Abstract
Purpose
To determine whether saccadic eye movements are altered in glaucoma patients.
Patients and methods
Sixteen patients with glaucoma and 21 control subjects were prospectively studied. Patients participated in a pro-saccade step task. Saccades were recorded using a noninvasive infrared oculometric device with head-mounted target projection. Medians of saccade reaction time, duration, amplitude, and peak velocity; frequency of express saccades; and percentage of trials with direction error were recorded. t-tests were used to compare the glaucoma and age-matched control groups. A correlation analysis of saccade parameters with visual field loss was also performed.
Results
Median saccade reaction times were significantly prolonged in glaucoma patients compared with controls (220.9 ± 49.02 ms vs 192.1 ± 31.24 ms; t-test: P = 0.036). Median duration, median amplitude, and median peak velocity of saccades did not show significant differences between glaucoma and control groups (P > 0.05). Frequency of express saccades was significantly decreased in glaucoma patients compared with controls (1.75 ± 2.32 vs 7.0 ± 6.99; t-test: P = 0.007). Saccade parameters in glaucoma patients showed no significant correlation with visual field loss.
Conclusion
Saccadic eye movements are significantly delayed in patients with early, moderate, or advanced glaucoma. Determination of median saccade reaction time may offer a novel functional test to quantify visual function in glaucoma patients. Further studies are needed to determine pathological processes implicated in delayed initiation of saccades, and to assess whether alteration of saccades affects daily activities in glaucoma patients.
Introduction
Glaucomatous optic neuropathy is a leading cause of irreversible blindness worldwide.Citation1 Loss of sight in glaucoma is caused by death in retinal ganglion cells,Citation2 and involves visual functions such as form, motion, and color.Citation3–Citation5 Functional tests for the diagnosis of glaucoma and for the assessment of progression are processed by retinal ganglion cells that project to the lateral geniculate nucleus,Citation6 a relay station to the primary visual cortex. In glaucoma, neurodegeneration in this major retino-geniculo-cortical pathway has been demonstrated.Citation7–Citation15
The effect of glaucoma on other visual functions such as eye movements that are controlled by alternative visual pathways is not well studied. Saccades are rapid eye movements that redirect the fovea to visual targets, such as a suddenly appearing visual stimulus.Citation16 They are generated by a complex network of brain structures, including the retinotectal pathway.Citation17 Saccades can be measured precisely with reliable parameters such as saccade reaction time, saccade duration,Citation18 amplitude,Citation19,Citation20 and peak velocity.Citation21,Citation22 Studies of these saccade parameters are used to assess the integrity of the saccade-generating neural network in various brain diseases.Citation23–Citation28 In addition, saccade parameters such as increased saccade reaction time are altered in various optic nerve pathologies affecting the nerve fibers that convey visual signals to the saccade-generating network.Citation29,Citation30 We hypothesize that eye movements may provide another functional marker of injury in glaucoma. The aim of this study was to determine whether saccadic eye movements are altered in glaucoma patients.
Material and methods
After study approval by St Michael’s Hospital Research Ethics Board, informed consent was obtained from glaucoma and age-matched participants between the ages of 40 and 80 years. Patients with primary open-angle glaucoma and a history of uncontrolled intraocular pressure (n = 16) were prospectively recruited from the practice of a glaucoma specialist (NG). Glaucoma was defined by characteristic optic nerve head findings with corresponding visual field changes assessed by white on white automated perimetry. Vision loss was estimated by the sum of mean deviations of right and left visual fields. Exclusion criteria included other nonglaucomatous eye disease, monocularity, incisional eye surgery within a month, central acuity of less than 20/50 OU, history of neurological disease, and use of psychotropic medications known to affect saccade velocity.Citation31 Control subjects (n = 21) were recruited from visitors or personnel working at the institution and from relatives of patients. All control subjects had normal eye examinations. Glaucoma and age-matched control subjects underwent full eye examinations. Age and visual acuity were not different between glaucoma and age-matched controls (–). Mean ages of glaucoma patients and controls were 63.2 ± 8.70 years and 60.9 ± 8.13 years, respectively (P = 0.42). Glaucoma patients showed minimal to advanced visual field loss ().
Table 1 DemographicsTable Footnotea
Table 2 Participant summary data for controls
Table 3 Participant summary data for glaucoma
Eye movement recordings
All glaucoma and control participants were tested under similar conditions under constant observation. Participants were seated 1.5 m away from an evenly lit wall with luminance measured at 500 cd/m2 (Minolta Luminance Meter LS-100, Osaka, Japan). A noninvasive infrared oculometric device with a head-mounted system of low-power laser-target projections (saccadometer) (Ober Consulting, Poznan, Poland) projected three high-contrast (13 cd/m2) discs subtending 0.1° in diameter, at 0°, 10° left, and 10° right, along the frontal plane at eye level. Viewing and recording were performed binocularly. Prior to proceeding with testing, all participants were required to report clearly seeing the red stimuli located at 0°, 10° left, and 10° right. Calibrations were made for both left and right stimuli under binocular viewing.
After a random foreperiod (500–1000 ms), the central fixation point was extinguished, and a randomly chosen 10° left or 10° right stimulus was projected. There were no gaps or overlaps, and the stimuli remained projected until either the participant performed a saccade or 2000 msec had elapsed. Each session consisted of 200 trials, measured over the course of 15 minutes. Saccade reaction time, duration, amplitude, peak velocity, and direction were recorded with a sampling rate of 1 kHz and a linear range within 7% for up to ±30° (Ober Consulting).Citation23 Blinks and head movements were automatically excluded by the Latency Meter Version 4.9 software (Ober Consulting), and analysis was performed on saccades made toward the stimulus. Trials with saccade reaction times between 50 ms and 600 ms were analyzed, removing anticipatory saccades (<50 ms) and latencies due to inattention (>600 ms).Citation22,Citation24–Citation26 Trials with saccade reaction times between 50 ms and 100 ms were defined as express saccades,Citation19,Citation32–Citation36 and their frequency was counted. Trials with direction error were analyzed separately.
Statistical analysis
Statistical analysis was performed using SPSS Version 14.0 (SPSS Inc, Chicago, IL) and SAS 9.2 (SAS Institute Inc, Cary, NC) with α level set at 0.05. Medians of saccade reaction time, duration, amplitude, and peak velocity, and frequency of express saccades were calculated. These variables in the glaucoma group were compared with age-matched controls using independent-samples t-tests. A P-value less than 0.05 was considered statistically significant. Bivariate correlations of the visual field loss (as measured by the sum of mean deviations of right and left visual fields) and various saccade variables were calculated using the Pearson correlation coefficient.
Results
shows a series of saccade recordings and histograms of saccade reaction times of representative glaucoma and control subjects. Saccade reaction times were prolonged in glaucoma patients compared with controls (). Median saccade reaction time was significantly increased in glaucoma patients compared with controls (220.9 ± 49.02 ms vs 192.1 ± 31.24 ms; P = 0.036) (). Median duration, median amplitude, and median peak velocity were not statistically different between groups (54.7 ± 5.16 ms vs 52.8 ± 4.45 ms, P = 0.25; 10.4 ± 1.88° vs 9.5 ± 1.69°, P = 0.12; 349.4 ± 72.01°/s vs 345.0 ± 83.41°/s, P = 0.87) ().
Figure 1 Traces of individual saccades for two subjects (left) and histograms of corresponding reaction times (right).a
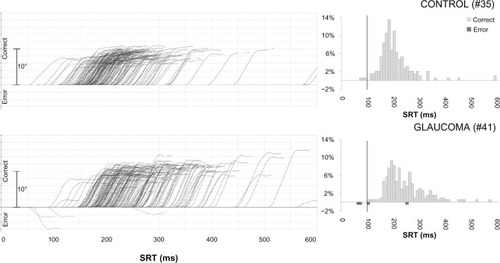
Figure 2 Saccade reaction times (SRT).a
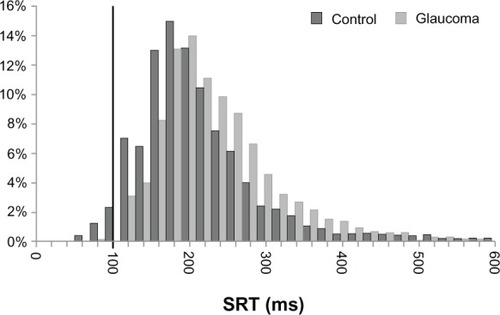
Figure 3 Box plots of median saccade reaction times per group.a
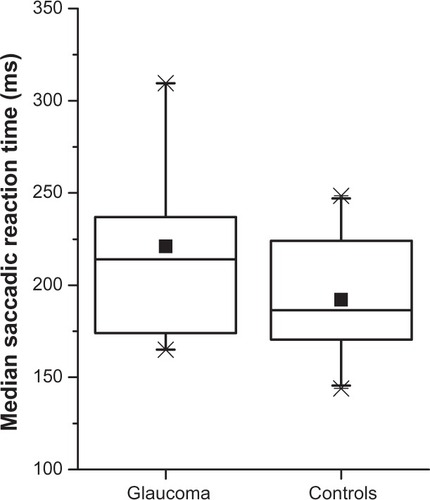
Table 4 Saccade parameters in glaucoma and controls
There was a significant reduction in the number of express saccades in the glaucoma patients compared with controls (1.75 ± 2.32 vs 7.0 ± 6.99; P = 0.007). The percentage of direction errors between the glaucoma and control groups was not statistically different (1.72 ± 1.82% vs 3.2 ± 5.89%; P = 0.34) (), and neither were direction errors significantly correlated with the degree of visual field loss in the glaucoma group (P = 0.54).
Median saccade reaction time, median duration, median amplitude, and median peak velocity were not statistically correlated with the degree of visual field loss (P = 0.64, P = 0.25, P = 0.14, and P = 0.38, respectively).
Discussion
This is the first study to demonstrate that saccade latencies are affected in glaucoma. Glaucoma patients were slower to initiate the saccade compared with age-matched controls. Normal saccade reaction times in control subjects in our study are consistent with previous work.Citation25,Citation27,Citation34
The fact that other saccade parameters such as duration, amplitude, and peak velocity are not altered suggests that patients were able to detect the targets located at 10° to the right and to the left of the fixation point, and that once the saccades were initiated, the accuracy and the motor characteristics of the saccades were not significantly affected. Therefore, the glaucomatous pathological process seems to alter mainly the initiation of saccades that involve visual input as well as a complex saccade-generation network.
The neural network underlying saccade generation involves a multitude of structures, including superior colliculus, areas of the frontal lobe, and the basal ganglia.Citation17 Visual input to the saccade-generating network is provided by a specific subpopulation of retinal ganglion cells that project directly to superior colliculus,Citation6,Citation37 and indirect visual input via the primary visual cortex also projects to the superior colliculus.Citation38 In view of the fact that delayed saccades have been described in various optic nerve diseases,Citation29,Citation30 the delayed saccades observed in patients with glaucomatous optic neuropathy are likely due to damage to retinal ganglion cells that provide direct retinal input to superior colliculusCitation6 and/or that drive the visual information through the visual cortex.Citation38,Citation39 It is interesting to note that delayed saccades are found in patients with mild visual field loss, as well as in advanced cases. It is not yet known whether the increased latency of nerve conduction in the retino-geniculate pathwayCitation40,Citation41 and/or the decreased amplitude of retinal ganglion cell responseCitation42 reported in some glaucoma patients contribute to delayed saccades in glaucoma patients. Further studies are needed to determine whether the retinal ganglion cells conveying visual information to the saccade-generation network are affected in glaucoma.
The superior colliculus is critical to saccade generation, with a role in both visual and motor components.Citation17 In addition to its sensory role as a visual information-recipient structure arranged in a retinotopic fashion, it plays an important role in influencing saccade generation through its direct projection to the brainstem reticular formation,Citation43 which, in turn, projects to oculomotor neurons.Citation44 Ablation of superior colliculus in nonhuman primates leads to increased saccade reaction times and eliminates a subpopulation of express saccades.Citation45 Interestingly, in addition to prolonged saccade reaction time, we noted a reduced frequency of express saccades in glaucoma. Investigations in nonhuman primate experimental glaucoma may help to determine whether the retinal ganglion cells and superior colliculus involved in the generation of saccades are, indeed, affected in glaucoma.
Few studies have looked at eye movements in glaucoma. Other types of gaze-shifting eye movements such as smooth pursuit have not yet been studied. Among the gaze-stabilizing eye movements, optokinetic nystagmus has been investigated and shown to exhibit characteristic differences in glaucoma patients as compared with controls, eg, in pursuit eye movement and optokinetic nystagmus elicitation tasks.Citation46,Citation47 Other studies have examined the eye movements of glaucoma patients as either pedestrians or drivers. Gaze behavior has been shown to differ in a group of glaucoma patients compared with controls when crossing a street,Citation48 whereas eye-tracking data have shown changes in the pattern of eye movements performed by glaucoma patients compared with controls while viewing a video of a driving scene.Citation49 Further studies are needed to determine whether delayed saccades in glaucoma may contribute to these altered eye movements.
Certain pharmacological agents are known to alter sac-cade parameters. Patients on psychotropic medications, including benzodiazepines, antipsychotics, and anticonvulsants, which are known to decrease saccade velocity,Citation31 were excluded in this study. This study shows that saccade reaction time is increased in glaucoma patients, and though topical treatment effects cannot be ruled out, no statistically significant difference in saccade reaction times (P = 0.42) was noted between glaucoma patients on topical β-blockers (n = 8) compared with those who were not (n = 8).
Glaucoma patients are known to be at increased risk for falls and motor vehicle collisions.Citation50 These tasks are dependent on a person’s ability to attend to and respond to visual stimuli. Further studies are needed to determine whether delayed saccades observed in glaucoma patients have functional implications and contribute to an increased risk for falls and motor vehicle collisions.
Overall, this study provides the first insight into saccade alterations in glaucoma. Further studies are needed to investigate the possible locations of neural damage in pathways and centers involved in saccades, to determine whether saccade changes correlate with disease development and/or progression, and to evaluate their possible functional impact in patients with glaucoma.
Acknowledgments
The authors would like to thank Joyce Lo and Lalaine Songalia for their excellent research assistance, and Barbara Thomson MSc for statistical analysis. This study was supported by the Glaucoma Research Society of Canada (NG, YY), Dorothy Pitts Fund (NG), and the University of Toronto CREMS program.
Disclosure
The authors report no conflicts of interest in this work.
References
- ResnikoffSPascoliniDEtya’aleDGlobal data on visual impairment in the year 2002Bull World Health Organ2004821184485115640920
- QuigleyHANeuronal death in glaucomaProg Retin Eye Res199918139579920498
- SamplePAJohnsonCAFunctional assessment of glaucomaJ Glaucoma2001105 Suppl 1S49S5211890275
- SamplePAMedeirosFARacetteLIdentifying glaucomatous vision loss with visual-function-specific perimetry in the diagnostic innovations in glaucoma studyInvest Ophthalmol Vis Sci20064783381388916877406
- JampelHDSinghKLinSCAssessment of visual function in glaucoma: a report by the American Academy of OphthalmologyOphthalmology20111185986100221539982
- PerryVHCoweyARetinal ganglion cells that project to the superior colliculus and pretectum in the macaque monkeyNeuroscience1984124112511376483194
- YucelYHZhangQWeinrebRNKaufmanPLGuptaNEffects of retinal ganglion cell loss on magno-, parvo-, koniocellular pathways in the lateral geniculate nucleus and visual cortex in glaucomaProg Retin Eye Res200322446548112742392
- GuptaNAngLCNoel de TillyLBidaiseeLYucelYHHuman glaucoma and neural degeneration in intracranial optic nerve, lateral geniculate nucleus, and visual cortexBr J Ophthalmol200690667467816464969
- DuncanROSamplePAWeinrebRNBowdCZangwillLMRetinotopic organization of primary visual cortex in glaucoma: comparing fMRI measurements of cortical function with visual field lossProg Retin Eye Res2007261385617126063
- GaraciFGBolacchiFCerulliAOptic nerve and optic radiation neurodegeneration in patients with glaucoma: in vivo analysis with 3-T diffusion-tensor MR imagingRadiology2009252249650119435941
- BoucardCCHernowoATMaguireRPChanges in cortical grey matter density associated with long-standing retinal visual field defectsBrain2009132Pt 71898190619467992
- GuptaNGreenbergGde TillyLNGrayBPolemidiotisMYucelYHAtrophy of the lateral geniculate nucleus in human glaucoma detected by magnetic resonance imagingBr J Ophthalmol2009931566018697810
- GuptaNYucelYHGlaucoma as a neurodegenerative diseaseCurr Opin Ophthalmol200718211011417301611
- YucelYGuptaNGlaucoma of the brain: a disease model for the study of transsynaptic neural degenerationProg Brain Res200817346547818929128
- YucelYHZhangQGuptaNKaufmanPLWeinrebRNLoss of neurons in magnocellular and parvocellular layers of the lateral geniculate nucleus in glaucomaArch Ophthalmol2000118337838410721961
- SaslowMGEffects of components of displacement-step stimuli upon latency for saccadic eye movementJ Opt Soc Am1967578102410296035296
- MunozDPSchallJDConcurrent, distributed control of saccade initiation in the frontal eye field and superior colliculusHallWCMoschovakisAThe superior colliculus: new approaches for studying sensorimotor integrationBoca Raton, FLCRC Press20043452
- WarabiTKaseMKatoTEffect of aging on the accuracy of visually guided saccadic eye movementAnn Neurol19841644494546497354
- FischerBWeberHBiscaldiMAipleFOttoPStuhrVSeparate populations of visually guided saccades in humans: reaction times and amplitudesExp Brain Res19939235285418454016
- BotzelKRottachKButtnerUNormal and pathological saccadic dysmetriaBrain1993116Pt 23373538461969
- PittMCRawlesJMThe value of measuring saccadic eye movement in the investigation of non-compressive myelopathyJ Neurol Neurosurg Psychiatry19895210115711612795041
- SharpeJAZackonDHSenescent saccades. Effects of aging on their accuracy, latency and velocityActa Otolaryngol19871045–64224283434263
- OberJKPrzedpelska-OberEGryncewiczWHandheld system for ambulatory measurement of saccadic durations of neurological patientsGajdaJModelling and measurement in medicineWarsaw, PolandKomitet Biocybernityki i Inzyneierii Biomedycznej PAN2003187198
- Pierrot-DeseillignyCRivaudSGaymardBAgidYCortical control of reflexive visually-guided saccadesBrain1991114Pt 3147314852065261
- MichellAWXuZFritzDSaccadic latency distributions in Parkinson’s disease and the effects of L-dopaExp Brain Res2006174171816544135
- RivaudSMuriRMGaymardBVermerschAIPierrot-DeseillignyCEye movement disorders after frontal eye field lesions in humansExp Brain Res199410211101207895787
- AntoniadesCAAlthamPMMasonSLBarkerRACarpenterRSaccadometry: a new tool for evaluating presymptomatic Huntington patientsNeuroreport200718111133113617589313
- PearsonBCArmitageKRHornerCWCarpenterRHSaccadometry: the possible application of latency distribution measurement for monitoring concussionBr J Sports Med200741961061217496064
- BrigellMGGoodwinJALoranceRSaccadic latency as a measure of afferent visual conductionInvest Ophthalmol Vis Sci1988298133113383417417
- ReulenJPLatency of visually evoked saccadic eye movements. II. Temporal properties of the facilitation mechanismBiol Cybern19845042632716509117
- ReillyJLLencerRBishopJRKeedySSweeneyJAPharmacological treatment effects on eye movement controlBrain Cogn200868341543519028266
- MunozDPBroughtonJRGoldringJEArmstrongITAge-related performance of human subjects on saccadic eye movement tasksExp Brain Res199812143914009746145
- Shafiq-AntonacciRMaruffPWhyteSTylerPDudgeonPCurrieJThe effects of age and mood on saccadic function in older individualsJ Gerontol B Psychol Sci Soc Sci1999546P361P36810625964
- IrvingELSteinbachMJLillakasLBabuRJHutchingsNHorizontal saccade dynamics across the human life spanInvest Ophthalmol Vis Sci20064762478248416723459
- SparksDRohrerWHZhangYThe role of the superior colliculus in saccade initiation: a study of express saccades and the gap effectVision Res200040202763277710960650
- FischerBRamspergerEHuman express saccades: extremely short reaction times of goal directed eye movementsExp Brain Res19845711911956519226
- MaysLESparksDLDissociation of visual and saccade-related responses in superior colliculus neuronsJ Neurophysiol19804312072326766178
- SchillerPHStrykerMCynaderMBermanNResponse characteristics of single cells in the monkey superior colliculus following ablation or cooling of visual cortexJ Neurophysiol19743711811944204566
- AbelPLO’BrienBJLiaBOlavarriaJFDistribution of neurons projecting to the superior colliculus correlates with thick cytochrome oxidase stripes in macaque visual area V2J Comp Neurol199737733133238989648
- ParisiVImpaired visual function in glaucomaClin Neurophysiol2001112235135811165541
- RodarteCHoodDCYangEBThe effects of glaucoma on the latency of the multifocal visual evoked potentialBr J Ophthalmol20069091132113616707520
- SehiMGrewalDSGoodkinMLGreenfieldDSReversal of retinal ganglion cell dysfunction after surgical reduction of intraocular pressureOphthalmology2010117122329233620920827
- RaybournMSKellerELColliculoreticular organization in primate oculomotor systemJ Neurophysiol1977404861878407334
- Buttner-EnneverJAHennVAn autoradiographic study of the pathways from the pontine reticular formation involved in horizontal eye movementsBrain Res19761081155164819096
- SchillerPHSandellJHMaunsellJHThe effect of frontal eye field and superior colliculus lesions on saccadic latencies in the rhesus monkeyJ Neurophysiol1987574103310493585453
- SevertWLMaddessTIbbotsonMREmploying following eye movements to discriminate normal from glaucoma subjectsClin Experiment Ophthalmol200028317217410981790
- TongJWangJSunFDual-directional optokinetic nystagmus elicited by the intermittent display of gratings in primary open-angle glaucoma and normal eyesCurr Eye Res200225635536212789542
- GeruschatDRHassanSETuranoKAQuigleyHACongdonNGGaze behavior of the visually impaired during street crossingOptom Vis Sci200683855055816909081
- CrabbDPSmithNDRauscherFGExploring eye movements in patients with glaucoma when viewing a driving scenePLoS One201153e9710
- HaymesSALeblancRPNicolelaMTChiassonLAChauhanBCRisk of falls and motor vehicle collisions in glaucomaInvest Ophthalmol Vis Sci20074831149115517325158