Abstract
Prosopagnosia is a selective visual agnosia characterized by the inability to recognize the identity of faces. There are both acquired forms secondary to brain damage and developmental forms without obvious structural lesions. In this review, we first discuss the diagnosis of acquired and developmental prosopagnosia, and the challenges present in the latter case. Second, we discuss the evidence regarding the selectivity of the prosopagnosic defect, particularly in relation to the recognition of other objects, written words (another visual object category requiring high expertise), and voices. Third, we summarize recent findings about the structural and functional basis of prosopagnosia from studies using magnetic resonance imaging, functional magnetic resonance imaging, and event-related potentials. Finally, we discuss recent attempts at rehabilitation of face recognition in prosopagnosia.
Introduction
Face recognition is usually effortless and rapid. In different places and times, despite changes in expression, hairstyle, and clothing, we easily recognize colleagues, friends, and family. Our visual expertise with faces likely exceeds that for any other type of object, and this ability to identify people is a cornerstone of our social interactions as human beings. Subjects with prosopagnosia, however, cannot recognize that they have seen a face before, an impairment that affects both faces well known to them and those recently encountered. This is not due to more general problems with vision, object recognition, or memory. The term “impaired face recognition” should be used rather than “prosopagnosia” when this symptom is part of a broader problem, as with macular degeneration, general memory problems in Alzheimer’s disease, and cognitive issues in schizophrenia, for example. These subjects realize that a face is a face and not a car or a tree, but simply cannot say whether they have seen it before or whose face it is. These subjects rely on other cues to identity, such as hairstyle, gait, or voice, and make mistakes if these cues change (eg, hairstyle). They relate surprising and sometimes embarrassing stories, such as not recognizing themselves in a mirror, or walking past siblings or spouses as if they were strangers.
Prosopagnosia can be either acquired or developmental. In acquired prosopagnosia, poor face recognition is the result of brain injury. While the first case of acquired prosopagnosia was reported 150 years ago,Citation1,Citation2 the modern study of this condition began with Bodamer’sCitation3 report in 1947, which described impaired face recognition in wounded soldiers. Subsequently, it has been recognized that acquired prosopagnosia can arise from many different pathologies, including trauma, stroke, encephalitis, tumors, degenerative atrophy, or temporal lobe resections.Citation4
Developmental prosopagnosia has been more recently described and is less well understood. Subjects with this condition fail to develop face recognition skills despite otherwise normal vision and memory, and do not have obvious lesions on brain imaging.Citation5,Citation6 Developmental prosopagnosia may have a genetic basis. It can run in families, with some pedigrees showing as many as ten affected members across two generations,Citation7–Citation9 observations that parallel findings that normal face recognition skills also have a heritable component, with monozygotic twins having more similar face recognition abilities than dizygotic twins.Citation10,Citation11 While the acquired form is rare, the developmental form may be relatively common. Some suggest that as many as 2.5% of the population has developmental prosopagnosia,Citation12,Citation13 although this number will vary with the statistical criteria used and may confound those subjects with a developmental problem with those on the low end of normal face recognition ability (see Barton and CorrowCitation14 for a discussion on the prevalence and diagnosis of developmental prosopagnosia).
Prosopagnosia has significant implications for those who have it. Adults with developmental prosopagnosia often report that their failure to recognize others creates traumatic social experiences, leading to chronic anxiety, feelings of embarrassment and guilt, and a limited social circle.Citation15 Our subjects with acquired prosopagnosia acknowledge similar difficulties. Children with developmental prosopagnosia and their parents describe the same problems, but with additional implications for the school environment and safety.Citation16
Models of face recognition
Face recognition is a multistage process ending with the identification of a person. These stages are reflected in cognitive models of face recognition, the most influential being that of Bruce and YoungCitation17 (). Each box in the model represents a distinct cognitive process: while it is not necessary that these different stages occur in separate anatomic structures, some neuroanatomic models suggest that this may be the case.Citation4,Citation18 The model begins with creating a “facial percept”, the encoding of the structural information about the face. This percept is matched to stores of face memories, termed “face recognition units”, to determine whether the face has been seen before. Some argue that a correct match at this stage produces a feeling of familiarity with the face.Citation19–Citation21 A correct match also activates a “person identity node”, which allows access to semantic information and the name of the person to whom the face belongs. This model continues to be useful and has been elaborated to incorporate parallel sources of information from other cues (eg, voice),Citation22,Citation23 hemispheric lateralization of these cues,Citation20 and more extensive bidirectional influences between modules.Citation19,Citation22–Citation25
Figure 1 Adaption of the Bruce and Young model.
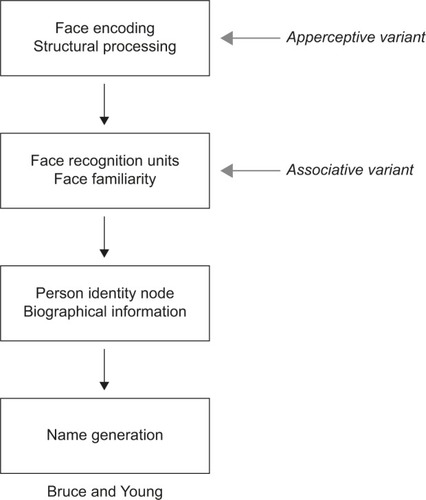
These models are reflected in our concepts about prosopagnosia. There are functional variants that may correspond to dysfunction of different cognitive stages.Citation26 Impairments in the ability to see differences between faces, or their structures, suggest an “apperceptive variant”, a failure in encoding the facial percept. Other prosopagnosic subjects can perceive facial structure accurately but on tests of facial imagery cannot recall the faces of familiar people, indicating an “associative or amnestic variant”. However, this is a relative rather than absolute dichotomy: subtle defects in face perception can be seen in patients with an associative variant,Citation27–Citation30 while those with an apperceptive variant have milder deficits on face imagery tests.Citation4 Nonetheless, this distinction remains useful, and these variants have distinct neural correlates (see “Neuroimaging” section).Citation4
Diagnosis
Tests of face familiarity
The hallmark of prosopagnosia is the reduced ability of subjects to realize that they have seen a face before: hence, key diagnostic tests probe the sense of familiarity for previously seen faces. Earlier tests of face recognition may have been less sensitive because their stimuli could allow subjects to use alternative strategies to circumvent poor face recognition, such as remembering hairstyles and clothing.Citation31,Citation32 Newer tests have addressed those limitations by minimizing those extraneous cues. The most commonly used test of familiarity for recently viewed faces is the Cambridge Face Memory Test (CFMT),Citation33 a test with high internal reliability.Citation34 While the original version of this test used only adult Caucasian faces, other versions have been created, such as the CFMT-Chinese,Citation35 CFMT-Australian,Citation36 and pediatric versions, such as the CFMT-CCitation37 and the CFMT-Kids.Citation38
Tests that use anonymous faces like the CFMT have the advantage that, as none of the faces are familiar to subjects prior to learning, all subjects taking the test have the same degree of short-term familiarity with the faces seen during the test. Tests of familiarity for famous faces are also used, but such tests depend on the person having seen those celebrities before, and are therefore affected by age, education, and cultural background. In prosopagnosic subjects, this can be compounded by the fact that these subjects may lose interest in films and television because they cannot keep track of the characters, thus limiting their exposure to newer celebrities.
Tests of face perception
Tests of face perception – that is, the ability to perceive differences between faces – do not establish the diagnosis of prosopagnosia. What they can do is demonstrate if prosopagnosia is due to impaired encoding of the facial structure, and therefore is an apperceptive variant, or if such encoding is intact, which would point to an associative variant. Deficits in face perception have been measured by the Cambridge Face Perception TestCitation39 and the Glasgow Face Matching Test,Citation40 which involve sorting or matching faces by their identity with minimal demands on memory. The Dartmouth Face Perception Test is useful for children.Citation41
Questionnaires of social impact
Questionnaires can evaluate everyday experiences with face recognition. There is a 15-item self-report questionnaireCitation13 that contains questions on face recognition, attractiveness judgments, and expression recognition, and a more recent 20-item questionnaire for face identity (Prosopagnosia Index, “PI20”,Citation42). However, these should be supplemented by objective tests for diagnosis.
Exclusionary tests
Establishing impaired face recognition is not sufficient for the diagnosis of prosopagnosia. One must also show that this is not due to more general problems with vision and memory. The assessment of acuity and visual fields can exclude low-level impairments of vision as a cause of poor face recognition: indeed one of the problems of subjects with macular degeneration is difficulty recognizing faces.Citation43 Beyond this, to exclude a more general visual agnosia, subjects with prosopagnosia should have normal object recognition at a “basic” level (ie, identifying that an object is a face, a bicycle, a lamp, etc). Some may have difficulties identifying specific examples of these objects (ie, which bicycle or which lamp): this is not grounds for rejecting a diagnosis of prosopagnosia, but is relevant to the debate about whether the recognition problem in prosopagnosia is truly specific for faces alone (see “Face Specificity” section). For this reason, challenging tests of object recognition that include measures of reaction time and premorbid expertiseCitation44 are useful (see “Objects” section).
Finally, face identity recognition deficits can occur in the context of other disorders, and the diagnostic process should consider whether any of these are present. In children, this includes conditions such as autismCitation45–Citation48 and Turner’s syndrome,Citation49 while in adults impaired face recognition has been reported in schizophrenia,Citation50,Citation51 Alzheimer’s disease,Citation52–Citation54 and Parkinson’s disease,Citation55 for example. The diagnosis of prosopagnosia should be reserved for cases in which poor face recognition cannot be explained by one of these other conditions. Suggested criteria for the diagnosis of acquired and developmental prosopagnosia are outlined in . Greater detail regarding guidelines and available tests can be found in a recent review.Citation56
Table 1 Suggested inclusion and exclusion criteria for the diagnosis of acquired and developmental prosopagnosia
Face specificity
Are prosopagnosic subjects impaired in the recognition of faces only? Here, we comment on four aspects of this question about specificity. First, a long-standing debate in face research is whether the mechanisms used to process faces are dedicated to faces alone, ie, “face specific”, or if they are involved in processing other objects, particularly those for which we possess perceptual expertise.Citation57–Citation59 Second, new theories have proposed that words and faces, two visual classes for which literate humans have great expertise, share and compete for resources, leading to predictions that prosopagnosic subjects may have subtle deficits in word processing.Citation60,Citation61 Third, questions have arisen as to whether some prosopagnosic subjects may actually have a multimodal problem in recognizing people.Citation19,Citation62,Citation63 If so, they should also have impairment of recognition of people by voice and name; however, voice recognition has seldom been objectively evaluated in prosopagnosia. Finally, an issue of less theoretical but some practical interest is the array of other visual deficits that likely reflect damage to neighboring structures and networks, particularly with acquired prosopagnosia.
Objects
All objects share visual processing in the striate and early extrastriate cortex: whether the processing of faces and objects diverges later is the question. Neuroimaging studies of healthy individuals show that face processing depends on a cortical network of regions that is partially overlapping but distinct from areas involved in object processing.Citation64–Citation67 Transcranial magnetic stimulation has demonstrated a double dissociation between face and object processing: stimulation of face areas interrupts face processing more than object processing and stimulation of object areas results in the reverse.Citation68
The contribution of prosopagnosia research to this debate is mixed. While there are studies that report intact ability to distinguish between members of other object categories,Citation28,Citation69–Citation76 others describe cases who have difficulty.Citation63,Citation77 If prosopagnosia is about expert processing, though, a notable omission from many of these studies is the failure to consider the premorbid expertise of the prosopagnosic subject for the objects being used in the testing. A recent advance is the development of a method to use verbal semantic knowledge about a type of object as an index of their premorbid expertise and to adjust visual recognition scores for the degree of expertise. When this was done, nine of ten subjects with acquired prosopagnosia were impaired in expertise-adjusted car recognition.Citation44,Citation78
Similar mixed results have been obtained in adults with developmental prosopagnosia, with several studies describing cases in which the recognition deficit affected only facesCitation9,Citation79–Citation84 and some cases in which the recognition of other objects was also impaired.Citation8,Citation9,Citation79,Citation80,Citation85–Citation88 This is true for studies of children too.Citation9,Citation49,Citation89–Citation92 A study of six children with developmental prosopagnosia found face-specific deficits in four, and more general deficits for both faces and objects in one.Citation93 These differences across cases and studies may reflect a real heterogeneity rather than methodological issues.
One study has also attempted to evaluate the effect of object expertise on recognition ability in developmental prosopagnosia.Citation34 Using the Cambridge Car Memory Test,Citation94 this study reported that, at the group level, those with developmental prosopagnosia did not differ from controls after controlling for car expertise. However, individual data were analyzed before controlling for expertise, making it difficult to know whether expertise-adjusted car recognition was intact in each subject.
Words
Next to faces, words may be the stimulus category for which we have the highest degree of visual expertise. Although face processing is more active in the right hemisphere and word processing on the left, both show bilateral networks that overlap.Citation95 A recent theory proposes that face processing and word processing compete for neural resources during development and that incomplete hemispheric lateralization is a result of this competition.Citation60,Citation96,Citation97 The prediction of this theory is that prosopagnosic subjects should have subtle impairments in the processing of words, even if their lesions are limited to the right hemisphere.
Several recent studies have tested this prediction in acquired prosopagnosia. One study found subtle impairments in word processing in three subjects,Citation61 but these may have had a more general integrative visual agnosia rather than prosopagnosia.Citation81,Citation98 A second study of five subjects found normal performance on seven different reading tasks.Citation99 A third studyCitation100 found that only prosopagnosic subjects with bilateral fusiform lesions showed an increased word-length effect (the time taken to read a word as a function of the number of letters), and slow sorting of printed cards by their word content. On the other hand, even subjects with right hemisphere lesions alone were impaired when they had to sort the same cards by their font or handwriting. This suggested that the right hemisphere makes a critical contribution to the processing of stylistic properties of written text, rather than analyzing their word content. However, a similar recent study in developmental prosopagnosia has not found any deficit in processing the words or style of writing.Citation101 This may indicate that the style-processing impairments in acquired prosopagnosia are related to damage to adjacent processing areas rather than damage to the mechanisms involved in face processing.
Voices
Another question of specificity has examined whether individuals with prosopagnosia have difficulty in only the recognition of faces, or whether they struggle with person recognition more generally, such as the recognition of voices. On the other hand, years of relying on voice cues to recognize others may produce superior voice recognition in prosopagnosic subjects.Citation102
A recent study of acquired prosopagnosia found that only subjects with bilateral anterior temporal lesions had deficits in the recognition of voices, and therefore were better classified as having a multimodal disorder of person recognition.Citation62 However, the recognition deficit was specific to faces and did not involve voices or names in those with right anterior temporal lesions alone or occipitotemporal lesions. A second study of 12 subjects with developmental prosopagnosia found impaired voice recognition in only one subject;Citation103 nevertheless, this provides more evidence for heterogeneity in the developmental variant.
Other deficits from damage to adjacent structures
The classic tetrad found with acquired prosopagnosia, particularly when due to occipitotemporal lesions, is superior field deficits, dyschromatopsia and topographic disorientation. Cerebral dyschromatopsia is associated with damage to the lingual and fusiform gyri, in the vicinity of the collateral sulcus, almost always with bilateral but rarely with right unilateral lesions.Citation104,Citation105 It is characterized primarily by an accentuation of the tritanopic-like patterns seen in healthy subjects.Citation104 This last study did not find color impairments in subjects with developmental prosopagnosia.
Topographic disorientation, a disorder in which subjects get lost in familiar surroundings, is commonly reported with acquired prosopagnosia. A recent review found mention of topographic difficulty in 29% of 147 cases.Citation106 One possible explanation is the close proximity of the parahippocampal place area,Citation107 an area activated when viewing scenes, to the fusiform face area (FFA),Citation108 which is activated by viewing faces. In developmental prosopagnosia, there are anecdotal reports of both impairedCitation94–Citation96,Citation109 and preservedCitation84,Citation110,Citation111 navigational abilities. A recent study found that most patients with acquired prosopagnosia, regardless of lesion location, were impaired in scene and landmark recognition, while those with occipitotemporal lesions were also impaired in the ability to form cognitive maps,Citation112 and either deficit was rare in developmental prosopagnosia.
Neuroimaging
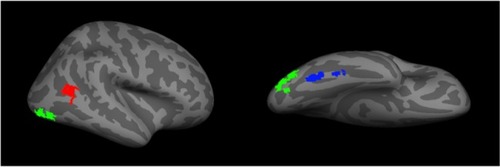
The advent of functional imaging has revolutionized cognitive brain science. In face research, it has delineated networks of regions active during face perception. This includes a core face network that includes the FFA,Citation108 the occipital face area (OFA), and the posterior portion of the superior temporal sulcusCitation113,Citation114 (). There is also an extended network that includes the anterior temporal face area and other regions such as the inferior frontal gyrus and precuneus.Citation4,Citation115 While faces activate these areas in both hemispheres,Citation95,Citation114 the effect is stronger on the right.Citation108
Figure 2 A representation of the core face network – including the fusiform face area (blue), the occipital face area (green), and the posterior superior temporal sulcus (red).
Studies of acquired prosopagnosia have been advanced by the improved functional and structural capabilities of magnetic resonance imaging (MRI). A key fact is that a variety of lesions can cause prosopagnosia,Citation116 an observation that makes sense when one considers the widely distributed networks involved in face processing. Two key observations have been made from the study of acquired prosopagnosia. First, lesions may be bilateral or unilateral, and when unilateral they are far more likely to be on the right.Citation4,Citation117,Citation118 A few prosopagnosic subjects with left-sided lesions have been described, but most have been left-handed,Citation119–Citation121 raising the possibility that they may have had anomalous hemispheric lateralization to begin with. Second, there is a useful division between occipitotemporal and anterior temporal damage. Recent functional MRI work has shown that occipitotemporal damage is associated with loss of activation of core components such as the FFA and OFA,Citation78 while activation in anterior areas may be spared.Citation122 Conversely, activation of the FFA and OFA may be spared in individuals with anterior temporal lesions.Citation78
These modern neuroimaging observations have generated structural correlates for functional variants of prosopagnosia that had long been hypothesized (see “Models of face recognition” section and ).Citation26 Recent studies show that those with fusiform lesions are more likely to have the apperceptive variant,Citation4,Citation78,Citation123 whereas those with anterior temporal lesions are more likely to have the associative variantCitation4,Citation78,Citation124,Citation125 (). The main conclusion is that acquired prosopagnosia is not a single disorder, but a family of disorders with different mechanisms and different lesions that nevertheless lead to the same end result of impaired face recognition.Citation78
Figure 3 Examples of lesions that produce acquired prosopagnosia.
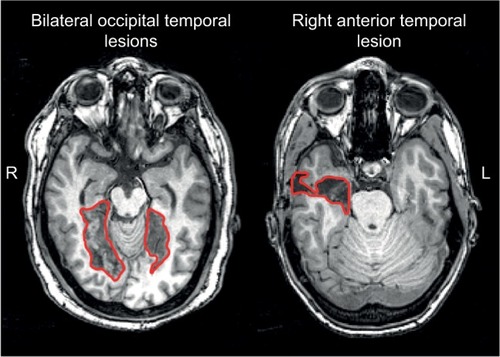
The structural correlates of developmental prosopagnosia are still debated. By definition, there is no obvious structural lesion, and early studies examining the evidence of abnormal activation of the core face network produced mixed results, with some reporting normal activationCitation126,Citation127 and another reporting activation for faces that did not differ from activation for other object types.Citation126–Citation128 Recent work with more advanced imaging methods has begun to uncover both structural and functional anomalies in developmental prosopagnosia, but there is disagreement. Some have suggested that there are anatomicalCitation84 or functionalCitation129–Citation131 abnormalities in the FFA and localized differences in white matter fibers around the right FFA.Citation132,Citation133 Others maintain that the core face network is largely normal and that abnormalities lie instead in the anterior temporal cortex,Citation134,Citation135 other regions of the extended face network,Citation136 or the long-range white matter tracts that connect the core regions in occipitotemporal cortex with the anterior temporal face area, namely the inferior longitudinal fasciculus.Citation134,Citation137 Both groups claim that the degree of altered white matter connectivity in their results correlated with behavioral measures of impaired face recognition.Citation133,Citation137 Whether these discrepancies reflect a real heterogeneity that exists in developmental prosopagnosia remains to be determined.
Event-related potentials
While event-related potentials do not have as good spatial resolution as MRI, they have a much finer resolution in time and can advance our understanding of the temporal dynamics of face recognition. Studies of face recognition in healthy subjects identify three components. The N170 component is prominent in right lateral occipitotemporal areas: it shows larger responses to faces than other objects and is associated with perceptual aspects of face processing.Citation138–Citation140 The N250 is also right-dominant and is the first component to show effects linked to the appearance of a specific facial identity, rather than just faces in general.Citation18,Citation141,Citation142 The P600 is seen when subjects can recognize a person by stating their name or providing information about them.Citation142,Citation143
Studies of acquired prosopagnosia support the association of the N170 with both an occipitotemporal location and perceptual aspects of face processing. Dalrymple et alCitation144 found that the face-selective aspect in the N170 was absent in subjects with apperceptive prosopagnosia whose lesions included at least two components of the core network (eg, FFA and OFA). However, it was intact in those with associative prosopagnosia whose lesions were restricted to anterior temporal cortex. Another study supported this finding by demonstrating preserved N170 face-selectivity in a subject with a right OFA lesion but preserved right fusiform gyrus,Citation145 and other studies reported its absence in a subject with impaired face perception.Citation143,Citation146
The findings in developmental prosopagnosia are less straightforward.Citation139 There are reports of both normalCitation147–Citation149 and abnormal N170 components,Citation111,Citation129,Citation147,Citation150 including one on the analogous M170 component detected by magnetoencephalography.Citation151 Larger studies have found heterogeneous results across subjects that can explain this inconsistency.Citation147,Citation152–Citation154 It is also possible that there are more subtle abnormalities in the N170 component. For example, the amplitude of the N170 component is usually larger when viewing upside-down faces, likely because it is harder to process them, but one study found that the majority of 16 subjects with developmental prosopagnosia failed to show an orientation effect in the N170 amplitude.Citation152
With regard to the later potentials, a study of 12 subjects with developmental prosopagnosia found normal N250 and P600 components on the few trials on which these subjects did identify a face, suggesting relatively normal processing when face recognition is successful. About half also exhibited N250 components for famous faces they did not recognize, which may indicate some unconscious processing. There was no P600 component under those circumstances, implying that this potential reflects conscious face identification.Citation155 Even though these studies indicate that the processes indexed by these later potentials can still be activated in developmental prosopagnosia, a recent Event Related Potential study claimed that these components are delayedCitation156 (see Towler et alCitation157 for a recent review of ERP findings in developmental prosopagnosia).
Treatment and rehabilitation
Can training improve prosopagnosia? The answer may be that it depends. In acquired prosopagnosia, one might speculate that the efficacy of any training could be affected by age at onset, duration since onset, and lesion size, laterality, and location, particularly with regard to how much of the face network and its connections are compromised.Citation158 Given the rarity of acquired prosopagnosia, it will be very difficult to establish the impact of each of these factors. To date, there have been few remedial attempts for the acquired variant, and most focus on enhancing coping strategies to circumvent poor face recognition.Citation158,Citation159 Only one published study attempted to improve face recognition, in a child with diffuse damage after meningococcal meningitis: 18 months of training did not improve matters.Citation160 More recently, two training studies have been reported at conferences. DeGutis et alCitation159,Citation161 attempted to train a 46-year-old with a right occipital-temporal lesion to categorize faces based on the distances between face parts. Unfortunately, this did not help. A second study trained 12 subjects to discriminate increasingly subtler differences in face shape across variations in expression and viewpoint, over 11 weeks. Some improvement was found, but this was more modest for the recognition of faces not used during training.Citation162
Given the lack of overt brain damage, one might wonder whether training may be more effective in developmental prosopagnosia. One group trained 25 subjects with developmental prosopagnosiaCitation163,Citation164 to perceive the spacing between facial features and found improvements that generalized to new faces but did not help recognition when viewpoint varied.Citation164 A different therapeutic approach was used in a randomized, placebo-controlled, double-blind study examining the effect of intranasal inhalation of oxytocin, a drug associated with the regulation of social behaviors, on face identity processing.Citation165 The authors reported transient improvement of face perception and recognition in ten subjects with developmental prosopagnosia after oxytocin administration.
While these reports are encouraging, there may be limitations. Given the heterogeneity of deficits in prosopagnosia, it may be that a specific training program will not be appropriate for all subjects. How much of a residual face network one needs in acquired prosopagnosia to benefit from training is unknown. The belief that the subtler structural alterations of developmental prosopagnosia imply a better chance of having the neural substrate to generate benefit from training is unproven.
Conclusion
A prevailing theme in prosopagnosia research is the heterogeneity of findings across both acquired and developmental prosopagnosia. There is heterogeneity in the mechanism of prosopagnosia (ie, apperceptive versus associative), the location, lateralization, and extent of structural damage in the acquired form, and the presence or absence of impairments in other perceptual domains (eg, object, word, and voice processing). Heterogeneity is expected when one is dealing with a complex process such as person recognition, but it does create challenges that require particular care and rigor in experimental study and analysis.
For one, it is important to ensure that heterogeneity is not the inadvertent result of experimental factors. To this end, care is required in establishing the diagnosis of prosopagnosia and excluding other conditions (). First, besides excluding more general failures in object recognition and memory, tests of voice and name recognition are needed to establish where a patient is more accurately characterized as having a multi-modal disorder of person recognition, whose mechanisms may differ from prosopagnosia. Second, uniform diagnostic criteria are needed. This is particularly an issue for developmental prosopagnosia. Currently, there is no diagnostic consensus: inclusion criteria range from purely self-report measuresCitation12,Citation13 to various conglomerations of self-report, behavioral tests of face familiarity, and tests of face naming/identification,Citation156,Citation166 and few require imaging to exclude brain lesions that would point to an early-onset acquired variant rather than developmental prosopagnosia. Another diagnostic issue that reflects the current lack of definitive genetic or radiologic markers for the developmental form is the challenge of distinguishing subjects with true pathology resulting from aberrant development of face recognition networks from those who are simply at the low end of a spectrum of normal face-processing skill (see Barton and CorrowCitation14 for a discussion).
Nevertheless, it remains a possibility that there is real heterogeneity in developmental prosopagnosia, just as there is in acquired prosopagnosia. This accounts for the current trend to use single-subject methods of analysis, using Crawford’s T tests, for example. However, it may be difficult for subtle anomalies to achieve statistical significance at the individual level, as illustrated by recent ERP work.Citation151,Citation152 Further work may benefit from the definition of more homogeneous variants, and supplementing the single-subject methods with group analyses on these subgroups. This will necessitate the collection of larger samples of these patients. Such efforts should advance our knowledge of the neuroanatomic and functional origins of these intriguing conditions.
Acknowledgments
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Disclosure
This work was supported by CIHR operating grant (MOP-102567) to JB. JB was supported by a Canada Research Chair and the Marianne Koerner Chair in Brain Diseases. SC was supported by National Eye Institute of the National Institutes of Health under award number F32 EY023479-02 and Loan Repayment Program. The authors report no other conflicts of interest in this work.
References
- QuaglinoABorelliGEmiplegia sinistra con amaurosi–guarigione–perdita totale della percezione dei colori e della memoria della configurazione degli oggetti. [Left hemiplegia with amaurosis–healing–total loss of color perception and the configuration memory of the objects]Giornale di Oftalmologia Italiano186710106117 Italian
- Della SalaSYoungAWQuaglino’s 1867 case of prosopagnosiaCortex200339353354012870826
- BodamerJDie Prosop-Agnosie. [The Prosopagnosic]Archiv fur Psychiatrie und Nervenkrankheiten1947179653
- BartonJJStructure and function in acquired prosopagnosia: lessons from a series of 10 patients with brain damageJ Neuropsychol20082Pt 119722519334311
- DuchaineBNakayamaKDevelopmental prosopagnosia: a window to content-specific face processingCurr Opin Neurobiol200616216617316563738
- SusiloTDuchaineBAdvances in developmental prosopagnosia researchCurr Opin Neurobiol201323342342923391526
- SchmalzlLPalermoRColtheartMCognitive heterogeneity in genetically based prosopagnosia: a family studyJ Neuropsychol2008219911719334307
- DuchaineBGermineLNakayamaKFamily resemblance: ten family members with prosopagnosia and within-class object agnosiaCogn Neuropsychol200724441943018416499
- LeeYDuchaineBWilsonHRNakayamaKThree cases of developmental prosopagnosia from one family: detailed neuropsychological and psychophysical investigation of face processingCortex201046894996419726036
- WilmerJBGermineLChabrisCFHuman face recognition ability is specific and highly heritableProc Natl Acad Sci U S A2010107115238524120176944
- ZhuQSongYHuSHeritability of the specific cognitive ability of face perceptionCurr Biol201020213714220060296
- KennerknechtIGrueterTWellingBFirst report of prevalence of non-syndromic hereditary prosopagnosia (HPA)Am J Med Genet A2006140151617162216817175
- KennerknechtIHoNYWongVCPrevalence of hereditary prosopagnosia (HPA) in Hong Kong Chinese populationAm J Med Genet A2008146A222863287018925678
- BartonJJCorrowSLThe problem of being bad at facesNeuropsychologia20168911912427312748
- YardleyLMcDermottLPisarskiSDuchaineBNakayamaKPsychosocial consequences of developmental prosopagnosia: a problem of recognitionJ Psychosom Res200865544545118940375
- DalrympleKAFletcherKCorrowS“A room full of strangers every day”: the psychosocial impact of developmental prosopagnosia on children and their familiesJ Psychosom Res20147714415025077856
- BruceVYoungAUnderstanding face recognitionBr J Psychol198677Pt 33053273756376
- SchweinbergerSRBurtonAMCovert recognition and the neural system for face processingCortex200339193012627750
- BartonJJCorrowSLRecognizing and identifying people: a neuropsychological reviewCortex20167513215026773237
- GainottiGCognitive models of familiar people recognition and hemispheric asymmetriesFront Biosci (Elite Ed)2014614815824389149
- FarahMJO’ReillyRCVeceraSPDissociated overt and covert recognition as an emergent property of a lesioned neural networkPsychol Rev199310045715888255950
- BelinPFecteauSBédardCThinking the voice: neural correlates of voice perceptionTrends Cogn Sci20048312913515301753
- EllisHDJonesDMMosdellNIntra- and inter-modal repetition priming of familiar faces and voicesBr J Psychol199788Pt 11431569061895
- BurtonAMBruceVI recognize your face but I can’t remember your name: a simple explanation?Br J Psychol199283Pt 145601559145
- BredartSValentineTCalderAGassiLAn interactive activation model of face namingQ J Exp Psychol A19954824664867610275
- De RenziEFaglioniPGrossiDNichelliPApperceptive and associative forms of prosopagnosiaCortex19912722132211879150
- BusignyTVan BelleGJemelBHoseinAJoubertSRossionBFace-specific impairment in holistic perception following focal lesion of the right anterior temporal lobeNeuropsychologia20145631233324503392
- BusignyTJoubertSFelicianOCeccaldiMRossionBHolistic perception of the individual face is specific and necessary: evidence from an extensive case study of acquired prosopagnosiaNeuropsychologia201048144057409220875437
- WhiteDRivoltaDBurtonAMAl-JanabiSPalermoRFace matching impairment in developmental prosopagnosiaQ J Exp Psychol Epub2016422
- BartonJJZhaoJKeenanJPPerception of global facial geometry in the inversion effect and prosopagnosiaNeuropsychologia200341121703171112887994
- DuchaineBCNakayamaKDevelopmental prosopagnosia and the benton facial recognition testNeurology20046271219122015079032
- DuchaineBCWeidenfeldAAn evaluation of two commonly used tests of unfamiliar face recognitionNeuropsychologia200341671372012591028
- DuchaineBNakayamaKThe Cambridge Face Memory Test: results from neurologically intact individuals and an investigation of its validity using inverted stimuli and prosopagnosic participantsNeuropsychologia200644457658516169565
- EsinsJSchultzJStemperCKennerknechtIBülthoffIFace perception and test reliabilities in congenital prosopagnosia in seven testsIperception201671137
- McKoneEStokesSLiuJA robust method of measuring other-race and other-ethnicity effects: the Cambridge Face Memory Test formatPLoS One2012710e4795623118912
- McKoneEHallAPidcockMFace ethnicity and measurement reliability affect face recognition performance in developmental prosopagnosia: evidence from the Cambridge Face Memory Test-AustralianCogn Neuropsychol201128210914622122116
- CroydonAPimpertonHEwingLDuchaineBCPellicanoEThe Cam-bridge Face Memory Test for Children (CFMT-C): a new tool for measuring face recognition skills in childhoodNeuropsychologia201462606725054837
- DalrympleKGomezJDuchaineBCFMT-Kids: a new test of face memory for childrenJ Vis2012129492
- DuchaineBYovelGNakayamaKNo global processing deficit in the Navon task in 14 developmental prosopagnosicsSoc Cogn Affect Neurosci2007210411318985129
- BurtonAWhiteDMcNeillAThe Glasgow Face Matching TestBehav Res Methods201042128629120160307
- DalrympleKAGarridoLDuchaineBDissociation between face perception and face memory in adults, but not children, with developmental prosopagnosiaDev Cogn Neurosci201410102025160676
- ShahPGauleASowdenSBirdGCookRThe 20-item prosopagnosia index (PI20): a self-report instrument for identifying developmental prosopagnosiaR Soc Open Sci20152614034326543567
- BarnesCSDe l’AuneWSchuchardRAA test of face discrimination ability in aging and vision lossOptom Vis Sci201188218819921150678
- BartonJJHanifHAshrafSRelating visual to verbal semantic knowledge: the evaluation of object recognition in prosopagnosiaBrain2009132Pt 123456346619805494
- BartonJJCherkasovaMVHefterRCoxTAO’ConnorMManoachDSAre patients with social developmental disorders prosopagnosic? perceptual heterogeneity in the asperger and socio-emotional processing disordersBrain2004127Pt 81706171615215211
- WeigeltSKoldewynKKanwisherNFace identity recognition in autism spectrum disorders: a review of behavioral studiesNeurosci Biobehav Rev20123631060108422212588
- DuchaineBMurrayHTurnerMWhiteSGarridoLNormal social cognition in developmental prosopagnosiaCogn Neuropsychol200926762063420191404
- WilsonCEPalermoRSchmalzlLBrockJSpecificity of impaired facial identity recognition in children with suspected developmental prosopagnosiaCogn Neuropsychol2010271304520623389
- HongDScaletta KentJKeslerSCognitive profile of turner syndromeDev Disabil Res Rev200915427027820014362
- FrithCDStevensMJohnstoneECOwensDGCrowTJIntegration of schematic faces and other complex objects in schizophreniaJ Nerv Ment Dis1983171134396848647
- ArcherJHayDCYoungAWFace processing in psychiatric conditionsBr J Clin Psychol199231Pt 145611559117
- RoudierMMarciePGrancherASTzortzisCStarksteinSBollerFDiscrimination of facial identity and of emotions in Alzheimer’s diseaseJ Neurol Sci199815421511589562305
- BäckmanLHerlitzAThe relationship between prior knowledge and face recognition memory in normal aging and Alzheimer’s diseaseJ Gerontol1990453P94P1002335731
- HodgesJRSalmonDPButtersNRecognition and naming of famous faces in Alzheimer’s disease: a cognitive analysisNeuropsychologia19933187757888413900
- DewickHCHanleyJRDaviesADPlayferJTurnbullCPerception and memory for faces in Parkinson’s diseaseNeuropsychologia19912987858021944878
- DalrympleKAPalermoRGuidelines for studying developmental prosopagnosia in adults and childrenWiley Interdiscip Rev Cogn Sci201671738726681428
- DiamondRCareySWhy faces are and are not special: an effect of expertiseJ Exp Psychol Gen198611521071172940312
- GauthierISkudlarskiPGoreJCAndersonAWExpertise for cars and birds recruits brain areas involved in face recognitionNat Neurosci20003219119710649576
- XuYLiuJKanwisherNThe M170 is selective for faces, not for expertiseNeuropsychologia20054358859715716149
- BehrmannMPlautDCDistributed circuits, not circumscribed centers, mediate visual recognitionTrends Cogn Sci201317521021923608364
- BehrmannMPlautDCBilateral hemispheric processing of words and faces: evidence from word impairments in prosopagnosia and face impairments in pure alexiaCereb Cortex201424241102111823250954
- LiuRPancarogluRHillsCSDuchaineBBartonJJVoice recognition in face-blind patientsCereb Cortex20162641473148725349193
- NeunerFSchweinbergerSRNeuropsychological impairments in the recognition of faces, voices, and personal namesBrain Cogn200044334236611104530
- Grill-SpectorKThe neural basis of object perceptionCurr Opin Neurobiol20031315916612744968
- HaxbyJGobbiniMDistributed neural systems for face perceptionCalderARhodesGJohnsonMHaxbyJThe Oxford andbook of Face PerceptionOxford, United KingdomOxford University Press201193110
- HaxbyJVGobbiniMIFureyMLIshaiASchoutenJLPietriniPDistributed and overlapping representations of faces and objects in ventral temporal cortexScience200129355392425243011577229
- KanwisherNDomain specificity in face perceptionNat Neurosci20003875976310903567
- PitcherDCharlesLDevlinJTWalshVDuchaineBTriple dissociation of faces, bodies, and objects in extrastriate cortexCurr Biol200919431932419200723
- FarahMJLevinsonKLKleinKLFace perception and within-category discrimination in prosopagnosiaNeuropsychologia19953366616747675159
- SusiloTYovelGBartonJJDuchaineBFace perception is category-specific: evidence from normal body perception in acquired prosopagnosiaCognition20131291889423856076
- RezlescuCPitcherDDuchaineBAcquired prosopagnosia with spared within-class object recognition but impaired recognition of degraded basic-level objectsCogn Neuropsychol201229432534723216309
- RezlescuCBartonJJPitcherDDuchaineBNormal acquisition of expertise with greebles in two cases of acquired prosopagnosiaProc Natl Acad Sci U S A2014111145123512824706834
- RiddochMJJohnstonRABracewellRMBoutsenLHumphreysGWAre faces special? a case of pure prosopagnosiaCogn Neuropsychol200825132618340601
- McNeilJEWarringtonEKProsopagnosia: a face-specific disorderQ J Exp Psychol A19934611108446761
- FarahMJWilsonKDDrainHMTanakaJRThe inverted face inversion effect in prosopagnosia: evidence for mandatory, face-specific perceptual mechanismsVision Res19953514208920937660612
- HenkeKSchweinbergerSRGrigoAKlosTSommerWSpecificity of face recognition: recognition of exemplars of non-face objects in prosopagnosiaCortex19983422892969606594
- GauthierIBerhmannMTarrMJCan face recognition really be dissociated from object recognition?J Cogn Neurosci199911434937010471845
- Davies-ThompsonJPancarogluRBartonJAcquired prosopagnosia: structural basis and processing impairmentsFront Biosci (Elite Ed)2014615917424389150
- DuchaineBNakayamaKDissociations of face and object recognition in developmental prosopagnosiaJ Cogn Neurosci200517224926115811237
- GarridoLFurlNDraganskiBVoxel-based morphometry reveals reduced grey matter volume in the temporal cortex of developmental prosopagnosicsBrain2009132Pt 123443345519887506
- SusiloTMcKoneEDennettHFace recognition impairments despite normal holistic processing and face space coding: evidence from a case of developmental prosopagnosiaCogn Neuropsychol201027863666422074472
- TreeJWilkieJFace and object imagery in congenital prosopagnosia: a case seriesCortex20104691189119820434142
- DuchaineBYovelGButterworthENakayamaKProsopagnosia as an impairment to face-specific mechanisms: elimination of the alternative hypotheses in a developmental caseCogn Neuropsychol200623571474721049351
- NunnJPostmaPPearsonRDevelopmental prosopagnosia: should it be taken at face value?Neurocase200171152711239073
- BehrmannMAvidanGCongenital prosopagnosia: face-blind from birthTrends Cogn Sci20059418018715808500
- De HaanEHCampbellRA fifteen year follow-up of a case of developmental prosopagnosiaCortex19912744895091782786
- DuchaineBCNieminen-von WendtTNewJKulomakiTDissociations of visual recognition in a developmental agnosic: evidence for separate developmental processesNeurocase20039538038914972753
- DuchaineBCParkerHNakayamaKNormal recognition of emotion in a prosopagnosicPerception200332782783812974568
- JonesRDTranelDSevere developmental prosopagnosia in a child with superior intellectJ Clin Exp Neuropsychol200123326527311404805
- ArielRSadehMCongenital visual agnosia and prosopagnosia in a childCortex1996127682
- McConachieHRDevelopmental prosopagnosia. A single case reportCortex197612176821261287
- BrunsdonRColtheartMNickelsLJoyPDevelopmental prosopagnosia: a case analysis and treatment studyCogn Neuropsychol200623682284021049355
- DalrympleKAElisonJTDuchaineBFace-specific and domain-general visual processing deficits in children with developmental prosopagnosiaQ J Exp Psychol (Hove) Epub201654
- DennettHWMcKoneETavashmiRThe Cambridge Car Memory Test: a task matched in format to the Cambridge Face Memory Test, with norms, reliability, sex differences, dissociations from face memory, and expertise effectsBehav Res Methods201244258760522012343
- NestorABehrmannMPlautDCThe neural basis of visual word form processing: a multivariate investigationCereb Cortex20132371673168422693338
- DundasEMPlautDCBehrmannMThe joint development of hemispheric lateralization for words and facesJ Exp Psychol Gen2013142234835822866684
- DehaeneSPegadoFBragaLWHow learning to read changes the cortical networks for vision and languageScience201033060091359136421071632
- MarottaJJMcKeeffTJBehrmannMThe effects of rotation and inversion on face processing in prosopagnosiaCogn Neuropsychol2002191314720957530
- SusiloTWrightVTreeJJDuchaineBAcquired prosopagnosia without word recognition deficitsCogn Neuropsychol201532632133926402384
- HillsCSPancarogluRDuchaineBBartonJJWord and text processing in acquired prosopagnosiaAnn Neurol201578225827125976067
- RubinoCCorrowSLCorrowJCDuchaineBBartonJJWord and text processing in developmental prosopagnosiaCogn Neuropsychol2016335627386744
- HooverAEDemonetJFSteevesJKSuperior voice recognition in a patient with acquired prosopagnosia and object agnosiaNeuropsychologia201048133725373220850465
- LiuRRCorrowSLPancarogluRDuchaineBBartonJJThe processing of voice identity in developmental prosopagnosiaCortex20157139039726321070
- MorozDCorrowSLCorrowJCBartonARDuchaineBBartonJJLocalization and patterns of Cerebral dyschromatopsia: a study of subjects with prospagnosiaNeuropsychologia20168915316027312747
- BouvierSEEngelSABehavioral deficits and cortical damage loci in cerebral achromatopsiaCereb Cortex200616218319115858161
- SchmidtDNeuro-ophthalmological findings in patients with acquired prosopagnosiaGraefes Arch Clin Exp Ophthalmol2015253333333425550096
- EpsteinRKanwisherNA cortical representation of the local visual environmentNature199839266765986019560155
- KanwisherNMcDermottJChunMMThe fusiform face area: a module in human extrastriate cortex specialized for face perceptionJ Neurosci19971711430243119151747
- TempleCMDevelopmental memory impairment: faces and patternsCampbellRMental Lives: Case Studies in CognitionOxford, United KingdomBlackwell1992199215
- DuchaineBCDevelopmental prosopagnosia with normal configural processingNeuroreport2000111798310683834
- BentinSDeouellLYSorokerNSelective visual streaming in face recognition: evidence from developmental prosopagnosiaNeuroreport199910482382710208555
- CorrowJCCorrowSLLeeEGetting lost: topographic skills in acquired and developmental prosopagnosiaCortex2016768910326874939
- GobbiniMIHaxbyJVNeural systems for recognition of familiar facesNeuropsychologia2007451324116797608
- HaxbyJVHoffmanEAGobbiniMIThe distributed human neural system for face perceptionTrends Cogn Sci20004622323310827445
- JonasJRossionBBrissartHBeyond the core face-processing network: intracerebral stimulation of a face-selective area in the right anterior fusiform gyrus elicits transient prosopagnosiaCortex20157214015526143305
- DamasioARTranelDDamasioHFace agnosia and the neural substrates of memoryAnnu Rev Neurosci199013891092183687
- DamasioARDamasioHVan HoesenGWProsopagnosia: anatomic basis and behavioral mechanismsNeurology19823243313417199655
- De RenziEProsopagnosia in two patients with CT scan evidence of damage confined to the right hemisphereNeuropsychologia19862433853893736820
- MattsonAJLevinHSGrafmanJA case of prosopagnosia following moderate closed head injury with left hemisphere focal lesionCortex200036112513710728902
- TzavarasAMerienneLMasureMCProsopagnosie, amnésie et troubles du langage par lésion temporale gauche chez un sujet gaucher [Prosopagnosia, amnesia and language disorders by left temporal lesion in a left-handed subject]Encephale1973624382394 French4794621
- BartonJJProsopagnosia associated with a left occipitotemporal lesionNeuropsychologia20084682214222418374372
- YangHSusiloTDuchaineBThe anterior temporal face area contains invariant representations of face identity that can persist despite the loss of right FFA and OFACereb Cortex20162631096110725527821
- BartonJJPressDZKeenanJPO’ConnorMLesions of the fusiform face area impair perception of facial configuration in prosopagnosiaNeurology2002581717811781408
- KanwisherNBartonJThe functional architecture of the face system: Integrating evidence from fMRI and patient studiesRhodesGCalderAJohnsonMHaxbyJVOxford Handbook of Face PerceptionOxfordUnited Kingdom2011
- BartonJJCherkasovaMFace imagery and its relation to perception and covert recognition in prosopagnosiaNeurology200361222022512874402
- HassonUAvidanGDeouellLYBentinSMalachRFace-selective activation in a congenital prosopagnosic subjectJ Cogn Neurosci200315341943112729493
- AvidanGHassonUMalachRBehrmannMDetailed exploration of face-related processing in congenital prosopagnosia: 2. functional neuroimagining findingsJ Cogn Neurosci20051771150116716102242
- HadjikhaniNde GelderBNeural basis of prosopagnosia: an fMRI studyHum Brain Mapp200216317618212112771
- BentinSDeGutisJMD’EspositoMRobertsonLCToo many trees to see the forest: performance, event-related potential, and functional magnetic resonance imaging manifestations of integrative congenital prosopagnosiaJ Cogn Neurosci200719113214617214570
- FurlNGarridoLDolanRJDriverJDuchaineBFusiform gyrus face selectivity relates to individual differences in facial recognition abilityJ Cogn Neurosci20112371723174020617881
- ZhangJLiuJXuYNeural decoding reveals impaired face configural processing in the right fusiform face area of individuals with developmental prosopagnosiaJ Neurosci20153541539154825632131
- GomezJPestilliFWitthoftNFunctionally defined white matter reveals segregated pathways in human ventral temporal cortex associated with category-specific processingNeuron201585121622725569351
- SongSGarridoLNagyZLocal but not long-range micro-structural differences of the ventral temporal cortex in developmental prosopagnosiaNeuropsychologia20157819520626456436
- AvidanGTanzerMHadj-BouzianeFLiuNUngerleiderLGBehrmannMSelective dissociation between core and extended regions of the face processing network in congenital prosopagnosiaCereb Cortex20142461565157823377287
- BehrmannMAvidanGGaoFBlackSStructural imaging reveals anatomical alterations in inferotemporal cortex in congenital prosopagnosiaCereb Cortex200717102354236317218483
- AvidanGBehrmannMFunctional MRI reveals compromised neural integrity of the face processing network in congenital prosopagnosiaCurr Biol200919131146115019481456
- ThomasCAvidanGHumphreysKJungKJGaoFBehrmannMReduced structural connectivity in ventral visual cortex in congenital prosopagnosiaNat Neurosci2009121293119029889
- BentinSAllisonTPuceAPerezEMcCarthyGElectrophysiological studies of face perception in humansJ Cogn Neurosci19968655156520740065
- TowlerJEimerMElectrophysiological studies of face processing in developmental prosopagnosia: neuropsychological and neurodevelopmental perspectivesCogn Neuropsychol2012295–650352923066851
- EimerMThe face-sensitive N170 component of the event-related brain potentialCalderAJRhodesGJohnsonMHaxbyJThe Oxford Handbook of Face PerceptionOxford, United KingdomOxford University Press2011329344
- TanakaJWCurranTPorterfieldALCollinsDActivation of preexisting and acquired face representations: the N250 event-related potential as an index of face familiarityJ Cogn Neurosci20061891488149716989550
- GoslingAEimerMAn event-related brain potential study of explicit face recognitionNeuropsychologia20114992736274521679721
- EimerMEvent-related brain potentials distinguish processing stages involved in face perception and recognitionClin Neurophysiol2000111469470510727921
- DalrympleKAOrucIDuchaineBThe anatomic basis of the right face-selective N170 IN acquired prosopagnosia: a combined ERP/fMRI studyNeuropsychologia20114992553256321601585
- PrietoEACaharelSHensonRRossionBEarly (N170/M170) face-sensitivity despite right lateral occipital brain damage in acquired prosopagnosiaFront Human Neurosci20115138
- EimerMMcCarthyRAProsopagnosia and structural encoding of faces: evidence from event-related potentialsNeuroreport199910225525910203318
- HarrisAMDuchaineBCNakayamaKNormal and abnormal face selectivity of the M170 response in developmental prosopagnosicsNeuropsychologia200543142125213616243056
- RivoltaDPalermoRSchmalzlLWilliamsMAInvestigating the features of the M170 in congenital prosopagnosiaFront Human Neurosci2012645
- de GelderBStekelenburgJJNaso-temporal asymmetry of the N170 for processing faces in normal viewers but not in developmental prosopagnosiaNeurosci Lett20053761404515694271
- KressTDaumIEvent-related potentials reflect impaired face recognition in patients with congenital prosopagnosiaNeurosci Lett2003352213313614625041
- LueschowAWeberJECarbonC-CThe 170ms response to faces as measured by MEG (M170) is consistently altered in congenital prosopagnosiaPLoS One2015109e013762426393348
- TowlerJGoslingADuchaineBEimerMThe face-sensitive N170 component in developmental prosopagnosiaNeuropsychologia201250143588359923092937
- RighartRde GelderBImpaired face and body perception in developmental prosopagnosiaProc Natl Acad Sci U S A200710443172341723817942679
- MinnebuschDASuchanBRamonMDaumIEvent-related potentials reflect heterogeneity of developmental prosopagnosiaEur J Neurosci20072572234224717439500
- EimerMGoslingADuchaineBElectrophysiological markers of covert face recognition in developmental prosopagnosiaBrain2012135254255422271660
- ParketnyJTowlerJEimerMThe activation of visual face memory and explicit face recognition are delayed in developmental prosopagnosiaNeuropsychologia20157553854726169316
- TowlerJFisherKEimerMThe cognitive and neural basis of developmental prosopagnosiaQ J Exp Psychol (Hove)2016129
- BateSBennettsRJThe rehabilitation of face recognition impairments: a critical review and future directionsFront Hum Neurosci2014849125100965
- DeGutisJMChiuCGrossoMECohanSFace processing improvements in prosopagnosia: successes and failures over the last 50 yearsFront Human Neurosci20148561
- EllisHDYoungATraining in face-processing skills for a child with acquired prosopagnosiaDev Neuropsychol198844283294
- DeGutisJCohanSKahnDAAguirreGKNakayamaKFacial expression training improves emotion recognition and changes neural tuning in a patient with acquired emotion recognition deficits and prosopagnosiaJ Vis2013139993993
- Davies-ThompsonJFletcherKHillsCCorrowSPancarogluRBartonJJSPerceptual training of faces in rehabilitation of acquired prosopagnosiaPaper presented at: 38th European Conference on Visual PercpetionAugust 23–27; 2015Liverpool, United Kingdom
- DeGutisJMBentinSRobertsonLCD’EspositoMFunctional plasticity in ventral temporal cortex following cognitive rehabilitation of a congenital prosopagnosicJ Cogn Neurosci200719111790180217958482
- DeGutisJCohanSNakayamaKHolistic face training enhances face processing in developmental prosopagnosiaBrain2014137Pt 61781179824691394
- BateSCookSJDuchaineBTreeJJBurnsEJHodgsonTLIntranasal inhalation of oxytocin improves face processing in developmental prosopagnosiaCortex201450556324074457
- TowlerJParketnyJEimerMPerceptual face processing in developmental prosopagnosia is not sensitive to the canonical location of face partsCortex201674536626649913
