Abstract
Wnt-signaling, a ubiquitous pathway that directs differentiation, cell polarity, and tissue specificity, has been implicated as an important gene-expression pathway in retinal development. An increasing body of evidence supports the importance of Wnt-signaling, and specifically, norrin-mediated Wnt-signaling in retinal development and retinal maintenance. Gene mutations affecting the Wnt-signaling pathways result in a variety of inherited vitreoretinopathies. Additionally, there is growing evidence that prematurity and associated retinopathy are associated with alterations in the Wnt-signaling pathways. Further investigations may allow for improved diagnoses, management, and therapies in the future.
Background
Wnt-signaling is a ubiquitous pathway that modulates cellular and tissue differentiation. The Wnt gene family consists of 19 structurally related genes, which encode cysteine-rich secreted glycoproteins that act as extracellular signaling factors. Wnt proteins can signal in a variety of ways, depending on the cell type and the receptors and/or co-receptors expressed. Extracellular Wnt ligands interact with their transmembrane Fzd receptors of which there are currently ten identified isoforms in humans, and thereby, activate three diverse signaling pathways.Citation1–Citation4 Binding of the Wnt ligand to its Fzd receptor simultaneously with a co-receptor, the low-density LRP5/6, initiates the canonical pathway. This leads to modulation in the transcription of over 50 genes in mammals.Citation1
The canonical Wnt/β-Catenin pathway is the most thoroughly studied. In the retina, activation is initiated by ligand binding to both the Fzd4 receptor and its co-receptor, LRP5. Multiple Wnt ligands (3a, 5a, 7a, 10a, norrin) are able to bind the Fzd4 receptor. Binding of the receptor complex activates the Dsh protein. This ultimately results in dephosphorylation of β-Catenin and its subsequent translocation to the nucleus.Citation1,Citation2 Nuclear β-Catenin participates as a transcriptional activator of the TCF/LEF-1 family of DNA binding proteins. Target genes include c-myc, CCND1, and VEGF, which presumably regulates cell proliferation in specific tissues.
The planar cell polarity pathway also involves activation of Dsh protein following Fzd4 binding. Unlike the canonical pathway, a co-receptor (LRP5) is not needed for signal activation. One of two small GTPases (Rho or Rac) is thereby activated, which in turn activate an alternative signal transduction pathway.Citation1,Citation2 The planar cell polarity pathway mediates cytoskeletal organization and cell migration during development and tissue maturation.
The Wnt-Ca2+ pathway (noncanonical signaling) is the least characterized cascade. In the Wnt-Ca2+ pathway, Fzd receptor activation stimulates an intracellular Ca2+ release, activating CamKII and PKC. This pathway directs cell adhesion and cell movement and is necessary for normal development. Wnt5a is a potent activator of this pathway.Citation3
In regards to eye development, a particular Wnt-pathway, norrin-Fzd4, has been identified as playing an essential role in normal retinal angiogenesis. Signaling through the norrin-Fzd4 (canonical and noncanonical) pathways is necessary for the development and maintenance of retinal vasculature.Citation4–Citation7 Conversely, increased expression of other Wnt ligands (Wnts 3a, 5a, 7a, and 10a) has been associated with pathologic angiogenesis (neovascularization) in animal models of retinopathy and proliferative diabetic retinopathy.Citation8 Without proper activation, dysregulated angiogenesis leads to pathological vascular changes, such as breakdown of tight junctions, capillary drop-out, endothelial budding, and neovascularization, and in extreme cases development of primitive vascular trees (vasculogenesis only). Patients with gene mutations affecting Wnt signaling result in several pediatric vitreoretinopathies, such as Norrie disease, familial exudative vitreoretinopathy (FEVR), and pseudoglioma and osteoporosis syndrome.Citation9–Citation16 Additionally, retinopathy of prematurity (ROP) has been associated with gene mutations affecting Wnt signaling.Citation14, Citation17–Citation20 Although Coats’ disease and persistent fetal vasculature (PFV) are generally unilateral and sporadic, Wnt-pathway mutations have been reported. Citation21–Citation23
Retinal vasculogenesis and angiogenesis
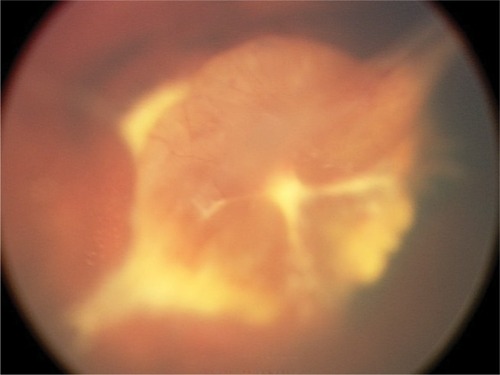
Vasculogenesis is the de novo synthesis of blood vessels. In the human retina the early development of vasculature begins posteriorly, at the optic nerve head. There is clear evidence that vascularization beyond the early stages of vasculogenesis does not occur without norrin-Fzd4 signaling.Citation18 Infants with Norrie disease often express mutations in norrin that either eliminates norrin protein translation or affects the tertiary structure, thereby disallowing norrin-Fzd4 binding. Point mutations of the conserved cysteine residues result in improper protein folding. Patients with these mutations have primitive vasculogenesis only and minimal regression of the primary hyaloid structures ().
Figure 1 Example of severe dysgenesis with unregressed stalk tissue and primitive retinal development.
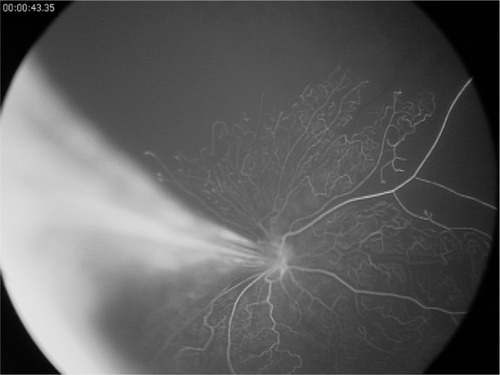
Angiogenesis is the growth of new vessels from existing vessels by way of endothelial budding. When properly controlled, normal retinal development with branching vessels and capillary networks occur. When this is dysregulated, incomplete vascularization and/or pathologic angiogenesis develop ().Citation1,Citation4,Citation7 This can often represent a spectrum of disease severity. In the premature infant, the earliest sign of abnormal angiogenesis is seen as a cessation of retinal vasculature growth. This is primarily an effect of decreased circulating VEGF but is exacerbated by other proangiogenic factors being dysregulated as well. It has been shown in animal models that Wnt pathway activation by norrin is able to overcome this delay in vascular development.Citation24,Citation25 Intravitreal injection of norrin following oxygen exposure greatly accelerates the retinal recovery and vascular maturation in the oxygen-induced retinopathy mouse model.Citation24 Endogenous expression of norrin in the eye by way of virally transfected lens epithelial cells is protective from oxygen-induced retinopathy and no abnormal retinal development is seen.Citation25
Figure 2 Fluorescein angiogram of a Wnt-associated vitreoretinopathy patient with an Fzd4 mutation.
Norrin may protect the developing eye from abnormal angiogenesis and retinopathy by modulating Wnt signaling as well. A comparison of room air mice and oxygen-induced retinopathy (OIR) mice showed significant changes in the expression of Wnt ligands that bind and activate the Fzd4 receptor. Messenger RNA is significantly increased for Wnts 3a, 7a, and 10a but not for norrin during the development of retinopathy.Citation26 This indicates that norrin provides a protective effect, in part, by modulating the activity of other Wnts when they are pathologically upregulated.
Nonfenestrated vessels
Retinal vessels are highly specialized nonfenestrated vessels, as seen in the central nervous tissue. This requires vessels to have tight junctions, formed by claudins and occludens, which prevents passive movement of fluids and proteins through the vessel walls.Citation27,Citation28
Claudins are integral transmembrane proteins necessary for tight junction formation.Citation28 CLD5 is expressed by retinal endothelial cells. At the cellular level, Wnt signaling modulates tight-junction maintenance in the retinal vessels by upregulating expression of CLD5.Citation29–Citation31 Disrupted norrin-Fzd4 binding leads to decreased expression of CLD5 and breakdown of the blood–retinal barrier.Citation4,Citation26 Although there is evidence that norrin activates all three of the intracellular Wnt pathways,Citation32–Citation35 loss of the co-receptor LRP5 results in decreased expression of CLD5, indicating that tight junction formation and maintenance is primarily driven by the canonical pathway.Citation26
Neurogenesis
Norrin is a small secreted protein with a cysteine-knot motif.Citation14–Citation16 The cysteine-knot motif is highly conserved in many growth factors, including TGF-β, human chorionic gonadotropin, NGF, and PDGF. The structural similarity of norrin to other growth factors suggests that it may have a function in addition to traditional Wnt-signaling, despite the fact that it is best characterized as a Wnt-receptor ligand. This theory is supported by its lack of structural similarity to other Wnt proteins.Citation17
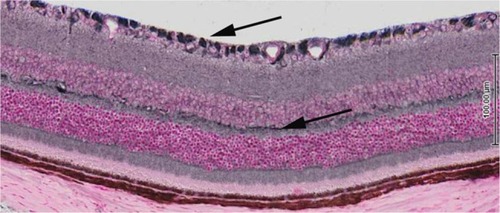
Norrin’s role in neurogenesis and neuroprotection is just being elucidated and appears to be multifactorial. Recent investigations have uncovered that norrin acts as a ligand for LGR4, a receptor for Wnt activation that regulates neurogenesis.Citation36 Recent mapping of LGR4 expression in the retina shows that it is expressed by Mueller cells, photoreceptor inner segments, retinal ganglion cells, and bipolar cells (). This suggests that norrin has multiple roles in the formation of the neurovascular unit in the retina. Norrin neuroprotection has been reported in models of retinal toxicity. Seitz et al reported increased survival of retinal ganglion cell (RGC) axons and decreased apoptosis in N-methyl-D-aspartate-injected mice following simultaneous injection with norrin.Citation37 The authors concluded that norrin’s protective effect on RGCs was caused by an increase of Muller cell neurotrophic growth factor expression. Norrin neuroprotection has been demonstrated in the photoreceptors, as well. Braunger and Tamm reported that norrin provided protection against light damage to photoreceptors in mice overexpressing norrin in their RPE cells.Citation5 The authors proposed that norrin provides protection to the photoreceptors by increasing the expression of neurotrophic growth factors, specifically BDNF. It is plausible that norrin provides protection to RGCs in OIR eyes by increasing the expression of Muller cell derived neurotrophic factors and, thereby, preventing excitotoxicity and subsequent loss of RGCs.
Clinical manifestations
To various degrees, eyes with Wnt-associated vitreoretinopathies exhibit incomplete peripheral vascularization, incomplete regression of the primary hyaloid system, capillary drop-out, endothelial budding, and breakdown of the blood–retina barrier, often leading to exudative and tractional retinal detachments.Citation15–Citation18 Norrie disease is an X-linked disorder and has the most severe phenotype resulting in blindness, often present at birth. It is often caused by mutations in the conserved cysteine residues of the NDP gene, which comprise the necessary tertiary structure involved in ligand–receptor binding.Citation18 In PFV, the premature vasculature of the eye does not regress appropriately, resulting in the formation of a fibrous stalk that extends from the optic disk to the posterior lens capsule. Since the fibrous stalks are common in both PFV and Norrie Disease, the two disorders are often indistinguishable if bilateral disease is present. FEVR occurs in X-linked (NDP), autosomal recessive (LRP5), and autosomal dominate inheritance patterns (Fzd4, TSpan12, ZNF408). Most reported cases of AdFEVR have been associated with heterozygous mutations in the Fzd4 gene.Citation14,Citation16 A unifying characteristic in these diseases is an aberration of retinal vascular development demonstrating varying degrees of peripheral avascular retina, abnormal vascularization with retinal neovascularization, and subretinal exudation.
Wnt signaling and ROP
ROP and FEVR have overlapping phenotypes and an ambiguous birth history may confuse the diagnosis. Generally, ROP is distinguished from FEVR by premature birth and lack of a family history of eye disease. A combination of incomplete peripheral retinal vessel development and exposure to a relatively high oxygen environment (compared to in utero) is believed to set the stage for development of ROP. However, gestational age (GA), birth weight, and systemic health alone do not predict the progression to retinopathy, thus requiring that all at-risk infants have routine screening exams every 1–2 weeks. The overlap in retinal characteristics has supported the hypothesis that a predisposition for ROP is created by genetic alterations. In fact, a twin study demonstrated that there is a likely genetic predisposition for ROP development in 80% of premature infants with retinopathy.Citation38 Mutations in both the NDP and Fzd4 genes have been reported in ROP cases and make Wnt-pathway mutations a likely candidate as a risk factor for developing ROP.Citation14,Citation17–Citation20,Citation39
Several studies have reported Fzd4, NDP, and LRP5 gene mutations in ROP patients and these appear to be particularly associated with more severe ROP.Citation13–Citation20,Citation30,Citation37 The first report discovered mutations located in the 5’ untranslated region of the NDP geneCitation18,Citation40 A subsequent study found 13% (7/53) of infants with advanced ROP had either Fzd4 (n=3) or LRP5 (n=4) mutations.Citation17 Similarly, Ells et al found three Fzd4 mutations (Arg466Trp, Ala370Gly, Lys203Asn) in a group of 71 severe ROP patients and no Fzd4 mutations in 33 patients with mild or no ROP.Citation20 Both studies concluded that Fzd4 mutations may have a role in the development or exacerbation of ROP. Ells et al also found the compound variation (P33S;P168S) or either mutation alone in 10% of their severe ROP patients and in only 3% of patients with mild to no ROP. However, since they also found (P33S;P168) in 7% of subjects in a group of random Caucasian samples, they could not make a conclusion regarding the causality of the variation. A recent publication confirmed this finding noting a high association with the Fzd4 compound variation (P33S;P168S) and ROP. In this study, a large patient database (n=519) was analyzed for mutations affecting the Wnt-signaling pathway.Citation41 Infants diagnosed with ROP had the highest frequency of the (P33S;P168S) variation (7.5%) compared to FEVR, possible FEVR, Norrie disease or bilateral PFV. In contrast, the rate in healthy newborns (3.1%) was not significantly different from that predicted based on the subjects in the 1,000 Genomes project, with a prevalence of the (P33S;P168S) variation in 20 out of 1,092 random subjects (1.8%).
Role of Wnt signaling in fetal development
The link between premature birth and ROP may lie with altered Wnt signaling. Studies have shown that norrin-Fzd4 signaling affects angiogenesis in the female reproductive system. Markers for angiogenesis and vascular formation are reduced in the corpora lutea of Fzd4 null mice and these mice are infertile.Citation42 Also, Fzd4 mRNA localizes to vessels and stroma surrounding the mouse embryo.Citation43 Similarly, norrin has been localized to the uterine blood vessels and decidual cells of rats,Citation44 and NDP knockout mice have defects in vascular development and decidualization in pregnancy that leads to embryonic loss.Citation45 In humans, the expression of NDP has been established in the placenta, and Fzd4 expression has been localized to placental villous mesenchymal cells.Citation45,Citation46
Infants expressing the (P33S;P168S) Fzd4 variant have significantly reduced weight for GA.Citation41 The lower than normal birth weights for GA present in the ROP patients with the (P33S;P168S) variant compared to the other premature infants supports the theory that the Fzd4 receptor is involved in placental formation and/or fetal growth. In fact, five of seven ROP patients with the variation were in the group that had the highest birth weight deficits and were defined as small for gestational age. Presumably, the presence and/or absence of the sequence variation in the mothers would also be a complicating factor in fertility and/or placental development as would additional mutations in NDP; Fzd4 pathway genes.Citation41
Summary
The Wnt signaling pathway plays a critical role in retinal development, in regard to both angiogenesis and neurogenesis. It is also vital for normal fetal growth and may be a predictor for placental insufficiency and preterm birth. Specifically, Wnt activation through proper norrin:Fzd4 binding is specific to retinal development. Alterations affecting this pathway often result in Wnt-associated vitreoretinopathies and may be a risk factor for developing ROP. Therefore, it may be beneficial to screen premature infants for the presence of Wnt signaling mutations so that these patients may be monitored more carefully. Additionally, patients with anomalous vasculature and displaying any of the characteristics commonly seen with Wnt-pathway mutations should be offered genetic testing.
Disclosure
The author has no conflicts of interest to disclose.
References
- TsaousiAMillCGeorgeSJThe Wnt pathways in vascular disease: lessons from vascular developmentCurr Opin Lipidol201122535035721841485
- BowermanBCell signaling. Wnt moves beyond the canonScience2008320587432732818420922
- KühlMThe WNT/calcium pathway: biochemical mediators, tools and future requirementsFront Biosci2004996797414766423
- WangYRattnerAZhouYNorrin/Frizzled4 signaling in retinal vascular development and blood brain barrier plasticityCell201215161332134423217714
- BraungerBMTammERThe different functions of NorrinAdv Exp Med Biol201272367968322183393
- OhlmannATammERNorrin: molecular and functional properties of an angiogenic and neuroprotective growth factorProg Retin Eye Res201231324325722387751
- YeXWangYNathansJThe Norrin/Frizzled4 signaling pathway in retinal vascular development and diseaseTrends Mol Med201016941742520688566
- ChenJStahlAKrahNMWnt signaling mediates pathological vascular growth in proliferative retinopathyCirculation2011124171871188121969016
- SimsKBNDP-related retinopathiesPagonRAAdamMPArdingerHHGeneReviews(R)Seattle (WA)University of Washington, Seattle1993
- ChamneySMcLooneEWilloughbyCEA mutation in the Norrie disease gene (NDP) associated with familial exudative vitreoretinopathyEye (Lond)201125121658
- ChenZYBattinelliEMFielderAA mutation in the Norrie disease gene (NDP) associated with X-linked familial exudative vitreoretinopathyNat Genet1993521801838252044
- DickinsonJLSaleMMPassmoreAMutations in the NDP gene: contribution to Norrie disease, familial exudative vitreoretinopathy and retinopathy of prematurityClin Experiment Ophthalmol200634768268816970763
- ChungBDKayseriliHAiMA mutation in the signal sequence of LRP5 in a family with an osteoporosis-pseudoglioma syndrome (OPPG)-like phenotype indicates a novel disease mechanism for tri-nucleotide repeatsHum Mutat200930464164819177549
- DrenserKADaileyWVinekarAClinical presentation and genetic correlation of patients with mutations affecting the FZD4 geneArch Ophthalmol2009127121649165420008721
- GilmourDFFamilial exudative vitreoretinopathy and related retinopathiesEye (Lond)201529111425323851
- ToomesCDowneyLFamilial exudative vitreoretinopathy, autosomal dominantPagonRAAdamMPArdingerHHGeneReviews(R)Seattle (WA)University of Washington, Seattle1993
- KondoHKusakaSYoshinagaAGenetic variants of FZD4 and LRP5 genes in patients with advanced retinopathy of prematurityMol Vis20131947648523441120
- WuWCDrenserKTreseMCaponeAJrDaileyWRetinal phenotype-genotype correlation of pediatric patients expressing mutations in the Norrie disease geneArch Ophthalmol2007125222523017296899
- HiraokaMTakahashiHOrimoHGenetic screening of Wnt signaling factors in advanced retinopathy of prematurityMol Vis2010162572257721151595
- EllsAGuernseyDLWallaceKSevere retinopathy of prematurity associated with FZD4 mutationsOphthalmic Genet2010311374320141357
- RobitailleJMWallaceKZhengBPhenotypic overlap of familial exudative vitreoretinopathy (FEVR) with persistent fetal vasculature (PFV) caused by FZD4 mutations in two distinct pedigreesOphthalmic Genet2009301233019172507
- BlackGCPerveenRBonshekRCoats’ disease of the retina (unilateral retinal telangiectasis) caused by somatic mutation in the NDP gene: a role for norrin in retinal angiogenesisHum Mol Genet19998112031203510484772
- RobitailleJMZhengBWallaceKThe role of Frizzled-4 mutations in familial exudative vitreoretinopathy and Coats diseaseBr J Ophthalmol201195457457921097938
- TokunagaCCMittonKPDaileyWEffects of anti-VEGF treatment on the recovery of the developing retina following oxygen-induced retinopathyInvest Ophthalmol Vis Sci201455318849224550366
- OhlmannASeitzRBraungerBNorrin promotes vascular regrowth after oxygen-induced retinal vessel loss and suppresses retinopathy in miceJ Neurosci20103011839320053900
- ChenJStahlAKrahNMRetinal expression of Wnt-pathway mediated genes in low-density lipoprotein receptor-related protein 5 (Lrp5) knockout micePLoS One201271e3020322272305
- HaseloffRFDithmerSWinklerLTransmembrane proteins of the tight junctions at the blood-brain barrier: structural and functional aspectsSemin Cell Dev Biol201538162525433243
- Van ItallieCMAndersonJMClaudin interactions in and out of the tight junctionTissue Barriers201313e2524724665401
- KotoTTakuboKIshidaSHypoxia disrupts the barrier function of neural blood vessels through changes in the expression of claudin-5 in endothelial cellsAm J Pathol2007170413899717392177
- LuoYXiaoWZhuXDifferential expression of claudins in retinas during normal development and the angiogenesis of oxygen-induced retinopathyInvest Ophthalmol Vis Sci2011521075566421862644
- ShenWLiSChungSHTyrosine phosphorylation of VE-cadherin and claudin-5 is associated with TGF-β1-induced permeability of centrally derived vascular endotheliumEur J Cell Biol20119043233221168935
- DescampsBSewduthRTojaisNFrizzled4 regulates arterial network organization through noncanonical Wnt/Planar Cell polarity signalingCir Res20124758
- YaoRNatsumeYNodaTMAGI-3 is involved in the regulation of the JNK signaling pathway as a scaffold protein for frizzled and LtapOncogene2004236023603015195140
- RobitailleJMacDonaldMLKaykasAMutant frizzled-4 disrupts retinal angiogenesis infamilial exudative vitreoretinopathyNat Genet20023232633012172548
- JungeHYangSBurtonJTSPAN12 Regulates retinal vascular development by promoting Norrin – but not Wnt-induced FZD4/β-Catenin signalingCell200913929931119837033
- DengCReddyPChengYMulti-functional norrin is a ligand for the LGR4 receptorJ Cell Sci2013126Pt 92060823444378
- HutchesonKAPaluruPCBernsteinSLNorrie disease gene sequence variants in an ethnically diverse population with retinopathy of prematurityMol Vis2005115018
- BizzarroMJHussainNJonssonBGenetic susceptibility to retinopathy of prematurityPediatrics2006118518586317079555
- MohamedSSchaaKCooperMEGenetic contributions to the development of retinopathy of prematurityPediatr Res2009652193718787502
- HiraokaMBerinsteinDMTreseMTInsertion and deletion mutations in the dinucleotide repeat region of the norrie disease gene in patients with advanced retinopathy of prematurityJ Hum Genet20014641788111322656
- DaileyWAGrycWGargPGFrizzled-4 variations associated with retinopathy and intrauterine growth retardation: A potential marker for prematurity and retinopathyOphthalmology2015122919172326119001
- MilhemRMBen-SalemSAl-GazaliLIdentification of the cellular mechanisms that modulate trafficking of frizzled family receptor 4 (FZD4) missense mutants associated with familial exudative vitreoretinopathyInvest Ophthalmol Vis Sci201455634233124744206
- HsiehMBoerboomDShimadaMMice null for Frizzled4 (Fzd4−/−) are infertile and exhibit impaired corpora lutea formation and functionBiol Reprod200573611354616093361
- HayashiKEriksonDWTilfordSAWnt genes in the mouse uterus: Potential regulation of implantationBiol Reprod2009805989100019164167
- KalogluCCesurIBulutHENorrin immunolocalization and its possible functions in rat endometrium during the estrus cycle and early pregnancyDev Growth Differ20115378879621899531
- LuhmannUFMeunierDShiWFetal loss in homozygous mutant norrie disease mice: A new role of norrin in reproductionGenesis20054242536216035034
- KnoflerMPollheimerJHuman placental trophoblast invasion and differentiation: A particular focus on wnt signalingFront Genet2013419024133501