosurgery200251489089412234395
- SoldàFWharramBGunapalaRBradaMFractionated stereotactic conformal radiotherapy for optic nerve sheath meningiomasClin Oncol2012248e106e112
- FinemanMSAugsburgerJJA new approach to an old problemSurv Ophthalmol199943651952410416794
- SubramanianPBresslerNMMillerNRRadiation retinopathy after fractionated stereotactic radiotherapy for optic nerve sheath menin-giomaOphthalmology2004111356556715019337
- MacleanJFershtNBremnerFStaceyCSivabalasinghamSShortSMeningioma causing visual impairment: outcomes and toxicity after intensity modulated radiation therapyInt J Radiat Oncol Biol Phys2013854e179e18623245285
- GrantWCainRBIntensity modulated conformal therapy for intra-cranial lesionsMed Dosim19982332372419783277
- MarchettiMBianchiSMilanesiIMultisession radiosurgery for optic nerve sheath meningiomas--an effective option: preliminary results of a single-center experienceNeurosurgery20116951116112221971489
- KlinkDFMillerNRWilliamsJPreservation of residual vision 2 years after stereotactic radiosurgery for a presumed optic nerve sheath meningiomaJ Neuroophthalmol19981821171209621268
- RomanelliPBianchiLMuacevicABeltramoGStaged image guided robotic radiosurgery for optic nerve sheath meningiomasComput Aided Surg201116625726621991922
- Danesh-MeyerHVRadiation-induced optic neuropathyJ Clin Neu-rosci200815295100
- FarzinMMollsMKampferSOptic toxicity in radiation treatment of meningioma: a retrospective study in 213 patientsJ Neurooncol2016127359760626852221
- BrownGCShieldsJASanbornGAugsburgerJJSavinoPJSchatzNJRadiation retinopathyOphthalmology19828912149415017162794
- FingerPTRadiation retinopathy is treatable with anti–vascular endo-thelial growth factor bevacizumab (Avastin)Int J Radiat Oncol Biol Phys200870497497718313522
- GuptaAMueckeJSTreatment of radiation maculopathy with intravit-real injection of bevacizumab (Avastin)Retina200828796496818698298
- SiddiquiJDLoefflerJSMurphyMARadiation optic neuropathy after proton beam therapy for optic nerve sheath meningiomaJ Neuroophthalmol201333216516823429064
- WrightJECallNBLiaricosSPrimary optic nerve meningiomaBr J Ophthalmol19806485535587426572
- FayazIGentiliFMackenzieIROptic nerve sheath meningiomaJ Neurol Neurosurg Psychiatr1999673408409
- CristanteLSurgical treatment of meningiomas of the orbit and optic canal: a retrospective study with particular attention to the visual outcomeActa Neurochir (Wien)1994126127328154318
- DelfiniRMissoriPTarantinoRCiappettaPCantoreGPrimary benign tumors of the orbital cavity: comparative data in a series of patients with optic nerve glioma, sheath meningioma, or neurinomaSurg Neurol19964521471548607065
- RoserFNakamuraMMartini-ThomasRSamiiMTatagibaMThe role of surgery in meningiomas involving the optic nerve sheathClin Neurol Neurosurg2006108547047616191463
- ZweckbergerKUnterbergAWSchickUPre-chiasmatic transection of the optic nerve can save contralateral vision in patients with optic nerve sheath meningiomsClin Neurol Neurosurg2013115122426243124113388
- SchickUDottUHasslerWSurgical management of meningiomas involving the optic nerve sheathJ Neurosurg2004101695195915597756
- SchickUJungCHasslerWPrimary optic nerve sheath meningiomas: a follow-up studyCent Eur Neurosurg2010710312613320127592
- DhootDSShultsWTNgJDJdNSuccessful use of mitomycin C to prevent recurrence of the cystic component of an optic nerve sheath meningiomaOphthal Plast Reconstr Surg2008243235236
- PausSKlockgetherTUrbachHSchlegelUMeningioma of the optic nerve sheath: treatment with hydroxyureaJ Neurol Neurosurg Psychiatr200374913481350
- BlackPCarrollRZhangJThe molecular biology of hormone and growth factor receptors in meningiomasActa Neurochir Suppl19966550538738495
- MafeeMFGoodwinJDorodiSOptic nerve sheath meningiomas. Role of MR imagingRadiol Clin North Am1999371375810026728
- GrunbergSMWeissMHSpitzIMTreatment of unresectable meningiomas with the antiprogesterone agent mifepristoneJ Neuro-surg1991746861866
- CassidyLMMoriartyPAGriffinJFKennedySMHormonal treatment of bilateral optic nerve meningiomaEye199711Pt 45665689425427
- FokasEHenzelMSurberGHammKEngenhart-CabillicRStereotactic radiation therapy for benign meningioma: long-term outcome in 318 patientsInt J Radiat Oncol Biol Phys201489356957524751409
- CombsSEAdebergSDittmarJOSkull base meningiomas: long-term results and patient self-reported outcome in 507 patients treated with fractionated stereotactic radiotherapy (FSRT) or intensity modulated radiotherapy (IMRT)Radiother Oncol2013106218619122906549
- LooJLTianJMillerNRSubramanianPSUse of optical coherence tomography in predicting post-treatment visual outcome in anterior visual pathway meningiomasBr J Ophthalmol201397111455145823966371
- SnellRSLempMAClinical Anatomy of the Eye2nd edNew JerseyWiley-Blackwell1997
- SubramanianPSBresslerNMMillerNRRadiation retinopathy after fractionated stereotactic radiotherapy for optic nerve sheath meningiomaOphthalmology2004111356556715019337
