Abstract
The pivotal role of the neurosensory retina in retinopathy of prematurity (ROP) disease processes has been amply demonstrated in rat models. We have hypothesized that analogous cellular processes are operative in human ROP and have evaluated these presumptions in a series on non-invasive investigations of the photoreceptor and post-receptor peripheral and central retina in infants and children. Key results are slowed kinetics of phototransduction and deficits in photoreceptor sensitivity that persist years after ROP has completely resolved based on clinical criteria. On the other hand, deficits in post-receptor sensitivity are present in infancy regardless of the severity of the ROP but are not present in older children if the ROP was so mild that it never required treatment and resolved without a clinical trace. Accompanying the persistent deficits in photoreceptor sensitivity, there is increased receptive field size and thickening of the post-receptor retinal laminae in the peripheral retina of ROP subjects. In the late maturing central retina, which mediates visual acuity, attenuation of multifocal electroretinogram activity in the post-receptor retina led us to the discovery of a shallow foveal pit and significant thickening of the post-receptor retinal laminae in the macular region; this is most likely due to failure of the normal centrifugal movement of the post-receptor cells during foveal development. As for refractive development, myopia, at times high, is more common in ROP subjects than in control subjects, in accord with refractive findings in other populations of former preterms. This information about the neurosensory retina enhances understanding of vision in patients with a history of ROP, and taken as a whole, raises the possibility that the neurosensory retina is a target for therapeutic intervention.
Introduction
Retinopathy of prematurity (ROP) is among the common retinal neovascular conditions that include diabetic retinopathy, age-related macular degeneration, and central vein occlusion.Citation1 ROP is distinguished from these conditions because it occurs in immature retina. Although the disease is mild and resolves spontaneously in the majority of cases, ROP remains a leading cause of avoidable blindness worldwide.Citation2,Citation3
ROP has its onset at preterm agesCitation4 when the retinal vasculatureCitation5–Citation8 and the neurosensory retinaCitation9 are immature (). The rod photoreceptors, which are ∼20 times more numerous than the cones, are the last retinal cells to mature, with the exception of the relatively small number of foveal cones.Citation10,Citation11 Even the small vessels in the interplexiform layers normally mature earlier than the rods.Citation6
Figure 1 The developmental increase in rhodopsin content of the retina and temporal retinal coverage by large vessels.
Abbreviation: ROP, retinopathy of prematurity.
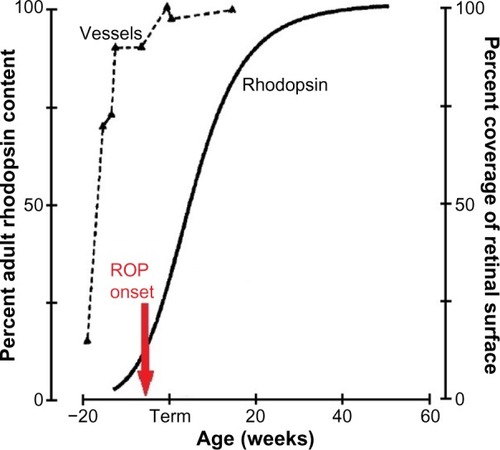
As shown in , the onset of ROPCitation4 is coincident with the rapid increase in the rhodopsin content of the developing retina. ROP resolves in early post-term weeksCitation12,Citation13 when rod outer segment development tails off. Thus, although we are all well aware that the accepted clinical hallmark of ROP is abnormal retinal vasculature, we cannot ignore the involvement of the neural retina in the ROP disease process and the burgeoning metabolic demands of the rapidly developing rods.
We have found evidence that the neurosensory retina is very much involved in the ROP disease process by studying children and rat models of ROP. The escalating metabolic needs of the oxygen-greedy rods are poised to be an instigating factor for ROP. In rat models, rod photoreceptor dysfunction is detectable before retinal vascular abnormalities manifest.Citation14 Our longitudinal study of rat models shows that early rod dysfunction predicts the vascular outcome, and not vice versa.Citation15–Citation18 We have shown that 1) recovery of the ROP rat’s post-receptor retinal sensitivity and retinal vasculature is under the cooperative control of growth factors, which in other neural tissues mediate neural–vascular crosstalkCitation19 and 2) pharmacological lessening of the developing rod’s metabolic needs improves the vascular outcome.Citation20 In children, there are significant effects on retinal and visual function and eye growth long after the clinical resolution of ROP; key results are presented below. In short, rods are involved before, during, and after active ROP.
The subjects in our studies met the criteria for ROP screeningCitation21 and underwent serial examinations in the neonatal intensive care unit, the frequency of which was based on the program of examinations in the multicenter ROP trials (CRYO-ROP and ETROP).Citation22,Citation23 We categorized the subjects as having had severe ROP, mild ROP, or no ROP, as shown in . This categorization was based on the International Committee for the Classification of Retinopathy of Prematurity (ICROP) systemCitation24 whereby the site of the disease is specified by zone (I–III from central to peripheral), the extent within the zone by number of affected clock hours (1–12), and disease severity by stage (1–5 from mild to complete retinal detachment).
Table 1 Classification of subjects in our ROP studies
Through a series of non-invasive studies of the neurosensory retina in human ROP subjects, we have found persistent effects on rod photoreceptor function and evidence of intralaminar re-organization of post-receptor retina. These studies have employed electroretinogram (ERG),Citation25 psychophysical,Citation26,Citation27 and retinal imaging procedures.Citation28,Citation29 We have also found significant departures from normal in eye growthCitation30 and refractive development.Citation31,Citation32
Peripheral retina in ROP
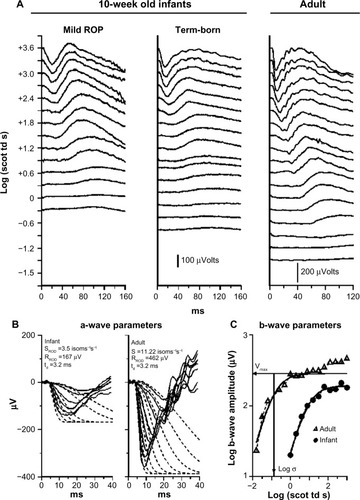
In our ERG studies, we recorded and analyzed rod photoreceptor activity represented in the a-wave and the rod-driven post-receptor activity represented in the b-wave () using procedures described previously.Citation33–Citation36 In infants with a history of ROP (median age 10 weeks post-term), both rod and rod-driven post-receptor sensitivity were low.Citation25 In children (median age 10 years), post-receptor sensitivity normalizes but the deficits in rod photoreceptor sensitivity persist even if the ROP had been mild.Citation25 These ERG data are evidence that after clinical healing (judged by inspection of the retinal vasculature), the post-receptor neural circuitry undergoes intralaminar reorganization.Citation37–Citation42 This is accompanied by effects on rod-mediated visual sensitivity that are demonstrated by our psychophysical studies.Citation26,Citation27
Figure 2 Sample rod-mediated ERG responses to full-field stimuli.
Abbreviations: ERG, electroretinogram; ROP, retinopathy of prematurity.
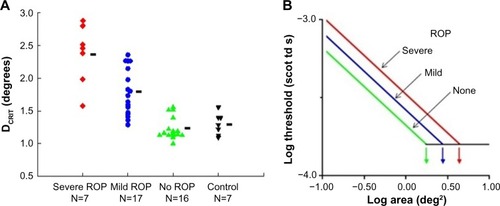
We tested rod-mediated vision to evaluate spatial and temporal summation in older children with a history of ROP and in control subjects.Citation26,Citation27 The underlying concept of spatial summation is that a large number of rods in a given retinal region connect to a neuron; the receptive field is the restricted post-receptor region onto which this group of photoreceptors converge. In our spatial summation test,Citation27 the diameter of a test spot was varied; the dark adapted threshold for the detection of the test spot was measured for eight different spot diameters, ranging from 0.4° (tiny) to 10° (big – about the diameter of a soft ball at arm’s length). The results () show that the critical diameter is larger in subjects with a history of ROP than in preterm subjects who never had ROP and term-born controls. In other words, visual signals are integrated over a larger area (larger receptive field) in ROP subjects; the larger receptive field benefits visual sensitivity. This is further evidence of intralaminar re-organization of the post-receptor ROP retina.
Figure 3 Rod-mediated spatial summation.
Abbreviations: DCRIT, critical diameter; ROP, retinopathy of prematurity.
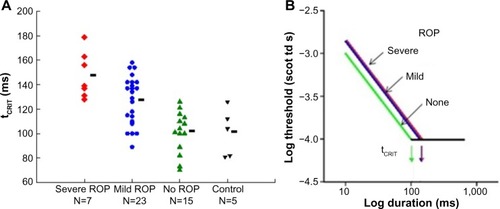
Temporal summation is an indicator of the kinetics of phototransduction in the photoreceptors. In our rod-mediated temporal summation test,Citation26 the duration of a constant diameter test spot (10°) was varied and the dark adapted threshold for the detection of the test spot was measured for eight different durations, ranging from brief (10 ms) to long (640 ms). The results () show that the critical duration is longer in subjects with a history of ROP than in preterm subjects who never had ROP and term-born controls. This is a consequence of the slow kinetics of activation of rod phototransduction in ROP, in accord with the ERG a-wave results.Citation5
Figure 4 Rod-mediated temporal summation.
Abbreviations: tCRIT, critical duration; ROP, retinopathy of prematurity.
For both spatial and temporal summation, reciprocity prevailed.Citation26,Citation27 That is, as indicated by the diagonal lines with slope −1 on the log–log plots shown in the right panels in and , the subjects could detect a light ten times dimmer if the stimulus was ten times bigger or ten times longer. Once a critical large size or long duration was reached, the threshold remained about the same as the stimulus size or duration increased.
In an adaptive optical coherence tomography (OCT) study of the retinal laminae at 18 degrees temporal eccentricity, we found a higher ratio of post-receptor to photoreceptor thickness in ROP subjects than in term-born control subjects.Citation43 In retinal degenerative disorders, thickened inner retinal laminae are reported in those retinal regions where the photoreceptors are disturbed or lostCitation44 and are postulated to be the retina’s compensation for altered photoreceptor inputs to the post-receptor retina.
Central retina in ROP
This region includes the fovea and the macula. Both cones and rods are found in ROP zone 1 ().Citation24 The most commonly measured visual function, visual acuity, is mediated by the foveal cones. Acuity deficits in ROP patients are common, even if the ROP had been successfully treated or was mild and, by clinical criteria, resolved completely. Most of the 15-year alumni of the CRYO-ROP studyCitation45 and two-thirds of the 6-year alumni of the ETROP studyCitation46 had acuity poorer than 20/40.
Figure 5 Diagram of the International Classification of Retinopathy of Prematurity zonesCitation24 with a superimposed fundus photograph on which the optic disc and fovea are indicated.
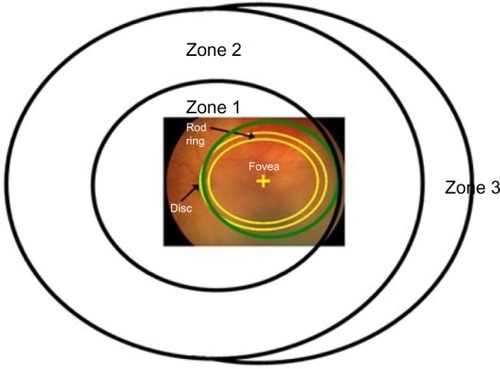
The central retina matures relatively late. For instance, the outer segments of the rods central to the ring undergo later developmental elongation than those peripheral to the ring. There is a functional parallel. In healthy infants, dark adapted visual thresholds central to the ring mature more slowly than those peripheral to the ring.Citation47,Citation48
We hypothesized that this late maturing central region is particularly vulnerable to ROP. Through a series of non-invasive studies of the central retina in subjects with a history of mild ROP, we found 1) delayed maturation of rod-mediated retinal sensitivity using psychophysical procedures,Citation49 2) deficits in cone-driven post-receptor activity using the multifocal ERG (mfERG),Citation50 and 3) persistent abnormalities of the intraretinal vasculature using adaptive optics retinal imaging.Citation28
In our longitudinal psychophysical study of infants with a history of mild ROP,Citation49 we found that, even though the clinical disease had resolved spontaneously and completely by term, dark adapted visual thresholds showed a protracted course of development that continued until 18 months post-term, whereas in term-born controls, the thresholds were mature by age 6 months.
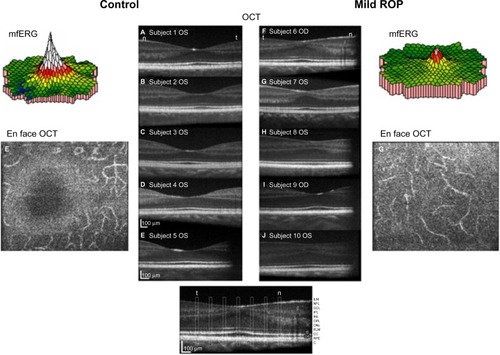
The mfERG provides topographical information about the central retina.Citation51 Cone-driven bipolar cells (post-receptor retina) are the main contributors to mfERG responses.Citation52 We found that mfERG responses were significantly smaller in subjects with a history of mild ROP than in control subjects (, color 3-D plots).Citation50 This result led us to hypothesize that bipolar cell density differs between ROP and control subjects. Using adaptive optics retinal imaging (),Citation28 we found that the foveal pit in mild ROP eyes was significantly shallower than in control eyes and that the inner retinal laminae of foveal and extrafoveal regions were significantly thicker in ROP eyes than in control eyes. This is evidence of failure of centrifugal movement of the bipolar cells during foveal development in ROP, as others have also concluded.Citation53
Figure 6 Adaptive optics OCT images of ROP and control subjects.
Abbreviations: mfERG, multifocal ERG; OCT, optical coherence tomography; OD, right; OS, left; ROP, retinopathy of prematurity; ILM, inner limiting membrane; NFL, nerve fiber layer; GCL, ganglion cell layer; IPL, inner plexiform layer; INL, inner nuclear layer; OPL, outer plexiform layer; ONL, outer nuclear layer; ELM, external limiting membrane; CC, connecting cilium; RPE, retinal pigment epithelium; C, choroid.
What leads to abnormal foveal structureCitation28 and functionCitation50 in ROP? What goes wrong in the neurovascular development of the central retina in prematurely born subjects? These important issues remain under investigation. The absence of foveal avascular zone and hypoperfusion of this important retinal region during development have been discussed as contributing factorsCitation54 There is also OCT evidence of cystoid macular edema in ROP infants.Citation55
Refractive development
Numerous studies have shown a high incidence of refractive errors, particularly myopia, in infants born prematurely.Citation31,Citation56–Citation62 Although these findings suggest a disturbance in the normal regulation of ocular growth, the mechanisms have yet to be specified. It seems unlikely that the mechanisms that are operational in experimental myopiaCitation63–Citation65 are applicable to ROP. Interestingly, we have noted that in ROP infants, low rod photoreceptor sensitivity, as determined by analysis of the scotopic ERG a-wave, predicted later myopia.Citation32 Deficits in cone ERG responses have been reported in chicks with form deprivation myopia.Citation66
We have developed a model of normal eye growth and applied it to the growth of ROP eyes.Citation30 Through analysis of extant magnetic resonance images (MRI), we found that the growth of the ROP eye is slow and results in eyes that are shorter and have steeper corneas and thicker lenses compared with those in preterm eyes without a history of ROP and in term-born eyes. Refractive development has also been studied in a rat model of ROP;Citation67,Citation68 more work is needed to translate these findings to the human ROP eye.
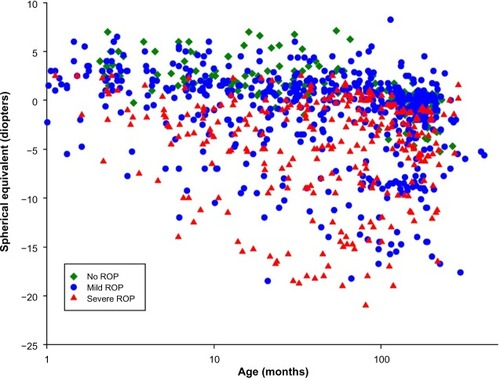
shows the results of refractions performed on 279 of our ROP subjects. These data are in reasonable accord with refractions in other populations of former preterms. Compared with the prediction limits for normal refractive development,Citation69,Citation70 myopia was more frequent in subjects with ROP than in preterm born subjects who never developed ROP and term-born control subjects. In the preterm subjects who never had ROP, myopia seldom occurred, whereas hyperopia was quite common. Previously reported data showed that, in ROP subjects with myopia, the magnitude of myopia typically increased with age.Citation31 We are attempting to unravel the mechanisms that underlie refractive development in ROP by analyzing animal models of ROP,Citation67,Citation68 and have developed a human model eye to facilitate studies in infants and children.Citation30
Figure 7 Spherical equivalent refraction (diopters) as a function of age (months).
Abbreviations: ROP, retinopathy of prematurity; PL, prediction limit.
Conclusion
Non-invasive investigation of former preterms, conceived within a framework of molecular and cellular processes known to occur in normal developing human retina and rat models of ROP,Citation6,Citation19,Citation71–Citation73 yields a numeric description of retinal, visual and refractive development in these infants and children. Taken as a whole, these data derived from electroretinographic, psychophysical and retinal imaging studies link the children’s results to the molecular and cellular processes. From a practical perspective, this body of information contributes to the understanding of vision in children with a history of ROP. Recognition that the neurosensory retina has a role in ROP opens the possibility of future novel therapeutic approaches to ROP.
Acknowledgments
Supported by National Eye Institute Grant R01 EY-010597.
Disclosure
The authors report no conflicts of interest in this work.
References
- Al-ShabraweyMElsherbinyMNussbaumJOthmanAMegyerdiSTawfikATargeting neovascularization in ischemic retinopathy: recent advancesExpert Rev Ophthalmol20138326728625598837
- World Health OrganizationVision2020 Report: Global Initiative for the Elimination of Avoidable Blindness, Action Plan 2006–2011GenevaWorld Health Organization2007
- SteinkullerPGDuLGilbertCFosterACollinsMLCoatsDKChildhood blindnessJ Aapos199931263210071898
- PalmerEAFlynnJTHardyRJIncidence and early course of retinopathy of prematurity. The Cryotherapy for Retinopathy of Prematurity Cooperative GroupOphthalmology19919811162816401800923
- FultonABHansenRMMoskowitzAAkulaJDThe neurovascular retina in retinopathy of prematurityProg Retin Eye Res200928645248219563909
- ProvisJMDevelopment of the primate retinal vasculatureProg Retin Eye Res200120679982111587918
- ProvisJMHendricksonAEThe foveal avascular region of developing human retinaArch Ophthalmol2008126450751118413520
- ProvisJMLeechJDiazCMPenfoldPLStoneJKeshetEDevelopment of the human retinal vasculature: cellular relations and VEGF expressionExp Eye Res19976545555689464188
- HendricksonAEThe morphologic development of human and monkey retinaAlbertDMJakobiecFAPrinciples and Practice of Ophthalmology: Basic SciencesPhiladelphiaWB Saunders1994561577
- CurcioCASloanKRKalinaREHendricksonAEHuman photoreceptor topographyJ Comp Neurol199029244975232324310
- OsterbergGTopography of the layer of rods and cones in the human retinaActa Ophthal Suppl193561103
- NiYQHuangXXueKNatural involution of acute retinopathy of prematurity not requiring treatment: factors associated with the time course of involutionInvest Ophthalmol Vis Sci20145553165317024764065
- RepkaMXPalmerEATungBInvolution of retinopathy of prematurity. Cryotherapy for Retinopathy of Prematurity Cooperative GroupArch Ophthalmol2000118564564910815156
- ReynaudXHansenRMFultonABEffect of prior oxygen exposure on the electroretinographic responses of infant ratsInvest Ophthalmol Vis Sci19953610207120797657546
- AkulaJDHansenRMMartinez-PerezMEFultonABRod photoreceptor function predicts blood vessel abnormality in retinopathy of prematurityInvest Ophthalmol Vis Sci20074894351435917724227
- AkulaJDMockoJAMoskowitzAHansenRMFultonABThe oscillatory potentials of the dark-adapted electroretinogram in retinopathy of prematurityInvest Ophthalmol Vis Sci200748125788579718055833
- LiuKAkulaJDFalkCHansenRMFultonABThe retinal vasculature and function of the neural retina in a rat model of retinopathy of prematurityInvest Ophthalmol Vis Sci20064762639264716723481
- LiuKAkulaJDHansenRMMoskowitzAKleinmanMSFultonABDevelopment of the electroretinographic oscillatory potentials in normal and ROP ratsInvest Ophthalmol Vis Sci200647125447545217122135
- AkulaJDMockoJABenadorIYThe neurovascular relation in oxygen-induced retinopathyMol Vis2008142499250819112532
- AkulaJDHansenRMTzekovRVisual cycle modulation in neurovascular retinopathyExp Eye Res201091215316120430026
- FiersonWMAmerican Academy of Pediatrics Section on Ophthalmology, American Academy of Ophthalmology, American Association for Pediatric Ophthalmology and Strabismus, American Association of Certified Orthoptists. Screening examination of premature infants for retinopathy of prematurityPediatrics2013131118919523277315
- Cryotherapy for Retinopathy of Prematurity Cooperative GroupMulticenter trial of cryotherapy for retinopathy of prematurity cooperative groupArch Ophthalmol198810644714792895630
- Early Treatment for Retinopathy of Prematurity Cooperative GroupRevised indications for the treatment of retinopathy of prematurity: results of the early treatment for retinopathy of prematurity randomized trialArch Ophthalmol2003121121684169414662586
- International Committee for the Classification of Retinopathy of PrematurityThe international classification of retinopathy of prematurity revisitedArch Ophthalmol2005123799199916009843
- HarrisMEMoskowitzAFultonABHansenRMLong-term effects of retinopathy of prematurity (ROP) on rod and rod-driven functionDoc Ophthalmol20111221192721046193
- HansenRMMoskowitzATavorminaJLBushJNSoniGFultonABTemporal summation in children with a history of retinopathy of prematurityInvest Ophthalmol Vis Sci201556291491725604681
- HansenRMTavorminaJLMoskowitzAFultonABEffect of retinopathy of prematurity on scotopic spatial summationInvest Ophthalmol Vis Sci20145553311331324781938
- HammerDXIftimiaNVFergusonRDFoveal fine structure in retinopathy of prematurity: an adaptive optics Fourier domain optical coherence tomography studyInvest Ophthalmol Vis Sci20084952061207018223243
- RamamirthamRGarimaSAkulaJDExtrafoveal cone packing density and geometry in retinopathy of prematurity (ROP)Invest Ophthalmol Vis Sci201556 ARVO E-Abstract 4933
- MunroRJFultonABChuiTYEye growth in term- and preterm-born eyes modeled from magnetic resonance imagesInvest Ophthalmol Vis Sci20155653121313126024095
- LueCLHansenRMReisnerDSFindlOPetersenRAFultonABThe course of myopia in children with mild retinopathy of prematurityVision Res1995359132913357610594
- MoskowitzAHansenRMFultonABEarly ametropia and rod photoreceptor function in retinopathy of prematurityOptom Vis Sci200582430731715829858
- FultonABHansenRMElectroretinogram responses and refractive errors in patients with a history of retinopathy prematurityDoc Ophthalmol1995912871008813488
- FultonABHansenRMThe development of scotopic sensitivityInvest Ophthalmol Vis Sci20004161588159610798680
- FultonABHansenRMPetersenRAVanderveenDKThe rod photoreceptors in retinopathy of prematurity: an electroretinographic studyArch Ophthalmol2001119449950511296015
- FultonABHansenRMMoskowitzAThe cone electroretinogram in retinopathy of prematurityInvest Ophthalmol Vis Sci200849281481918235032
- JonesBWKondoMTerasakiHLinYMcCallMMarcRERetinal remodelingJpn J Ophthalmol201256428930622644448
- MarcREJonesBWAndersonJRNeural reprogramming in retinal degenerationInvest Ophthalmol Vis Sci20074873364337117591910
- MarcREJonesBWWattCBVazquez-ChonaFVaughanDKOrganisciakDTExtreme retinal remodeling triggered by light damage: implications for age related macular degenerationMol Vis20081478280618483561
- XuHPTianNPathway-specific maturation, visual deprivation, and development of retinal pathwayNeuroscientist200410433734615271261
- XuHPTianNRetinal ganglion cell dendrites undergo a visual activity-dependent redistribution after eye openingJ Comp Neurol2007503224425917492624
- XuHPTianNGlycine receptor-mediated synaptic transmission regulates the maturation of ganglion cell synaptic connectivityJ Comp Neurol20085091537118425804
- AkulaJDMocofanescuAFergusonRDRetinal remodeling in retinopathy of prematurityInvest Ophthalmol Vis Sci201454 ARVO E-Abstract 3505
- HuangWCCideciyanAVRomanAJInner and outer retinal changes in retinal degenerations associated with ABCA4 mutationsInvest Ophthalmol Vis Sci20145531810182224550365
- PalmerEAHardyRJDobsonV15-year outcomes following threshold retinopathy of prematurity: final results from the multicenter trial of cryotherapy for retinopathy of prematurityArch Ophthalmol2005123331131815767472
- Early Treatment for Retinopathy of Prematurity Cooperative GDobsonVQuinnGEGrating visual acuity results in the early treatment for retinopathy of prematurity studyArch Ophthalmol2011129784084621746974
- HansenRMFultonABDark-adapted thresholds at 10- and 30-deg eccentricities in 10-week-old infantsVis Neurosci19951235095127654607
- HansenRMFultonABThe course of maturation of rod-mediated visual thresholds in infantsInvest Ophthalmol Vis Sci19994081883188610393066
- BarnabyAMHansenRMMoskowitzAFultonABDevelopment of scotopic visual thresholds in retinopathy of prematurityInvest Ophthalmol Vis Sci200748104854486017898313
- FultonABHansenRMMoskowitzABarnabyAMMultifocal ERG in subjects with a history of retinopathy of prematurity Documenta ophthalmologicaAdvances in ophthalmology2005111171316502302
- SutterEETranDThe field topography of ERG components in man – I. The photopic luminance responseVision Res19923234334461604830
- HoodDCFrishmanLJSaszikSViswanathanSRetinal origins of the primate multifocal ERG: implications for the human responseInvest Ophthalmol Vis Sci20024351673168511980890
- YanniSEWangJChanMFoveal avascular zone and foveal pit formation after preterm birthBr J Ophthalmol201296796196622544530
- LeporeDMolleFPagliaraMMAtlas of fluorescein angiographic findings in eyes undergoing laser for retinopathy of prematurityOphthalmology2011118116817520709401
- MaldonadoRSO’ConnellRAscherSBSpectral-domain optical coherence tomographic assessment of severity of cystoid macular edema in retinopathy of prematurityArch Ophthalmol2012130556957822232366
- FledeliusHCPre-term delivery and subsequent ocular development. A 7–10 year follow-up of children screened 1982–84 for ROP. 3) Refraction. Myopia of prematurityActa Ophthalmol Scand19967432973008828731
- FielderARQuinnGEMyopia of prematurity: nature, nurture, or disease?Br J Ophthalmol1997811239135397
- O’ConnorARStephensonTJohnsonALong-term ophthalmic outcome of low birth weight children with and without retinopathy of prematurityPediatrics20021091121811773536
- LarssonEKRydbergACHolmstromGEA population-based study of the refractive outcome in 10-year-old preterm and full-term childrenArch Ophthalmol2003121101430143614557179
- O’ConnorARStephensonTJJohnsonATobinMJRatibSFielderARChange of refractive state and eye size in children of birth weight less than 1701 gBr J Ophthalmol200690445646016547327
- QuinnGEDobsonVDavittBVProgression of myopia and high myopia in the early treatment for retinopathy of prematurity study: findings to 3 years of ageOphthalmology200811561058106418423871
- QuinnGEDobsonVDavittBVProgression of myopia and high myopia in the early treatment for retinopathy of prematurity study: findings at 4 to 6 years of ageJ AAPOS201317212412823622444
- TroiloDNeonatal eye growth and emmetropisation – a literature reviewEye19926Pt 21541601624037
- TroiloDWallmanJThe regulation of eye growth and refractive state: an experimental study of emmetropizationVision Res1991317–8123712501891815
- WallmanJRetinal control of eye growth and refractionProg Retin Res199312133153
- WestbrookAMCrewtherDPCrewtherSGCone receptor sensitivity is altered in form deprivation myopia in the chickenOptom Vis Sci199976532633810375250
- ChuiTYBissigDBerkowitzBAAkulaJDRefractive Development in the “ROP Rat”J Ophthalmol20122012 Article ID 956705
- ZhangNFavazzaTLBaglieriAMThe rat with oxygen-induced retinopathy is myopic with low retinal dopamineInvest Ophthalmol Vis Sci201354138275828424168993
- MayerDLHansenRMMooreBDKimSFultonABCycloplegic refractions in healthy children aged 1 through 48 monthsArch Ophthalmol2001119111625162811709012
- ZadnikKMannyREYuJAOcular component data in schoolchildren as a function of age and genderOptom Vis Sci200380322623612637834
- HartnettMEPathophysiology and mechanisms of severe retinopathy of prematurityOphthalmology2015122120021025444347
- HendricksonATroiloDDjajadiHPossinDSpringerAExpression of synaptic and phototransduction markers during photoreceptor development in the marmoset monkey Callithrix jacchusJ Comp Neurol2009512221823119003975
- HendricksonAEPrimate foveal development: a microcosm of current questions in neurobiologyInvest Ophthalmol Vis Sci1994358312931338045707
- FultonABDodgeJHansenRMWilliamsTPThe rhodopsin content of human eyesInvest Ophthalmol Vis Sci19994081878188310393065
- HoodDCBirchDGRod phototransduction in retinitis pigmentosa: estimation and interpretation of parameters derived from the rod a-waveInvest Ophthalmol Vis Sci1994357294829618206712
- LambTDPughENJrA quantitative account of the activation steps involved in phototransduction in amphibian photoreceptorsJ Physiol19924497197581326052
- PughENJrLambTDAmplification and kinetics of the activation steps in phototransductionBiochim Biophys Acta199311412–31111498382952
- FultonABRushtonWAThe human rod ERG: correlation with psychophysical responses in light and dark adaptationVision Res1978187793800676087
