Abstract
The mammalian neocortex features distinct anatomical variation in its tangential and radial extents. This review consolidates previously published findings from our group in order to compare and contrast the spatial profile of neural activity coherence across these distinct cortical dimensions. We focus on studies of ongoing local field potential (LFP) data obtained simultaneously from multiple sites in the primary visual cortex in two types of experiments in which electrode contacts were spaced either along the cortical surface or at different laminar positions. These studies demonstrate that across both dimensions the coherence of ongoing LFP fluctuations diminishes as a function of interelectrode distance, although the nature and spatial scale of this falloff is very different. Along the cortical surface, the overall LFP coherence declines gradually and continuously away from a given position. In contrast, across the cortical layers, LFP coherence is discontinuous and compartmentalized as a function of depth. Specifically, regions of high LFP coherence fall into discrete superficial and deep laminar zones, with an abrupt discontinuity between the granular and infragranular layers. This spatial pattern of ongoing LFP coherence is similar when animals are at rest and when they are engaged in a behavioral task. These results point to the existence of partially segregated laminar zones of cortical processing that extend tangentially within the laminar compartments and are thus oriented orthogonal to the cortical columns. We interpret these electrophysiological observations in light of the known anatomical organization of the cortical microcircuit.
Introduction
The adoption of the Latin “cortex” to describe the outer gray matter covering of the brain paints this structure as a thin bark or rind enveloping the remainder of the brain.Citation1 The cerebral cortex, however, is not a homogeneous, two-dimensional mantle. Rather, the cortex is a multilayered sheet with rich laminar interconnections.Citation2–Citation6 Accordingly, there is a notable spatial anisotropy in the radial (laminar) arrangement of cortical cell bodies and their projections. What is the consequence of this anatomical organization for neural activity patterns measured in the cortical microcircuit? For example, might the intrinsically generated, spontaneous activity be highly coordinated within a cortical column but relatively independent between cortical columns? Addressing this type of question requires simultaneous measurements at known spatial intervals in the cortex. Recent advances in neurophysiological techniques have exploited high-density multicontact recordings of extracellular electrical activity in vivoCitation7–Citation9 to facilitate comparison of neuronal signals collected simultaneously. This approach allows one to assess the spatial characteristics of neural coordination between known positions within the three-dimensional cortical extent.
In this review, we highlight key findings from our group that shed light on neuronal activity patterns within the three-dimensional volume of the cerebral cortex. We focus on primate visual cortex as a model sensory system with well described anatomy that has been the subject of a large body of neurophysiological studies. To contextualize current work, we first provide an overview of the cortex’s anatomical features as well as methods to assess spatial coordination of neural processes. We then briefly summarize neurophysiological data that demonstrate the fundamental anisotropy of neural coherence in the tangential versus radial dimensions of the cortex.
Distinct anatomical features in the radial and tangential dimensions
As early as the 19th century, neuroanatomists began to parcel the mammalian cortical sheet across the tangential dimension into distinct areas that differ in their cytoarchitecture.Citation10 These areas were subsequently shown to exhibit distinct patterns of connectivity and neuronal function. Some of the neocortical areas defined in these ways are conserved among primates and to some extent among other mammalian orders in their topological position and pattern of interareal projections.Citation11–Citation13 Within each cortical area, certain axonal projections do not enter the white matter, but instead remain in the neuropil. These “horizontal” connections, which are laterally extending branches of pyramidal cell axons,Citation14,Citation15 are marked by a high degree of functional specificity and often target neurons or columns with broadly similar response properties.Citation16 The extent of tangential cortical distance covered by these horizontal connections varies considerably between areas and increases at higher stages in the cortical hierarchy.Citation17 Whereas axons in the primary visual cortex (V1) may reach 2 mm,Citation18 those in the inferior temporal cortical area (TE) have been shown to project up to 9 mm.Citation19 Horizontal connections tend to be reciprocal,Citation20 commonly remain within their cortical layer,Citation21,Citation22 and are largely excitatory both in origin as well as in their postsynaptic targets.Citation23,Citation24 Functionally speaking, horizontal connections are mostly modulatory in nature,Citation25 and likely contribute to the high activity coherence measured over short distances in the cortex (see Measuring the reach of activity coherence in the cortex).
Early neuroanatomists also recognized the importance of the radial dimension to the composition of the cortex, with sheets of cells arranged in segregated layers that form early in development.Citation26,Citation27 Four to six cytoarchitectonic layers can be visualized with a variety of histological staining techniques (). The exact delineation of the respective layer boundaries depends somewhat on the type of histological stain used, the cortical areas examined, and the animal species.Citation28 The resulting variability in layer count has prompted the adoption of slightly different labeling schemes over the years,Citation29,Citation30 but broad consensus follows neuroanatomist Korbinian Brodmann’s original plan of dividing the neocortex into six major laminae.Citation10 These individual layers are further grouped into three visibly distinct laminar domains. Layer 4 and its various sublayers are commonly denoted as granular, since the high density of spiny cell bodies in this domain appears grainy in Nissl-type histological stains. Superficial layers 1–3 thus occupy a supragranular position, whereas deeper layers 5 and 6 are infragranular.Citation31
Figure 1 Neurophysiological measures within a cortical volume.
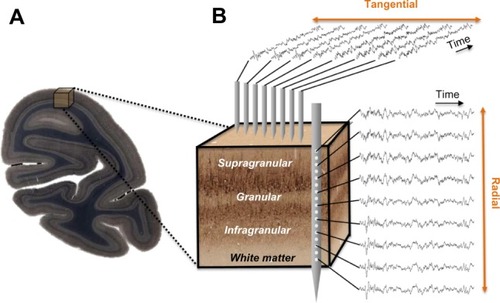
In contrast with the pronounced morphological differences between cortical layers, functional responses across layers are similar, and have given rise to the concept of a cortical column.Citation32–Citation36 The cortical column is conceptualized as a repeated motif of radial units, each supported by a stereotypical pattern of intrinsic connections,Citation37 the functional homogeneity of which is often considered a universal and critical feature of the cerebral cortex.Citation38 In the primary visual cortex, for example, the same basic orientation and eye preferences can be observed across layers of a given column.Citation39 Moreover, studies point to a basic model of sequential excitation across particular laminae within a column following the arrival of sensory information from the thalamus.Citation40,Citation41 This model suggests that thalamic input to cortical layer 4 is relayed to the supragranular layers, where it is integrated with various types of modulatory signals,Citation2,Citation42–Citation44 and then relayed to the infragranular layers. Infragranular neurons send local projections to granular and supragranular targets in the same cortical column, as well as long-range projections to cortical and subcortical structures inside and outside of the telencephalon.Citation45 While there are many ways in which information can be integrated across columns, and the generality of functional columns in the cortex remains a topic of debate,Citation46–Citation58 this idealized microcircuit model emphasizing intracolumnar processing has played an important role in understanding the complexity of cortical projection patterns and their bearing on measured sensory signals.Citation59,Citation60
To summarize, the anatomical organization of the cortex varies considerably across both the radial and tangential dimensions. Within a given area, horizontal connections account for much of the tangential connectivity, whereas the interlaminar projections account for the radial connectivity.
Measuring the reach of activity coherence in the cortex
The local field potential (LFP) represents a collective measure of neural activity, including subthreshold synaptic processes that are synchronized in space and time.Citation61–Citation66 Its inherent heterogeneity limits the precision of the LFP for probing details of microcircuit function.Citation67,Citation68 Nonetheless, empirical studies have shown that both sensory-evoked LFP responsesCitation69 and high frequency “gamma” range (>30 Hz) LFP powerCitation70,Citation71 are often well correlated with local spiking activity in the cortex. Thus, it is possible to use the LFP, and in particular its upper frequency range, to study neural coordination at different spatial scales. However, a conspicuous feature of the LFP signal is its 1/fβ spectral distribution, where f denotes frequency and β is the exponent of a power law.Citation72,Citation73 This means that the high frequency gamma range is at least one order of magnitude smaller in amplitude than the lower frequency fluctuations, which dominate the raw signal fluctuations.
Experimental approaches to measure the spatiotemporal coordination of neural signals involve simultaneous electrophysiological measurement from multiple electrodes (). Having obtained such simultaneous signals, most attempts to evaluate the interaction between sites amount to a measure of temporal correlation of two or more neural signals. To this end, neurophysiologists employ a wide range of correlational measures that will not be reviewed here. Here we describe one common measure, the mean squared coherence, which measures signal correlation as a function of frequency. Whether or not frequency is a natural domain across which to divide neural signals is a matter of debate, although this practice is very common. What is clear is that doing so allows one to isolate aspects of the LFP signal that correlate with particular behavioral or neural events, such as the close relationship between the local spiking and the low amplitude gamma range mentioned above. It is important to mention that the precision of LFP coherence as a measure of neural coordination is limited by electric volume conduction. In contrast with spiking activity, where the signal source can be identified as one or more nearby neurons, the LFP reflects an ambiguous combination of superimposed local and distant neural events.Citation65,Citation67
In the following section, we use mean squared coherence as a measure to assess the coherence across the different cortical dimensions. Of particular interest is how the pronounced anatomical anisotropy of the cortex described in the previous section influences the spatial coordination of neural activity across these different dimensions.
Distinct laminar sheets of coherent LFP
Given the columnar cortical architecture outlined above, one might predict that LFP coherence would also follow a columnar pattern. Would intrinsic LFP fluctuations show more coherence within a column than between columns? Interestingly, the data suggest nearly the opposite: the spatial stretch of LFP coordination is perpendicular to the cortical columns. The LFP is spatially coherent along zones extending tangentially, but is sharply discontinuous within a column.
Between electrodes spaced tangentially along the cortical surface, LFP coherence falls off continuously and in a frequency-dependent fashion. One study of the macaque visual cortex examined the coherence of ongoing activity across distances spanning from a few hundred microns to over a centimeter as monkeys rested idly in a dark room.Citation74 The main findings of that study were that LFP coherence falls off monotonically, and that the falloff is steeper for high frequency than for low frequency LFP components ( and ). A subsequent study showed that this falloff reflects the cortical distance rather than the absolute distance between the electrode contacts, since LFP coherence between electrodes placed on either side of a sulcus showed a drop reflecting the span of the infolded cortex.Citation75 This general pattern of coherence is qualitatively similar during rest and when monkeys perform a simple behavioral task (ie, when they are behaviorally engaged) and is comparable in amplitude and slope between areas V1, V2, and V4. The results are consistent with other findings that show steep (<5 mm) falloff in high frequency LFP and spikingCitation76 and more gradual (>5 mm) falloff in low frequency LFP coherence as a function of lateral cortical distance.Citation67,Citation69,Citation74,Citation77
Figure 2 LFP coherence as a function of tangential and radial distance.
Abbreviations: LFP, local field potential; sg, supragranular; g, granular; ig, infragranular.
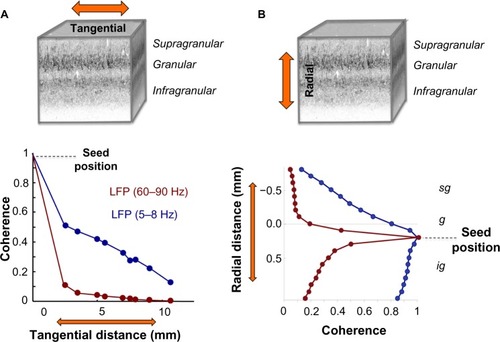
Figure 3 Volumetric profile of LFP coherence.
Abbreviations: LFP, local field potential; sg, supragranular; g, granular; ig, infragranular.
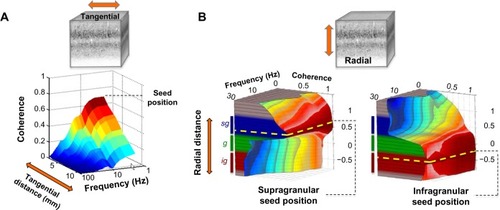
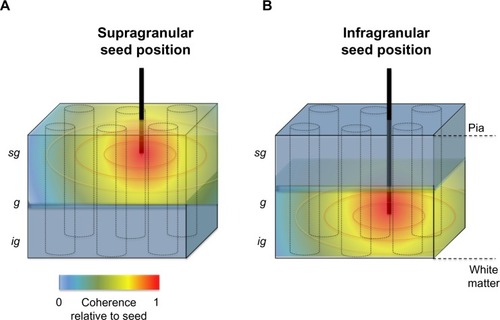
In the radial dimension, coherence has recently been assessed between contacts occupying different laminar falloff in LFP coherence along the cortical surface, the pattern of laminar coherence is discontinuous and strongly compartmentalized, particularly for frequency ranges below the gamma range ( and ).Citation78,Citation79 Probing the spatial pattern of coherence from different seeds reveals two prominent laminar zones of coherent LFP activity separated by the deep granular layer, with one zone dominating within the superficial layers (1–3) and another zone in the deep layers (5 and 6). While the LFP coherence within these zones was high, that between them was nearly zero across a wide frequency range (see ). This spatial structure was present in the ongoing activity both during rest and when the animals were engaged in a behavioral task. Taken together, the studies outlined above suggest that the spatial extent of ongoing LFP coherence spreads broadly in the tangential direction but is restricted in the radial direction. The resulting spatial pattern can be conceptualized as two laminar sheets of coherent activity (), which constitutes a marked deviation from the radial anisotropy predicted by the columnar architecture described above.
Figure 4 Schematic representation of the spatial anisotropy of LFP coherence along the tangential and radial dimensions of primate primary visual cortex.
Abbreviations: LFP, local field potential; sg, supragranular; g, granular; ig, infragranular.
The findings reviewed here point to an intricate three-dimensional structure of neural coherence that reflects a laminar compartmentalization emerging when the brain is not explicitly stimulated (with the animals being either at rest or behaviorally engaged). Neither known interlaminar connections nor innervation from the thalamus or other cortical areas can fully explain the strictly separate domains of intracortical coherenceCitation37,Citation80–Citation83 we observed in the upper and lower cortical layers. Nonetheless, it is interesting to speculate that in the absence of specific sensory input (ie, during periods of rest or between sensory events), cortical activity may switch from a “columnar mode”, where the sensory world is parsed by a large number of columnar units dedicated to extracting features of the world, to a “laminar mode”, where functional differences in the tangential direction become less important, and activity segregates by layer.
What might be the basis for this laminar segregation of intrinsic activity? One possibility is that it reflects a division between intracortical versus thalamocortical interactions, with one contributing most strongly to the upper layer fluctuations and the other to the lower layer fluctuations. While clearly a speculation at this point, this explanation would account for the apparently separate laminar sheets of ongoing cortical activity that are largely uncoordinated across their boundary in the granular layer.
At first sight, our observation of radially anisotropic ongoing activity seems at odds with the columnar structure of stimulus-evoked responses because of the apparent compartmentalization that has been reported in certain studies. However, several other neurophysiological phenomena have been described that exhibit similar laminar specificity. For example, neurophysiological recordings with multiple laminar electrodes inserted simultaneously into rat auditory cortex found that the spatial pattern of spiking activity within the supragranular layers was highly localized, sparse, and strongly specific to a small subset of sensory stimuli. In contrast, spiking within the infragranular layers was widespread, constant, and promiscuous with respect to sensory stimulation.Citation84 Furthermore, analysis of spontaneous LFP recorded in several visual areas found a peak of low frequency (∼10 Hz) coherence between LFP and multiunit spiking activity in the infragranular layers that was absent in the supragranular layers.Citation85 In contrast, the recently described repeating patterns of clustered cortical synchronization termed “neuronal avalanches” seem to be confined to the superficial layers of primate sensory cortex.Citation86 Stimulus-evoked LFP powerCitation87 and multiunit spiking activityCitation88 of macaque V1 have been demonstrated to exhibit pronounced anisotropies between upper and lower cortical layers. Moreover, reversible inactivation (cooling) of the supragranular layers of cat primary visual cortex had little influence, if any, on the response properties of infragranular neurons.Citation89,Citation90 Lastly, some authors have reported stark differences in orientation tuning between supragranular and infragranular neurons within the same cortical column of macaque visual cortex,Citation91–Citation93 which they interpreted as evidence for a functional dichotomy between deep and superficial layers.Citation94
These results, taken together with the coherence differences in LFP discussed above, hint at a strict functional division between the main laminar compartments of visual cortex. The laminar pattern of LFP activity described here thus may provide a unique window on the spatiotemporal structure of neuronal population activity that warrants further investigation into the relationship between structure and function within the cortical microcircuitry.
Acknowledgments
Authors would like to thank V Casagrande for comments on an earlier version of the manuscript as well as G Adams for his contributions to the figures. This research was supported in part by the Intramural Research Program of the NIMH. MAC is supported by a NSFGRF grant (DGE-0909667). AM is supported by grants from the Whitehall and Alfred P Sloan Foundation.
Disclosure
The authors report no conflicts of interest in this work.
References
- MonroAThe cortex of the encephalonAnat Nerves174214
- GilbertCDMicrocircuitry of the visual cortexAnnu Rev Neurosci1983612172476132585
- ShadlenMNNewsomeWTNoise, neural codes and cortical organizationCurr Opin Neurobiol1994445695797812147
- ReynoldsJHMapping the microcircuitry of attentionNat Neurosci200811886186218660837
- IzhikevichEMEdelmanGMLarge-scale model of mammalian thalamocortical systemsProc Natl Acad Sci U S A200810593593359818292226
- BastosAMUsreyWMAdamsRAMangunGRFriesPFristonKJCanonical microcircuits for predictive codingNeuron201276469571123177956
- LakatosPKarmosGMehtaADUlbertISchroederCEEntrainment of neuronal oscillations as a mechanism of attentional selectionScience2008320587211011318388295
- UlbertIHalgrenEHeitGKarmosGMultiple microelectrode-recording system for human intracortical applicationsJ Neurosci Methods20011061697911248342
- BuzsákiGLarge-scale recording of neuronal ensemblesNat Neurosci20047544645115114356
- BrodmannKLocalisation in the Cerebral CortexNew York, NY, USASpringer2007
- KaasJHThe evolution of brains from early mammals to humansWiley Interdiscip Rev Cogn Sci201341334523529256
- KaasJHEvolution of columns, modules, and domains in the neocortex of primatesProc Natl Acad Sci U S A2012109Suppl 1106551066022723351
- AlfanoCStuderMNeocortical arealization: evolution, mechanisms, and open questionsDev Neurobiol201373641144723239642
- GilbertCDWieselTNClustered intrinsic connections in cat visual cortexJ Neurosci198335111611336188819
- RocklandKSLundJSIntrinsic laminar lattice connections in primate visual cortexJ Comp Neurol198321633033186306066
- StettlerDDDasABennettJGilbertCDLateral connectivity and contextual interactions in macaque primary visual cortexNeuron200236473975012441061
- AmirYHarelMMalachRCortical hierarchy reflected in the organization of intrinsic connections in macaque monkey visual cortexJ Comp Neurol1993334119468408757
- AngelucciALevittJBWaltonEJHupeJ-MBullierJLundJSCircuits for local and global signal integration in primary visual cortexJ Neurosci200222198633864612351737
- TanigawaHWangQFujitaIOrganization of horizontal axons in the inferior temporal cortex and primary visual cortex of the macaque monkeyCereb Cortex200515121887189915758199
- AngelucciABullierJReaching beyond the classical receptive field of V1 neurons: horizontal or feedback axons?J Physiol Paris2003972–314115414766139
- MalachRAmirYHarelMGrinvaldARelationship between intrinsic connections and functional architecture revealed by optical imaging and in vivo targeted biocytin injections in primate striate cortexProc Natl Acad Sci U S A1993902210469104738248133
- YoshiokaTBlasdelGGLevittJBLundJSRelation between patterns of intrinsic lateral connectivity, ocular dominance, and cytochrome oxidase-reactive regions in macaque monkey striate cortexCereb Cortex1996622973108670658
- McGuireBAGilbertCDRivlinPKWieselTNTargets of horizontal connections in macaque primary visual cortexJ Comp Neurol199130533703921709953
- YoshimuraYSatoHImamuraKWatanabeYProperties of horizontal and vertical inputs to pyramidal cells in the superficial layers of the cat visual cortexJ Neurosci20002051931194010684894
- HirschJAGilbertCDSynaptic physiology of horizontal connections in the cat’s visual cortexJ Neurosci1991116180018091675266
- RakicPRadial unit hypothesis of neocortical expansionNovartis Found Symp2000228304210929315
- GoetzMBolzJFormation and preservation of cortical layers in slice culturesJ Neurobiol19922377838021431845
- DeFelipeJAlonso-NanclaresLArellanoJIMicrostructure of the neocortex: comparative aspectsJ Neurocytol2002313–529931612815249
- Billings-GagliardiSChan-PalayVPalaySLA review of lamination in area 17 of the visual cortex Macaca mulattaJ Neurocytol1974356196294142638
- Marín-PadillaMCajal-Retzius cells and the development of the neocortexTrends Neurosci199821264719498301
- ZekiSShippSThe functional logic of cortical connectionsNature198833561883113173047584
- MountcastleVBThe columnar organization of the neocortexBrain199712047017229153131
- LübkeJFeldmeyerDExcitatory signal flow and connectivity in a cortical column: focus on barrel cortexBrain Struct Funct2007212131717717695
- de NóRLCerebral cortex: architecture, intracortical connections, motor projectionsFultonJFPhysiology of the Nervous System3rd edOxford, UKOxford University Press1949
- HubelDHWieselTNUniformity of monkey striate cortex: a parallel relationship between field size, scatter, and magnification factorJ Comp Neurol197415832953054436457
- HubelDHWieselTNFerrier lecture. Functional architecture of macaque monkey visual cortexProc R Soc Lond B Biol Sci1977198113015920635
- CallawayEMLocal circuits in primary visual cortex of the macaque monkeyAnnu Rev Neurosci199821147749530491
- LundJSAngelucciABressloffPCAnatomical substrates for functional columns in macaque monkey primary visual cortexCereb Cortex2003131152412466211
- HubelDHWieselTNReceptive fields and functional architecture of monkey striate cortexJ Physiol196819512152434966457
- Bode-GreuelKMSingerWAldenhoffJBA current source density analysis of field potentials evoked in slices of visual cortexExp Brain Res19876912132193436389
- NowakLGMunkMHGirardPBullierJVisual latencies in areas V1 and V2 of the macaque monkeyVis Neurosci19951223713847786857
- RocklandKSPandyaDNLaminar origins and terminations of cortical connections of the occipital lobe in the rhesus monkeyBrain Res19791791320116716
- RocklandKSVirgaATerminal arbors of individual “Feedback” axons projecting from area V2 to V1 in the macaque monkey: a study using immunohistochemistry of anterogradely transported Phaseolus vulgaris-leucoagglutininJ Comp Neurol1989285154722754047
- AndersonJCMartinKAThe synaptic connections between cortical areas V1 and V2 in Macaque monkeyJ Neurosci20092936112831129319741135
- ThomsonAMBannisterAPInterlaminar connections in the neocortexCereb Cortex200313151412466210
- RockelAJHiornsRWPowellTPThe basic uniformity in structure of the neocortexBrain198010322212446772266
- DouglasRJMartinKAWhitteridgeDA canonical microcircuit for neocortexNeural Comput19891480488
- LundJSYoshiokaTLevittJBComparison of intrinsic connectivity in different areas of macaque monkey cerebral cortexCereb Cortex1993321481628490320
- RocklandKSComplex microstructures of sensory cortical connections199884545551
- SawaguchiTKubotaKA hypothesis on the primate neocortex evolution: column-multiplication hypothesisInt J Neurosci1986301–257643091521
- SilberbergGGuptaAMarkramHStereotypy in neocortical microcircuitsTrends Neurosci200225522723011972952
- Herculano-HouzelSCollinsCEWongPKaasJHLentRThe basic nonuniformity of the cerebral cortexProc Natl Acad Sci U S A200810534125931259818689685
- HaugHBrain sizes, surfaces, and neuronal sizes of the cortex cerebri: A stereological investigation of man and his variability and a comparison with some mammals (primates, whales, marsupials, insectivores, and one elephant)Am J Anat198718021261423673918
- RakicPConfusing cortical columnsProc Natl Acad Sci U S A200810534120991210018715998
- HortonJCAdamsDLThe cortical column: a structure without a functionPhilos Trans R Soc Lond B Biol Sci2005360145683786215937015
- NelsonSBCortical microcircuitsNeuron2002361192712367502
- Van HooserSDSimilarity and diversity in visual cortex: is there a unifying theory of cortical computation?Neuroscientist200713663965617911223
- SwindaleNVIs the cerebral cortex modular?Trends Neurosci199013124874921703679
- RocklandKSDrashGWCollateralized divergent feedback connections that target multiple cortical areasJ Comp Neurol199637345295488889943
- FellemanDJvan EssenDCDistributed hierarchical processing in the primate cerebral cortexCereb Cortex1991111471822724
- RaschMLogothetisNKKreimanGFrom neurons to circuits: linear estimation of local field potentialsJ Neurosci20092944137851379619889990
- ReimannMWAnastassiouCAPerinRHillSLMarkramHKochCA biophysically detailed model of neocortical local field potentials predicts the critical role of active membrane currentsNeuron201379237539023889937
- EinevollGTKayserCLogothetisNKPanzeriSModelling and analysis of local field potentials for studying the function of cortical circuitsNat Rev Neurosci2013141177078524135696
- BuzsákiGLogothetisNSingerWScaling brain size, keeping timing: evolutionary preservation of brain rhythmsNeuron201380375176424183025
- MitzdorfUCurrent source-density method and application in cat cerebral cortex: investigation of evoked potentials and EEG phenomenaPhysiol Rev1985651371003880898
- BuzsákiGAnastassiouCAKochCThe origin of extracellular fields and currents – EEG, ECoG, LFP and spikesNat Rev Neurosci201213640742022595786
- KajikawaYSchroederCEHow local is the local field potential?Neuron201172584785822153379
- ŁęskiSLindénHTetzlaffTPettersenKHEinevollGTFrequency dependence of signal power and spatial reach of the local field potentialPLoS Comput Biol201397e100313723874180
- KatznerSNauhausIBenucciABoninVRingachDLCarandiniMLocal origin of field potentials in visual cortexNeuron2009611354119146811
- LiuJNewsomeWTLiuJLocal field potential in cortical area MT: stimulus tuning and behavioral correlationsJ Neurosci200626307779779016870724
- RaySMaunsellJHDifferent origins of gamma rhythm and high- gamma activity in macaque visual cortexJ Neurosci201194e1000610
- FreemanWJNeurodynamicsLondon, UKSpringer2000
- BuzsákiGRhythms of the BrainOxford, UKOxford University Press2006
- LeopoldDAMurayamaYLogothetisNVery slow activity fluctuations in monkey visual cortex: implications for functional brain imagingCereb Cortex200313442243312631571
- LeopoldDALogothetisNKSpatial patterns of spontaneous local field activity in the monkey visual cortexRev Neurosci2003141–219520512929926
- SmithMAKohnASpatial and temporal scales of neuronal correlation in primary visual cortexJ Neurosci20082848125911260319036953
- CanoltyRTSpatiotemporal dynamics of word processing in the human brainFront Neurosci20071118519618982128
- HarrisKDThieleACortical state and attentionNat Rev Neurosci201112950952321829219
- MaierAAdamsGKAuraCLeopoldDADistinct superficial and deep laminar domains of activity in the visual cortex during rest and stimulationFront Syst Neurosci201043111120204156
- LundJSAnatomical organization of macaque monkey striate visual cortexAnnu Rev Neurosci19881112532883284442
- YamamoriTRocklandKSNeocortical areas, layers, connections, and gene expressionNeurosci Res2006551112716546282
- MarionRLiKPurushothamanGJiangYCasagrandeVAMorphological and neurochemical comparisons between pulvinar and V1 projections to V2J Comp Neurol2013521481383222826174
- KätzelDZemelmanBVBuetferingCWölfelMMiesenböckGThe columnar and laminar organization of inhibitory connections to neocortical excitatory cellsNat Neurosci201114110010721076426
- SakataSHarrisKDLaminar structure of spontaneous and sensory-evoked population activity in auditory cortexNeuron200964340441819914188
- BollimuntaAChenYSchroederCENeuronal mechanisms of cortical alpha oscillations in awake-behaving macaquesJ Neurosci200828409976998818829955
- PetermannTThiagarajanTCLebedevMANicolelisMAChialvoDRPlenzDSpontaneous cortical activity in awake monkeys composed of neuronal avalanchesProc Natl Acad Sci U S A200910637159211592619717463
- MaierAAuraCJLeopoldDAInfragranular sources of sustained local field potential responses in macaque primary visual cortexJ Neurosci20113161971198021307235
- XingDYehC-IBurnsSLaminar analysis of visually evoked activity in the primary visual cortexProc Natl Acad Sci U S A201210934138711387622872866
- WeyandTGMalpeliJGLeeCSchwarkHDCat area 17. III. Response properties and orientation anisotropies of corticotectal cellsJ Neurophysiol1986564108811013783231
- SchwarkHDMalpeliJGWeyandTGLeeCCat area 17. II. Response properties of infragranular layer neurons in the absence of supragranular layer activityJ Neurophysiol1986564107410873783230
- BauerRDowDBSnyderAZVautinROrientation shift between upper and lower layers in monkey visual cortexExp Brain Res19835011331456416881
- BauerRDowDBVautinRGLaminar distribution of preferred orientations in foveal striate cortex of the monkeyExp Brain Res198041154606780372
- BauerRDowBMComplementary global maps for orientation coding in upper and lower layers of the monkey’s foveal striate cortexExp Brain Res19897635035092792244
- MurphyPCMurphySillitoAMContinuity of orientation columns between superficial and deep laminae of the cat primary visual cortexJ Physiol19863811951103625547
