Abstract
Neurons in primary visual cortex (V1) integrate across the representation of the visual field through networks of long-range projecting pyramidal neurons. These projections, which originate from within V1 and through feedback from higher visual areas, are likely to play a key role in such visual processes as low contrast facilitation and extraclassical surround suppression. The extent of the visual field representation covered by feedback is generally much larger than that covered through monosynaptic horizontal connections within V1, and, although it may be possible that multisynaptic horizontal connections across V1 could also lead to more widespread spatial integration, nothing is known regarding such circuits. In this study, we used injections of the CVS-11 strain of rabies virus to examine disynaptic long-range horizontal connections within macaque monkey V1. Injections were made around the representation of 5° eccentricity in the lower visual field. Along the opercular surface of V1, we found that the majority of connected neurons extended up to 8 mm in most layers, consistent with twice the typically reported distances of monosynaptic connections. In addition, mainly in layer 6, a steady presence of connected neurons within V1 was observed up to 16 mm away. A relatively high percentage of these connected neurons had large-diameter somata characteristic of Meynert cells, which are known to project as far as 8 mm individually. Several neurons, predominantly in layer 6, were also found deep within the calcarine sulcus, reaching as far as 20° of eccentricity, based on estimates, and extending well into the upper visual field representation. Thus, our anatomical results provide evidence for a wide-ranging disynaptic circuit within V1, mediated largely through layer 6, that accounts for integration across a large region of the visual field.
Introduction
In primary visual cortex (V1), pyramidal neurons send axons laterally, forming an extensive horizontal lattice of connectivity within the superficial and deep layers.Citation1–Citation6 In higher visual species such as carnivore, primate, and tree shrew, horizontal connections are strikingly patchy and link together neurons found in similar orientation columns,Citation7–Citation13 providing a network that may lead to facilitation of responses at low stimulus contrastCitation14–Citation17 and enhanced selectivity to stimulus orientation.Citation16,Citation18–Citation20 In addition, because long-range lateral connections can integrate across the visual field representation, they are capable of providing a global context important for processing of visual scenes.Citation21–Citation24 One such process studied extensively in both monkey and cat is that of extraclassical surround suppression,Citation15,Citation16,Citation19,Citation23,Citation25–Citation29 which may be mediated indirectly through long-range lateral connections originating from excitatory pyramidal cells synapsing onto local inhibitory neurons,Citation13,Citation30–Citation32 or even directly through a subtype of long-range projecting inhibitory neurons.Citation33 However, the role of intrinsic V1 connections in surround suppression has been called into question.Citation15,Citation34–Citation36 This is because, even though long-range connections typically extend up to 3–4 mm, the region of visual space covered in V1 is relatively small compared to the full extent of the extraclassical surround.Citation12,Citation14,Citation37
Instead of long-range lateral connections, feedback from higher visual areas has been proposed as a more likely source of inputs to the full extraclassical surround, since the extent of the visual field representation covered by feedback is generally much larger.Citation14,Citation38 Through this circuit, surround suppression could be mediated via excitatory feedback projections synapsing onto local inhibitory neurons,Citation39 for which there is anatomical support, although the occurrence of feedback neurons synapsing onto local inhibitory cells is significantly lower than onto local excitatory neurons.Citation40–Citation42 Nevertheless, it is just as possible that the full extent of the extraclassical surround could be covered by a string of multisynaptic long-range lateral connections within V1.
In this study, we used a transneuronal rabies virus and limited the transport time to 72 hours to determine the extent of intrinsic V1 connections across two synapses. We found that the majority of connected neurons extended up to 8 mm in layers 2/3, 4A, 4B, 5, and 6, consistent with twice the typically reported distances of monosynaptic connections.Citation6,Citation14 An additional, steady presence of neurons was found up to 16 mm away, most prominently in layer 6. Moreover, several neurons, also predominantly in layer 6, were found deep within the calcarine sulcus. A relatively high percentage of these long-range layer 6 neurons had large cell body sizes characteristic of Meynert cellsCitation43–Citation46 – cells that can project as far as 8 mm individually.Citation47 Our anatomical results show that a disynaptic horizontal network within layer 6 is capable of reaching at least twice as far as the previously reported maximum distance for monosynaptic Meynert cell projections, allowing for integration across a large region of the visual field independent of feedback from higher visual areas.
Methods
Surgical procedures
One adult male macaque monkey was used following procedures approved by the Salk Institute Animal Care and Use Committee. Procedures involving rabies virus were conducted using biosafety level 2 precautions as described elsewhere.Citation48
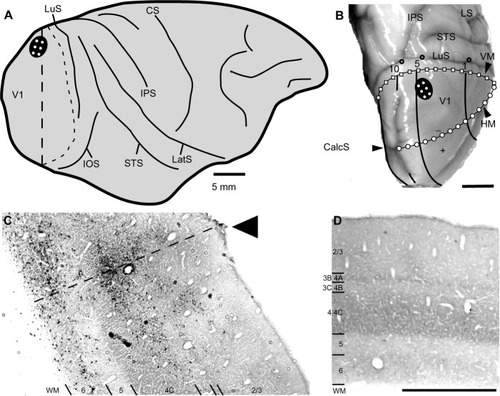
The animal was anesthetized as previously describedCitation49 and placed in a stereotaxic head frame. Under sterile conditions, the dorsolateral surface of V1 of the right hemisphere was exposed, and injections of the challenge virus strain-11 ([CVS-11] see Rabies virus strain and speed of transport section) of rabies virus were made at six locations spaced ~2 mm apart, forming a 2×3 grid just lateral to the medial lip in a region corresponding to ~5° of eccentricity in the lower visual quadrant (see ). At each location, 0.3 µL of rabies virus was injected at three depths spaced by 0.7 mm (0.7, 1.4, and 2.1 mm deep) using a glass pipette (tip diameter ~30 µm) attached to a Pneumatic Pico Pump (PV820; World Precision Instruments, Sarasota, FL, USA). Injections were placed first at the deepest penetration (2.1 mm) and then moved upward toward the surface for the subsequent injections.
Figure 1 Rabies virus injections in macaque monkey primary visual cortex.
Abbreviations: CalcS, calcarine sulcus; CS, central sulcus; HM, horizontal meridian; IOS, inferior occipital sulcus; IPS, intraparietal sulcus; LatS, lateral sulcus; LuS, lunate sulcus; STS, superior temporal sulcus; V1, primary visual cortex; V2, secondary visual cortex; VM, vertical meridian; WM, white matter.
Rabies virus strain and speed of transport
A 72-hour survival time following injections of the CVS-11 strain of rabies virus into V1 was used to allow disynaptic retrograde transport. Different preparations of rabies virus spread through the nervous system at different rates depending on the passage history of the virus.Citation48,Citation50,Citation51 The CVS-11 rabies virus strain that we used here was passaged four times in mouse brain, followed by five times in cultured chicken embryo-related cells, and had a titer of 1×104 plaque-forming units (PFU)/mL (supplied by Dr Donald Lodmell, National Institutes of Health/National Institute of Allergy and Infectious Diseases, Rocky Mountain Laboratory, Hamilton, MT, USA). The titer of our virus (~1×107 PFU/mL) was lower than that of the CVS-11 virus used by Kelly and Strick,Citation52 who confirmed that a 72-hour survival time limited infection to second-order neurons. Similar controls for our lower-titer virus were used in three previous publications.Citation49,Citation53,Citation54 Therefore, it is highly unlikely that our lower-titer virus would have infected beyond two synapses.
Histology
After the 72-hour survival time, the animal was sacrificed and perfused transcardially, first with saline and then with 4% paraformaldehyde in phosphate buffer (pH 7.4). The brain was removed, then postfixed and cryopreserved overnight in 4% paraformaldehyde and 30% sucrose in phosphate buffer. The brain was then kept in 30% sucrose without 4% paraformaldehyde for another 5 days. Brain sections were cut coronally at 40 µm on a freezing microtome. Every sixth section was processed for cytochrome oxidase (CO),Citation55 and then for the rabies nucleocapsid protein to reveal rabies virus-infected neurons. For this, immunohistochemistry was performed using the anti-nucleocapsid mouse monoclonal antibody (gifted by Dr Donald Lodmell) and the biotinylated horse anti-mouse secondary antibody (Vector Labs, Burlingame, CA, USA) and revealed through a diaminobenzidine reaction. V1 was identified through distinct CO staining patterns, including a thick, CO dark layer 4C (using the terminology of BrodmannCitation56), corresponding to the main geniculate input layer, and a thin, CO dark layer 4A (see ).
Data analysis
Every 24th section throughout the entire V1 region, and every 12th section near the injection sites, was examined for rabies-labeled neurons. Infected neurons were identified and plotted under bright-field illumination using a Zeiss Axioplan microscope (Carl Zeiss Microscopy GmbH, Jena, Germany) at low magnification (×10 objective, 0.45 NA) and a high-power digital camera (Cooke SensiCam QE; PCO-TECH Inc., Romulus, MI, USA) to send live images to a Neurolucida computerized reconstruction system (MBF Bioscience, Williston, VT, USA). Reconstructions of the laminar boundaries of V1 were aided by CO staining of the same sections that were stained for rabies nucleocapsid (for examples, see , , and ). In every section, cell counts were made for each layer and the number multiplied by 12 or 24 to provide an interpolated count, since one in 12 (near injection sites) or one in 24 sections were sampled. The distance of each neuron from the lateralmost or medial-most injection sites were also determined at 0.5 mm increments for neurons found on the opercular surface and immediately adjacent along the medial wall (see examples of distance measurements in ). Note that, because rabies virus does not leave an injection-site halo,Citation57 the uptake region from each injection site was estimated to have a radius of 0.5 mm. The distance of each counted neuron is relative to this estimated edge of the injection, not to the center of the injection track.
Figure 2 The distribution of rabies-infected neurons in two coronal sections of V1 of the right hemisphere.
Abbreviations: CalcS, calcarine sulcus; V1, primary visual cortex; WM, white matter.
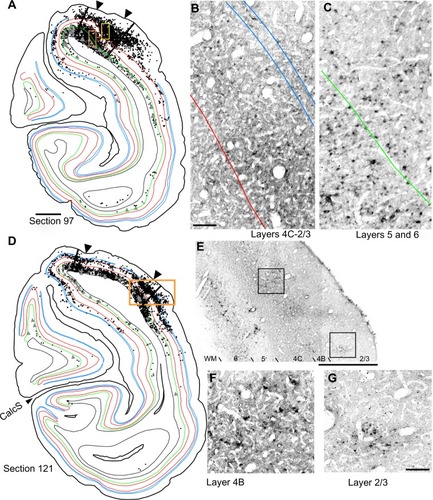
Figure 3 The distribution of rabies-infected neurons in a single cross-section of V1 of the right hemisphere.
Abbreviations: V1, primary visual cortex; WM, white matter.
Pyramidal neurons with cell somata >30 µm in diameter were labeled as “large” cells and identified within Neurolucida using an open circular curser size set to a 30 µm diameter; cells <30 µm were considered “small”. The 30 µm diameter was chosen as a conservative estimate of layer 6 Meynert cells and is based on previous reports showing that their average diameter is 22.3 µm, whereas the typical diameter of all other pyramidal neuron somata (in primary visual cortex) is around 13 µm.Citation43 Even though large diameters can be useful for identifying Meynert cells, not all Meynert cells necessarily have large cell bodies.Citation58 Therefore, our numbers of this cell type based on cell body diameter alone is likely to be an underestimate. In addition to layer 6 Meynert cells, we also identified a relatively small number of large-diameter pyramidal neurons in layer 4B, likely corresponding to Meynert–Cajal cells as reported by others.Citation45,Citation46
Digital images shown in – were captured by the Cooke SensiCam and were cropped and adjusted for brightness and contrast in Canvas software (version 11; ACD Systems International Inc., Seattle, WA, USA). None of the images was altered in any other way.
Results
To determine the extent of disynaptic long-range lateral connections within V1, we made several injections of the CVS-11 strain of rabies virus within a small 2×3 grid, covering 2×4 mm on the dorsal lateral surface of V1 in one hemisphere of a macaque monkey (). The injected region was estimated to be just within 5° of the lower visual field representation, nearer to the vertical meridian (). An example of rabies virus infection near one of the injection sites is shown in , where dense clusters of rabies-infected neurons are visible in layers 2/3–6. Note that, unlike traditional neuronal tracers, rabies virus does not leave a halo, making injected locations less prominent.Citation57 Nevertheless, this site could be verified based on the surface perturbation caused by the needle insertion and the denser clusters of infected neurons.
Reconstructions of entire coronal sections containing parts of several of the injection sites (black arrowheads) are shown in and . In each of these 40 µm-thick sections, which are composed almost entirely of V1, retrogradely connected neurons, while far more numerous within a few millimeters of the injections, are nevertheless found throughout the extent of V1, including along the lateral pole more than 18 mm away and within the lower bank of the calcarine sulcus. Infected neurons located nearer to the injection sites are more likely to represent both mono- and disynaptically labeled neurons, whereas neurons located further from the injection site are increasingly more likely to represent disynaptically labeled neurons. This is especially the case for neurons found further than 4 mm or 8 mm away, which represent the longest range reported for monosynaptic lateral connections in V1Citation6,Citation14,Citation47 for layers 2–5 and layer 6, respectively (see “Discussion”). Examining the laminar distributions, infected neurons were found at long range in all layers, but had a distinctive presence in layer 6 ( and [green line indicates the layer 5/6 border]), particularly beyond 4 mm. Also notable was the prevalence of neurons with relatively large-diameter somata in layer 6 ( and [displayed as gray triangles]), compared to other layers. For example, the cell bodies of the layer 6 pyramidal neurons shown in are each over 30 µm in diameter, more than twice the diameter of most neurons found in more superficial layers (). Based on their large size and laminar position, these neurons can be classified as Meynert cells (see “Methods” and “Discussion”).
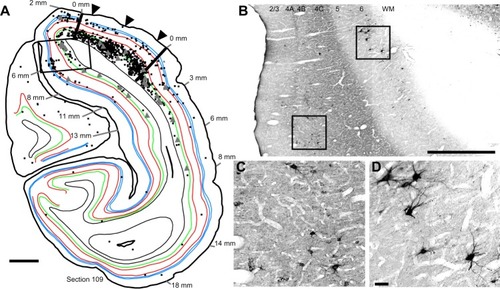
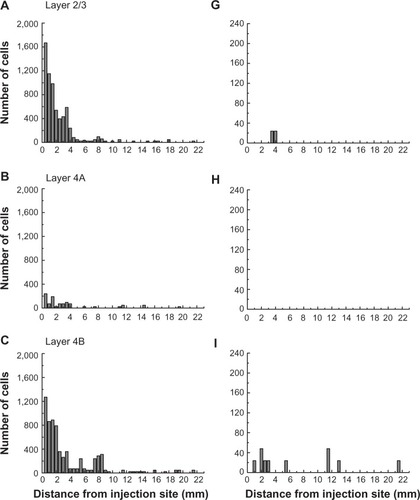
To examine the laminar pattern of long-range connections throughout more than 450 coronal sections containing V1, the position of every rabies-infected neuron was plotted for every 24th section (960 µm intervals). Near the injection sites, every 12th section was analyzed (sections 85–157). In all, infected V1 cells were found in 24 sections spanning over 20 mm from anterior to posterior. A sample of these sections is shown in , where V1 can be distinguished by the parallel red lines used to delineate layer 4C. The relative distance of each labeled neuron from the lateral- and medial-most injection sites was determined for neurons located along the opercular surface and medial wall. For detailed examples of cell distances from the medial- and lateralmost injection sites, refer to section 109 shown in . The results of this analysis are given for different layers in . In layers 2/3, 4B, and 6, there is a large number of neurons within the first 0.5 mm, ~3,200 in layer 6, and ~1,200–1,600 in layers 4B and 2/3, respectively (). The number gradually declines to about 400 cells in each of these three layers at around 3.5–4 mm, then plateaus somewhat until about the 8 mm mark. In the remaining layers, the number of neurons is fewer, but also extends to 4 mm for layer 4A () and to 8 mm for layers 4C and 5 (). Beyond 8 mm, especially within layer 6, a number of connected neurons within V1 were observed up to 16 mm away (). A relatively high number of very-long-range layer 6 neurons had large somata () characteristic of Meynert cells.Citation43–Citation46 These large-diameter neurons were virtually absent from all other layers except for a sporadic distribution in layer 4B ().
Figure 4 Serial reconstructions show the pattern of mono- and disynaptically connected neurons in visual cortex following rabies virus injections in V1.
Abbreviations: CalcS, calcarine sulcus; IOS, inferior occipital sulcus; IPS, intraparietal sulcus; LuS, lunate sulcus; STS, superior temporal sulcus; V1, primary visual cortex.
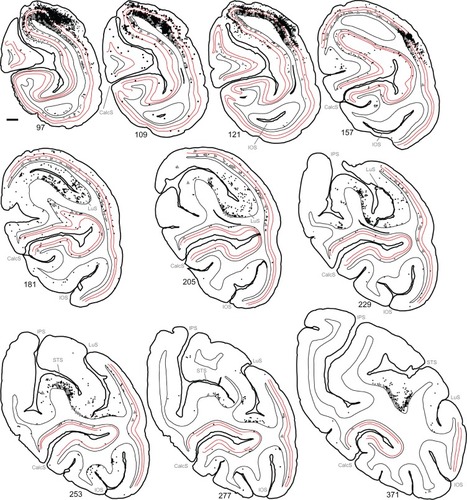
Figure 5 The numbers of retrogradely infected neurons found across several millimeters of the opercular surface of primary visual cortex.
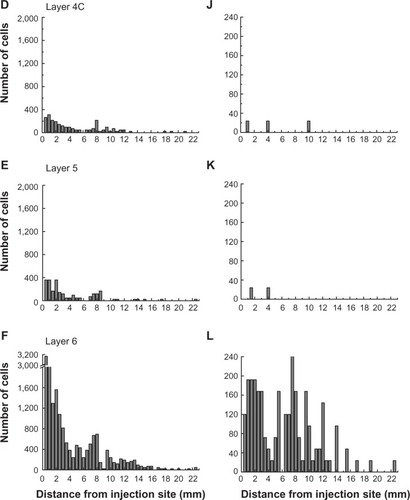
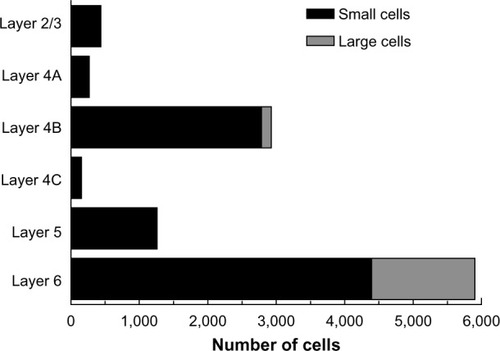
The laminar position of infected neurons found in the calcarine sulcus was also tabulated. For these cells, distances from the injection sites were not considered because of the difficulties in knowing the direct paths taken through the convolutions of the calcarine sulcus. Nevertheless, cells in the calcarine can be considered to be further than 8 mm away, based on the distance shown in . graphs the interpolated numbers of cells found in the calcarine sulcus and their distribution across layers. The most neurons were found in layer 6 (n=5,904). Fewer cells were found in layer 4B (n=2,928) and in layer 5 (n=1,248), with fewer still in layers 2/3 (n=432), 4A (n=264), and 4C (n=144). In layer 6, 26% of the infected neurons had somata over 30 µm in diameter, whereas in the only other layer with such large cells, layer 4B, these cells made up 5% of the labeled population.
Figure 6 The numbers of retrogradely infected V1 neurons present in the calcarine sulcus.
Abbreviation: VI, primary visual cortex.
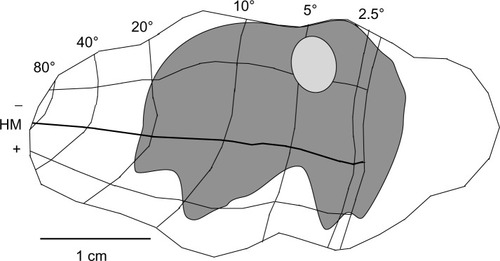
Using the full series of reconstructions, we made an estimate of the range of the visual field representation covered in V1. This estimate was based on the detailed receptive field maps provided by Van Essen et alCitation59 and Weller and KaasCitation60 that could be easily related to identifiable landmarks within the calcarine sulcus and along the opercular surface. As shown in , stemming from the injection at ~5° eccentricity in the lower visual field, disynaptic connections within V1 spanned 2°–20° of the visual field, and were found well within the upper field representation. Cells corresponding to the 20° representation of the visual field were found more than halfway through the anterior–posterior extent of the calcarine sulcus, on both the upper (lower visual field representation) and lower (upper field) banks of the calcarine (see sections 229–371 in ). As noted above, most of these cells are located in layer 6 and several of them have large-diameter somata characteristic of Meynert cells (large gray triangles in ). Similarly, cells found most anteriorly on the opercular surface, near the representation of 2° in eccentricity, are shown in section 277 (), are mainly found in layer 6, and are similar in size to Meynert cells (see also ).
Figure 7 The region of visual space covered by the very-long-range disynaptic connections is shown on an unfolded, two-dimensional view of the V1 surface.
Abbreviations: HM, horizontal meridian; V1, primary visual cortex.
Discussion
By using the CVS-11 strain of rabies virus as a transneuronal tracer, we were able to determine the length and laminar distribution of disynaptic long-range horizontal connections within macaque monkey V1. We found that the number of connected neurons in all layers was highest within 0.5 mm of the injection site and steadily declined over the first 4 mm. Between 4 and 8 mm, fewer neurons were found in most layers, but the number of cells in layer 6 remained relatively high. Beyond 8 mm, a steady presence of connected neurons was observed up to 16 mm away, primarily in layer 6. Between 4 and 16 mm, a relatively high percentage of these layer 6 neurons had large soma sizes characteristic of Meynert cells.Citation43–Citation46 Several neurons, the majority of which were in layer 6, were also found deep within the calcarine sulcus, reaching as far as 20° in eccentricity and extending well into the upper visual field representation. These anatomical results provide evidence for a far-ranging disynaptic circuit within V1, mediated in large part through layer 6, that accounts for integration across a wide expanse of the visual field.
While we are unable to differentiate monosynaptic from disynaptic long-range lateral connected neurons, previous work using traditional monosynaptic tracers indicates that long-range connected neurons are typically found up to 3–4 mm from the injection site,Citation6,Citation14,Citation37 with a subpopulation of layer 6 Meynert cells projecting as far as 8 mm.Citation47 Therefore, the densest connectivity within the first 4–8 mm in our results is largely consistent with monosynaptic connectivity (0–4 mm), and subsequent disynaptically labeled inputs (4–8 mm) to these monosynaptically connected neurons, with some of the neurons between 4 and 8 mm likely being monosynaptically labeled Meynert cells. In contrast, the sparser labeling of neurons between 8 and 16 mm likely reflects disynaptic rabies virus infection only and should involve the longer-range projecting Meynert cells, as either the first- and/or second-order neuron infected. Indeed, of the putative disynaptically connected neurons found further than 8 mm away, most were located in layer 6, and a high proportion were identified as Meynert cells based on their large cell body diameter ( and ).
An alternative, or additional, explanation could be that these longer-range (>8 mm away) layer 6 neurons were infected through a feedback loop between higher visual areas, such as the middle temporal visual area (MT), to which layer 6 neurons project.Citation44,Citation54,Citation58,Citation61 While there is no direct evidence for such a disynaptic circuit, this may help explain the handful of neurons found even further away than 16 mm. With our tracing approach, there is no way to rule out this alternative. Nevertheless, the documented distance of up to 8 mm for lateral axon projections of layer 6 Meynert cellsCitation47 correlates well with a disynaptic explanation of the ~16 mm we observed in layer 6.
The significance of this very-long-range disynaptic circuit is that, through V1 alone, the full extent of the extra-classical surround is covered. That is, while 3–4 mm-long projections make up the majority of the horizontal connections and can be tied only to the small, “near” component of the extraclassical surround (seeCitation14–Citation16), the longer projections of up to 8 mm reported for layer 6 Meynert cells,Citation47 and the very long disynaptic projections we find here, cover up to four times that of the near surround. Accordingly, these disynaptic projections covered 2°–20° of the visual field (), comparable to the visual field range covered by feedback.Citation14 This is not to say that feedback connections do not also play a role in surround modulation of V1 neurons. There is both a fastCitation62,Citation63 and a slowerCitation19,Citation27,Citation63 emerging component of the surround. Long-range lateral connections are unmyelinated, therefore the signal transmits too slowlyCitation64–Citation66 (~0.1–0.2 mm/millisecond) to account for early effects,Citation62 although the larger-diameter axons of Meynert cellsCitation61 could potentially propagate signals faster. Surround effects within the first 20 milliseconds are most likely mediated through faster feedforwardCitation63,Citation67 and feedback circuits.Citation14,Citation15,Citation62 In contrast, the slower surround component builds in strength over 70 milliseconds or more and is consistent with long-range lateral connections arising from mono- or disynaptic distances over 7 mm.Citation19
Consistent with our anatomical results, neurophysiological recordings have found that some layer 6 neurons in cat and monkey V1 integrate across a wider visual field representation than other neurons in more superficial layers.Citation25,Citation27,Citation68–Citation72 For example, we recently showed that ~20% of infragranular layer neurons respond maximally to drifting grating apertures as large as 30° – significantly larger than other neurons within the same cortical columns by more than 3 times (see Liu et alCitation27). The cells’ preference for this large stimulus was a result of a facilitative surround that emerged slowly, over a 100-millisecond time window, more consistent with slower propagation speeds of long-range lateral connections than faster feedback from higher visual areas. Within layer 6, therefore, long-range disynaptic connections between excitatory Meynert cells may be feeding this slowly emerging facilitative surround. In contrast, this integration across visual space in layer 6 may lead to later emerging suppression for neurons found in other layers.Citation27 For example, when layer 6 neurons in cat and mouse V1 are inactivated, this leads to a net excitatory effect on more superficial neurons;Citation73–Citation76 however, others have found the opposite effect for certain superficial layer cell types, such as hypercomplex cells,Citation77 in which responses are attenuated by layer 6 inactivation. Exactly what type of impact layer 6 has on different superficial cell types, and whether or not these ascending circuits are a part of this very-long-range lateral network we observed in macaque monkey V1, remains to be determined.
In a wide range of species, V1 neurons in layer 6 consist of several subtypes and are involved in numerous circuits, providing inputs to the lateral geniculate nucleus, pulvinar, claustrum, the reticular formation, superficial cortical layers within the cortical column, and higher visual areas (reviewed ThomsonCitation78). While several of these cell types are likely to be involved in long-range lateral connections, Meynert cells may play a highly specialized role. In primates, for example, Meynert cells are the only layer 6 cells that project to MT,Citation44,Citation58 providing direction-selective inputsCitation79 to this motion-sensitive region.Citation80,Citation81 Though not demonstrated in the same neurons, the direction selectivity of Meynert cellsCitation79 and their potential to integrate over a wide expanse of the visual fieldCitation47 has led some to speculate that such cells serve as an anatomical substrate for predator detection.Citation82 Consistent with this idea, many of the same MT-projecting Meynert cells also send axon collaterals to the superior colliculusCitation44,Citation58 that are likely to contribute to visually guided search behavior. In addition, layer 6 Meynert cells are implicated in the most direct feedforward relay of magnocellular and parvocellular inputs from the LGN to the MT.Citation54 This convergence of magno- and parvocellular inputs early in V1, and subsequent direct projection to the MT, may be instrumental in relaying a quick signal that covers a more complete range of spatial, temporal, luminance, and chromatic contrasts provided by the two LGN cell types, thus enhancing the effectiveness of a global early warning system.
Acknowledgments
We thank Dr Ed Callaway and the Salk Institute for generously providing the monkey and laboratory space for the rabies virus injection; Dr Jonathan Nassi for assistance with virus injections; and Dr Donald Lodmell for providing the CVS-11 strain of rabies virus and antibodies to the rabies nucleocapsid.
Disclosure
The authors report no conflicts of interest in this work.
References
- GilbertCDWieselTNClustered intrinsic connections in cat visual cortexJ Neurosci198335111611336188819
- LyonDCJainNKaasJHCortical connections of striate and extrastriate visual areas in tree shrewsJ Comp Neurol199840111091289802703
- RocklandKSAnatomical organization of primary visual cortex (area 17) in the ferretJ Comp Neurol198524122252364067016
- RocklandKSLundJSWidespread periodic intrinsic connections in the tree shrew visual cortexScience19822154539153215347063863
- RocklandKSLundJSIntrinsic laminar lattice connections in primate visual cortexJ Comp Neurol198321633033186306066
- YoshiokaTBlasdelGGLevittJBLundJSRelation between patterns of intrinsic lateral connectivity, ocular dominance, and cytochrome oxidase-reactive regions in macaque monkey striate cortexCereb Cortex1996622973108670658
- BoskingWHZhangYSchofieldBFitzpatrickDOrientation selectivity and the arrangement of horizontal connections in tree shrew striate cortexJ Neurosci1997176211221279045738
- GilbertCDWieselTNColumnar specificity of intrinsic horizontal and corticocortical connections in cat visual cortexJ Neurosci198997243224422746337
- MalachRAmirYHarelMGrinvaldARelationship between intrinsic connections and functional architecture revealed by optical imaging and in vivo targeted biocytin injections in primate striate cortexProc Natl Acad Sci U S A1993902210469104738248133
- RocklandKSLundJSHumphreyALAnatomical binding of intrinsic connections in striate cortex of tree shrews (Tupaia glis)J Comp Neurol1982209141587119173
- StettlerDDDasABennettJGilbertCDLateral connectivity and contextual interactions in macaque primary visual cortexNeuron200236473975012441061
- Ts’oDYGilbertCDWieselTNRelationships between horizontal interactions and functional architecture in cat striate cortex as revealed by cross-correlation analysisJ Neurosci198664116011703701413
- WelikyMKandlerKFitzpatrickDKatzLCPatterns of excitation and inhibition evoked by horizontal connections in visual cortex share a common relationship to orientation columnsNeuron19951535415527546734
- AngelucciALevittJBWaltonEJHupeJMBullierJLundJSCircuits for local and global signal integration in primary visual cortexJ Neurosci200222198633864612351737
- CavanaughJRBairWMovshonJANature and interaction of signals from the receptive field center and surround in macaque V1 neuronsJ Neurophysiol20028852530254612424292
- Hashemi-NezhadMLyonDCOrientation tuning of the suppressive extraclassical surround depends on intrinsic organization of V1Cereb Cortex201222230832621666124
- SceniakMPRingachDLHawkenMJShapleyRContrast’s effect on spatial summation by macaque V1 neuronsNat Neurosci19992873373910412063
- DasAGilbertCDTopography of contextual modulations mediated by short-range interactions in primary visual cortexNature1999399673765566110385116
- LiuYJHashemi-NezhadMLyonDCSharper orientation tuning of the extraclassical suppressive-surround due to a neuron’s location in the V1 orientation map emerges late in timeNeuroscience201322910011723159311
- NauhausIBenucciACarandiniMRingachDLNeuronal selectivity and local map structure in visual cortexNeuron200857567367918341988
- GilbertCDHorizontal integration and cortical dynamicsNeuron1992911131632964
- SerièsPLorenceauJFrégnacYThe “silent” surround of V1 receptive fields: theory and experimentsJ Physiol Paris2003974–645347415242657
- SillitoAMGrieveKLJonesHECudeiroJDavisJVisual cortical mechanisms detecting focal orientation discontinuitiesNature199537865564924967477405
- ZipserKLammeVASchillerPHContextual modulation in primary visual cortexJ Neurosci19961622737673898929444
- DeAngelisGCFreemanRDOhzawaILength and width tuning of neurons in the cat’s primary visual cortexJ Neurophysiol19947113473748158236
- LevittJBLundJSContrast dependence of contextual effects in primate visual cortexNature1997387662873769139823
- LiuYJHashemi-NezhadMLyonDCDynamics of extraclassical surround modulation in three types of V1 neuronsJ Neurophysiol201110531306131721228302
- SengpielFSenABlakemoreCCharacteristics of surround inhibition in cat area 17Exp Brain Res199711622162289348122
- ShushruthSNurminenLBijanzadehMIchidaJMVanniSAngelucciADifferent orientation tuning of near- and far-surround suppression in macaque primary visual cortex mirrors their tuning in human perceptionJ Neurosci201333110611923283326
- AhmedBAndersonJCDouglasRJMartinKANelsonJCPolyneuronal innervation of spiny stellate neurons in cat visual cortexJ Comp Neurol1994341139498006222
- AndersonJCDouglasRJMartinKANelsonJCSynaptic output of physiologically identified spiny stellate neurons in cat visual cortexJ Comp Neurol1994341116248006220
- HirschJAGilbertCDSynaptic physiology of horizontal connections in the cat’s visual cortexJ Neurosci1991116180018091675266
- KisvárdayZFTóthERauschMEyselUTOrientation-specific relationship between populations of excitatory and inhibitory lateral connections in the visual cortex of the catCereb Cortex1997776056189373017
- AngelucciABressloffPCContribution of feedforward, lateral and feedback connections to the classical receptive field center and extra-classical receptive field surround of primate V1 neuronsProg Brain Res20061549312017010705
- AngelucciABullierJReaching beyond the classical receptive field of V1 neurons: horizontal or feedback axons?J Physiol Paris2003972–314115414766139
- BullierJHupéJMJamesACGirardPThe role of feedback connections in shaping the responses of visual cortical neuronsProg Brain Res200113419320411702544
- LevittJBLundJSThe spatial extent over which neurons in macaque striate cortex pool visual signalsVis Neurosci200219443945212511077
- CantoneGXiaoJMcFarlaneNLevittJBFeedback connections to ferret striate cortex: direct evidence for visuotopic convergence of feedback inputsJ Comp Neurol2005487331233115892103
- SchwabeLIchidaJMShushruthSMangapathyPAngelucciAContrast-dependence of surround suppression in Macaque V1: experimental testing of a recurrent network modelNeuroimage201052377779220079853
- AndersonJCMartinACThe synaptic connections between cortical areas V1 and V2 in macaque monkeyJ Neurosci20092936112831129319741135
- JohnsonPBurkhalterAMicrocircuitry of forward and feedback connections within rat visual cortexJ Comp Neurol199636833833988725346
- LiuYJEhrengruberMUNegwerMShaoHJCetinAHLyonDCTracing inputs to inhibitory or excitatory neurons of mouse and cat visual cortex with a targeted rabies virusCurr Biol201323181746175523993841
- FriesWDistelHLarge layer VI neurons of monkey striate cortex (Meynert cells) project to the superior colliculusProc R Soc Lond B Biol Sci1983219121453596137827
- FriesWKeizerKKuypersHGLarge layer VI cells in macaque striate cortex (Meynert cells) project to both superior colliculus and prestriate visual area V5Exp Brain Res19855836136163839191
- HofPRGlezerIINimchinskyEAErwinJMNeurochemical and cellular specializations in the mammalian neocortex reflect phylogenetic relationships: evidence from primates, cetaceans, and artiodactylsBrain Behav Evol200055630031010971015
- HofPRUngerleiderLGWebsterMJNeurofilament protein is differentially distributed in subpopulations of corticocortical projection neurons in the macaque monkey visual pathwaysJ Comp Neurol199637611121278946287
- RocklandKSKnutsonTAxon collaterals of Meynert cells diverge over large portions of area V1 in the macaque monkeyJ Comp Neurol2001441213414711745640
- KellyRMStrickPLRabies as a transneuronal tracer of circuits in the central nervous systemJ Neurosci Methods20001031637111074096
- LyonDCNassiJJCallawayEMA disynaptic relay from superior colliculus to dorsal stream visual cortex in macaque monkeyNeuron201065227027920152132
- MorimotoKHooperDCCarbaughHFuZFKoprowskiHDietz-scholdBRabies virus quasispecies: implications for pathogenesisProc Natl Acad Sci U S A1998956315231569501231
- UgoliniGUse of rabies virus as a transneuronal tracer of neuronal connections: implications for the understanding of rabies pathogenesisDev Biol (Basel)200813149350618634512
- KellyRMStrickPLCerebellar loops with motor cortex and prefrontal cortex of a nonhuman primateJ Neurosci200323238432844412968006
- LyonDCRabideauCLack of robust LGN label following transneuronal rabies virus injections into macaque area V4J Comp Neurol2012520112500251122237967
- NassiJJLyonDCCallawayEMThe parvocellular LGN provides a robust disynaptic input to the visual motion area MTNeuron200650231932716630841
- Wong-RileyMChanges in the visual system of monocularly sutured or enucleated cats demonstrable with cytochrome oxidase histochemistryBrain Res197917111128223730
- BrodmannKVergleichende Lokalisationslehre der Grosshirnrinde [Comparative Localization Theory of the Cerebral Cortex]LeipzigBarth1909 German
- LyonDCMorphological approaches to the anatomical dissection of neuronal circuitsFellinTHalassaMNeuronal Network Analysis: Concepts and Experimental ApproachesNew YorkSpringer Science Humana Press2012375389
- NhanHLCallawayEMMorphology of superior colliculus- and middle temporal area-projecting neurons in primate primary visual cortexJ Comp Neurol20125201528021674487
- Van EssenDCNewsomeWTMaunsellJHThe visual field representation in striate cortex of the macaque monkey: asymmetries, anisotropies, and individual variabilityVision Res19842454294486740964
- WellerREKaasJHRetinotopic patterns of connections of area 17 with visual areas V-II and MT in macaque monkeysJ Comp Neurol198322032532796315784
- RocklandKSBistratified distribution of terminal arbors of individual axons projecting from area V1 to middle temporal area (MT) in the macaque monkeyVis Neurosci1989321551702487098
- BairWCavanaughJRMovshonJATime course and time-distance relationships for surround suppression in macaque V1 neuronsJ Neurosci200323207690770112930809
- WebbBSDhruvNTSolomonSGTailbyCLenniePEarly and late mechanisms of surround suppression in striate cortex of macaqueJ Neurosci20052550116661167516354925
- BringuierVChavaneFGlaeserLFrégnacYHorizontal propagation of visual activity in the synaptic integration field of area 17 neuronsScience199928354026956999924031
- GirardPHupéJMBullierJFeedforward and feedback connections between areas V1 and V2 of the monkey have similar rapid conduction velocitiesJ Neurophysiol20018531328133111248002
- GrinvaldALiekeEEFrostigRDHildesheimRCortical point-spread function and long-range lateral interactions revealed by real-time optical imaging of macaque monkey primary visual cortexJ Neurosci1994145 Pt 1254525688182427
- AlittoHJUsreyWMOrigin and dynamics of extraclassical suppression in the lateral geniculate nucleus of the macaque monkeyNeuron20081057113514618184570
- BolzJGilbertCDThe role of horizontal connections in generating long receptive fields in the cat visual cortexEur J Neurosci19891326326812106157
- GilbertCDLaminar differences in receptive field properties of cells in cat primary visual cortexJ Physiol19772682391421874916
- HenryCAJoshiSXingDShapleyRMHawkenMJFunctional characterization of the extraclassical receptive field in macaque V1: contrast, orientation, and temporal dynamicsJ Neurosci201333146230624223554504
- SceniakMPHawkenMJShapleyRVisual spatial characterization of macaque V1 neuronsJ Neurophysiol20018551873188711353004
- ShushruthSIchidaJMLevittJBAngelucciAComparison of spatial summation properties of neurons in macaque V1 and V2J Neurophysiol200910242069208319657084
- AllisonJDCasagrandeVABondsABThe influence of input from the lower cortical layers on the orientation tuning of upper layer V1 cells in a primateVis Neurosci19951223093207786852
- BolzJGilbertCDGeneration of end-inhibition in the visual cortex via interlaminar connectionsNature198632060603623653960119
- EydingDMacklisJDNeubacherUFunkeKWörgötterFSelective elimination of corticogeniculate feedback abolishes the electroencephalogram dependence of primary visual cortical receptive fields and reduces their spatial specificityJ Neurosci200323187021703312904463
- OlsenSRBortoneDSAdesnikHScanzianiMGain control by layer six in cortical circuits of visionNature20124837387475222367547
- GrieveKLSillitoAMA re-appraisal of the role of layer VI of the visual cortex in the generation of cortical end inhibitionExp Brain Res19918735215291783022
- ThomsonAMNeocortical layer 6, a reviewFront Neuroanat201041320556241
- MovshonJANewsomeWTVisual response properties of striate cortical neurons projecting to area MT in macaque monkeysJ Neurosci19961623773377418922429
- AlbrightTDDesimoneRGrossCGColumnar organization of directionally selective cells in visual area MT of the macaqueJ Neurophysiol198451116316693933
- FellemanDJKaasJHReceptive-field properties of neurons in middle temporal visual area (MT) of owl monkeysJ Neurophysiol19845234885136481441
- SherwoodCCLeePWRivaraCBEvolution of specialized pyramidal neurons in primate visual and motor cortexBrain Behav Evol2003611284412626860
- LyonDCKaasJHEvidence for a modified V3 with dorsal and ventral halves in macaque monkeysNeuron200233345346111832231
- HasslerRComparative anatomy in the central visual systems in day-and night-active primatesHasslerRStephanHEvolution of the ForebrainSpringer Science + Business MediaNew York1966419434
