Abstract
Purpose
To report the reduction in the incidence and severity of retinopathy of prematurity (ROP) in rural India over a 4-year period following the introduction of improved neonatal care practices.
Methods
The Karnataka Internet Diagnosis of Retinopathy of Prematurity program (KIDROP), is a tele-medicine network that screens for ROP in different zones of Karnataka state in rural India. North Karnataka is the most underdeveloped and remote zone of this program and did not have any ROP screening programs before the intervention of the KIDROP in 2011. Six government and eleven private neonatal centers in this zone were screened weekly. Specific neonatal guidelines for ROP were developed and introduced in these centers. They included awareness about risk factors, oxygen regulation protocols, use of pulse oxymetry, monitoring postnatal weight gain, nutritional best practices, and management of sepsis. The incidence and severity of ROP were compared before the guidelines were introduced (Jan 2011 to Dec 2012) and after the guidelines were introduced (July 2013 to June 2015).
Results
During this 4-year period, 4,167 infants were screened over 11,390 imaging sessions. The number of enrolled infants increased from 1,825 to 2,342 between the two periods (P<0.001). The overall incidence of any stage ROP reduced significantly from 26.8% to 22.4% (P<0.001). The incidence of treatment-requiring ROP reduced from 20.7% to 16% (P=0.06), and of the treated disease, aggressive posterior ROP reduced from 20.8% to 13.1% (P=0.23) following introduction of the guidelines.
Discussion
Rural neonatal centers in middle-income countries have a large, unscreened burden of ROP. Improving neonatal care in these centers can positively impact the incidence and severity of ROP even in a relatively short period. A combined approach of a robust ROP screening program and improved neonatal care practices is required to address the challenge.
Introduction
Middle-income countries (like India) are currently suffering from the “third epidemic” of retinopathy of prematurity (ROP).Citation1,Citation2 Over the past 2 decades, owing to improved neonatal care, infant mortality in India has considerably reduced, leading to an increased survival of these infants. Furthermore, the incidence of premature babies surviving has increased manifold. The World Health Organization reported in 2012 that 3.5 million infants are born premature in India annually, more than any other nation in the world.Citation3
With >60% of the nation’s population still residing in rural areas combined with the improved survival of babies even in rural hospitals, the “at-risk” population for ROP in rural India has become a challenge to manage. Furthermore, the number of ROP specialists in India is dismally low. Although the absolute number and expertise of ROP specialists are currently unknown, it is believed that there are <100 such specialists, almost all of whom practice in urban centers. This leaves a large segment of the rural premature infants at risk of unscreened and untreated ROP blindness.Citation4
To address this unmet challenge, the Karnataka Internet Diagnosis of Retinopathy of Prematurity (KIDROP) was initiated in 2008 with the purpose of employing trained and accredited nonphysicians to screen in remote and rural outreach centers. The salient features of this program include the use of wide-field digital imaging to screen at-risk infants in their neonatal care units, reporting on-site using a decision-based algorithm, and relying on an indigenously developed tele-medicine network.Citation5,Citation6 There is a short turnaround cycle of receiving reports from ROP specialists situated in the city, who use their smart phone to report and make the decision accessible to these technicians.Citation7
We have previously reported data from our multicenter rural program from two geographically defined zones, North and Central Karnataka.Citation6 The report included data from 7,106 premature infants screened in 36 rural neonatal centers, over 77 months of activity. The incidence of treatment-requiring ROP was 3.57% of the babies screened. However, large regional variations between the zones were observed. In this paper, we describe the declining incidence and improving profile of the type of ROP in the north zone over a 4-year period following introduction of neonatal care guidelines that were introduced in this zone after the first 2 years and the impact of the same in the latter 2 years of the study.
Methods
ROP screening
The ROP screening program has been previously described in detail.Citation5,Citation6 Briefly, the service of the KIDROP program in the north and central zones is under the aegis of a public–private partnership with the National Rural Health Mission (NRHM), Ministry of Health and Family Welfare, Government of Karnataka, since 2009. The region for this study, ie, the North Karnataka zone, has six districts, namely, Raichur (headquarters), Gulbarga, Bidar, Bijapur, Bagalkot, and Koppal. The Institutional Review Board, the Research Committee, and the Ethics Committee of the Narayana Nethralaya Postgraduate Institute of Ophthalmology, Bangalore, approved this program.
Active screening in this zone began in February 2011. Prior to this, in 2010, a specialized team comprising a technician and a project manager were trained at the headquarters in Bangalore. Our training methods, scoring, and criteria for qualifying to be certified as a Level 3 technician have been previously published.Citation5 A Level 3 technician captures images on the RetCam Shuttle and can decide on follow-up based on a three-way algorithm – “requires treatment”, “requires follow-up”, and “can be discharged”. The decisions agreed with 94.3% of those of an ROP specialist, with the Level 3 technician missing only 0.4% of those needing treatment.Citation5 The NRHM provides financial and logistic support of the recurring costs of the team, although the private hospital (Narayana Nethralaya Postgraduate Institute of Ophthalmology) managing the program offers screening and treatment at no cost. The team travels on a fixed schedule and visits one district each day of the week.
In the study zone, the team visits six public and eleven private hospitals weekly. Infants born <2,000 g at birth and/or <34 weeks of gestation were enrolled into the study in accordance with the national ROP guidelines.Citation8 At each session, technicians performed the “KIDROP sequence” of image acquisition for each infant. This included a minimum of seven images per eye.Citation9 Standard ROP screening guidelines were followed to determine the follow-up.Citation8 Treatment in the program was performed by laser photoablation using the Early Treatment for Retinopathy of Prematurity guidelines with the 532 nm green laser.Citation10 As far as possible, the treatment was performed at the rural center by the ROP specialist traveling from Bangalore, thereby obviating the need for the infant to travel to the city. In those cases where this was not possible, the baby was transferred to Bangalore for treatment.
Data review
The diagnoses of the infants screened are shared with the treating neonatologists of the respective centers. A register is maintained in each neonatal intensive care unit (NICU) and is updated at each visit. In addition, the team maintains a data register in hard copy and online. All images are stored and archived on a secure server dedicated for this program. Furthermore, images of the babies are also shown to their respective mothers and other decision makers of the family at the end of the imaging session wherever possible. This is always done for babies who require treatment. This enhances family participation and improves compliance of follow-up. Data of any NICU are not shared with any other center, and patient information is anonymized in all cases.
Every month a summary of the data with a detailed review of the incidence of any stage ROP, treatment-requiring ROP (Type 1), and aggressive posterior ROP (APROP) is computed and submitted to the NRHM. Every quarter, there is a more detailed review of the data and suggestions to improve the program. Every 6 months, there is a review with the government where feedback on improving the enrollment and expansion of the program is discussed. Constant feedback on the incidence and severity of ROP in each center is discussed with the treating neonatologists or pediatricians.
The program director visits the NICUs periodically and discusses measures to reduce the incidence of ROP. Knowledge sharing and skill transfer activities, including sharing best care practices in neonatal care, training and retraining of the nursing staff and the paramedical staff encountered during the ROP screening, sharing publications, reports, and good clinical practice guidelines with the NICU administrators and doctors, increasing awareness among the mothers to act as ambassadors to educate other potential mothers in their villages about ROP, promoting good newborn care practices, including breast-feeding and kangaroo mother care, and promoting health education about immunization are carried out in several sessions.
Improving neonatal care practices
Centers that had the highest incidence of APROP were specially targeted to promote best neonatal care practices in an attempt to reduce severe ROP. These guidelines have been laid down by a special ROP subcommittee of the national neonatology foundation, Karnataka state chapter, and published elsewhere.Citation11
A summary of these guidelines includes the following:
Reducing iatrogenic premature births as far as possible. We promoted the discussion between the obstetrician and the neonatologist regarding the timing of the neonatal birth and promoted the use of antenatal steroid injection in the mother.
Promoting the judicious use of oxygen – which included using pulse oxymetry soon after birth, during resuscitation, transport into the NICU or to another facility, and during the stay in the NICU.
Avoiding the use of oxygen, especially 100% and unblended soon after birth especially if there were spontaneous respiratory efforts.
Promoting the concept of fraction of inspired oxygen (FiO2) to resuscitate preterm infants. This was not possible in all cases because of the lack of equipment in these rural centers.
Removal of the reservoir from the bag to ventilate the baby when using bag and mask for positive-pressure ventilation ensuring the FiO2 of <0.40.
Altering the FiO2 based on the pulse oxymetry readings.
Promoting the concept of target saturation (SaO2) between 88% and 92%.
Avoiding large fluctuations of FiO2 during oxygen desaturation episodes.
Not to turn off the desaturation alarms in the NICU, especially at night.
Promoting good postnatal weight gain and promoting the concept of total parenteral nutrition. Starting enteral feeds early as soon as the baby’s condition is stable.
Preventing late-onset sepsis with strict aseptic protocols and intravenous fluid handling.
Pasting printed guidelines for ROP screening on the NICU wall and constant retraining of all shifts of nurses attending the NICU.
A majority of these guidelines were introduced in mid-2013. We reviewed the incidence of the profile of ROP in the first 2 years of the program (2011–2013) and compared it with the profile 2 years (2013–2015) later to study the effect of these practices on the incidence and severity of ROP in these centers.
All statistical analysis was done using MedCalc Statistical Software Version 15.6.1 (MedCalc Software bvba; MedCalc, Ostend, Belgium; https://www.medcalc.org; 2015). A proportion statistics was applied to analyze the data in the two study periods and to determine the difference between them.
Results
The study period was from January 2011 to June 2015. The details of the overall study cohort are summarized in , and their demographic details are summarized in . During this 4-year period, 4,167 infants were screened in 11,390 imaging sessions and 158,406 RetCam images were analyzed. The mean birth weight of babies enrolled in the two periods and the mean periods of gestation were 1,592.7 and 1,578.8 g and 31.7 and 31.6 weeks, respectively; both were comparable (P>0.05).
Table 1 Demographics of the study zone
Table 2 Birth weight and gestational age distribution of the study cohort
The incidence of any stage ROP during the 4-year period was 24.5% and of treatment-requiring ROP was 4.5%. The overall distribution of the disease profile is summarized in and the disease burden between the two study periods in . The number of enrolled neonatal centers remained constant throughout both periods; however, the number of enrolled infants increased from 1,825 to 2,342 between the two periods (P<0.001). The overall incidence of any stage ROP reduced significantly from 26.8% in the first 2 years when compared to 22.4% in the latter 2-year period (P<0.001). The number of babies undergoing treatment also reduced between the two study periods from 101 (20.7% of those with ROP) to 84 (16% of those with ROP), but this was not statistically significant (P=0.06).
Table 3 ROP distribution in the study cohort
Table 4 ROP distribution compared during the two study periods
Similarly, the number of babies with APROP reduced from 21 infants (20.8% of those treated) to eleven infants (13.1% of those treated) (P=0.23). Although this difference was not statistically significant, clinically, there was an improvement in the type and severity of the disease observed (). In the first study period, all babies with APROP (21/21 babies) had posterior zone 1 disease with large capillary nonperfusion retinal beds extending irregularly like “tongues” into the central macula and accompanied by very severe plus disease ( and ). In the latter study period, 36.4% (4/11) were in zone 1, while the remaining (7/11) had less severe presentation with the disease observed in zone 2 posterior ( and ). Following laser photoablation with 532 nm Nd:YAG laser (Iridex, CA, USA), five out of 42 eyes (11.9%) treated in the first period progressed to unfavorable outcome compared to 0% in the second period. Overall, 92.2% had a successful outcome.
Figure 1 The graph depicts the trend of decline in the disease profile over the first 2 years and the latter 2-year period following the introduction of better neonatal care practices.
Abbreviations: ROP, retinopathy of prematurity; APROP, aggressive posterior ROP.
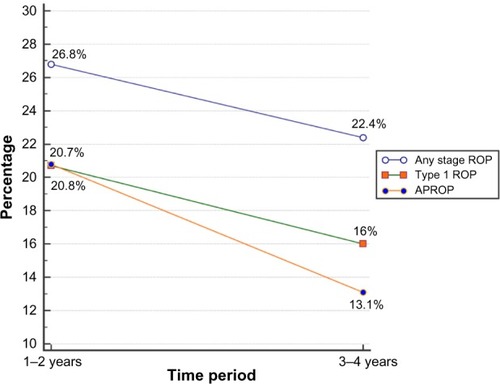
Figure 2 Left eye of a female infant born at 33 weeks weighing 1,750 g in a private rural neonatal center showing aggressive posterior retinopathy of prematurity with neovascularization in zone 1 (blue arrows), severe plus disease, and closed capillary loops.
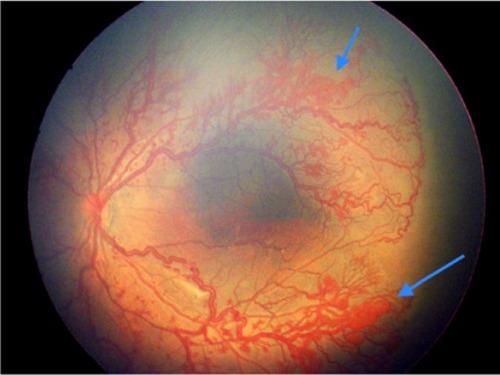
Figure 3 Left eye of a male infant born at 32 weeks weighing 1,300 g in a rural center showing occluded and closed loops of vessels abruptly stopping in posterior zone 1.
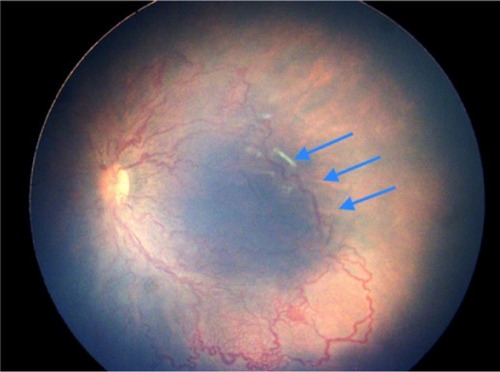
Figure 4 Left eye of a female infant born at 32 weeks weighing 1,400 g shows aggressive posterior ROP.
Abbreviation: ROP, retinopathy of prematurity.
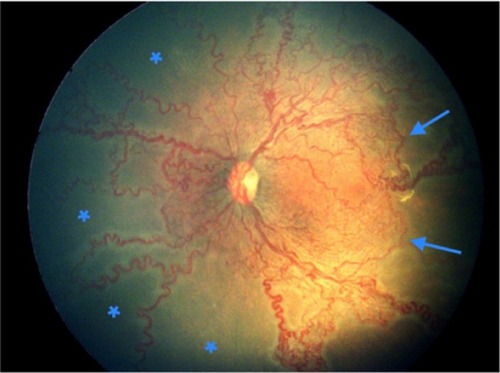
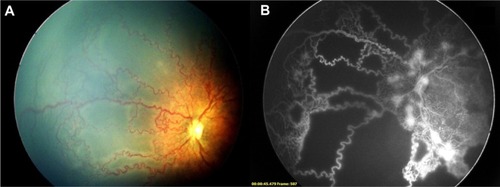
Figure 5 (A) Left eye nasal quadrant of a male baby born at 33 weeks weighing 1,500 g shows ischemic loops enclosing areas of capillary drop out extending until the anterior edge of zone 2. (B) The angiogram on the same eye confirms the capillary non-perfusion within these loops. Leaking neovascularization adjacent to the optic disc is also noted.
Discussion
ROP is one of the leading causes of preventable infant blindness in the world. With improving neonatal care, upperCitation12–Citation21 and lowerCitation22–Citation30 middle-income countries are reporting an incidence of severe ROP as high as 44%. A summary of ROP incidence in some of these countries is detailed in .
Table 5 Incidence of ROP in upper and lower middle-income countries
ROP in rural India has recently been reported and appears to contribute to a significant burden of infant blindness. Though it has been reported from urban centers for over 2 decades in India,Citation22–Citation24,Citation31–Citation34 rural data had been scarce.Citation5,Citation6,Citation27,Citation35 The first prospective study of rural ROP in 2012 reported an overall incidence of 41.5% and treatment-requiring incidence of 10.2%.Citation27 More recently, a multicenter rural study revealed an overall incidence of 22.39% and 3.57% requiring treatment.Citation6 While APROP in heavier babies in urban settings is known,Citation33,Citation36 it has more recently been reported in rural centers with a high variation of up to 13%.Citation37
The demographics of babies admitted in rural NICUs in our setting suggest that 4% of admissions were <1,000 g at birth and 29% were <30 weeks of gestation, indicating that smaller and lighter babies are increasingly surviving in rural centers.Citation6 This indicates an overall improvement in the general neonatal care. However, even within the same state, there are large variations in the ROP profile. Comparing two adjoining zones in Karnataka state, the incidence of any stage ROP was reported as 24.5% vs 19.6% and the treatment-requiring disease was 4.5% vs 2.4%, despite the ethnic and demographic features of the two zones being comparable. This allows speculation on the factors that may influence the severity of ROP in rural settings. Rural data have also shown that a significantly larger proportion of babies needing ROP treatment were from private hospitals rather than public hospitals (7.1% vs 1.7%).Citation6 It is possible that this gap could be attributed to aggressive neonatal management in the private hospitals since there may be a compulsion to improve survival. However, this has not been confirmed in any formal study till date.
The most striking difference encountered between rural zones reported thus far is the skewed distribution of APROP between two adjoining areas of the same state. Over 84% of all reported cases of APROP from a state came from one zone compared to only 16% from another adjoining zone.Citation6 All other factors being comparable between the two zones, the cause of this large difference could be attributed to the level of neonatal care. The zone with the higher incidence of APROP (North Karnataka) is the subject of this current manuscript.
APROP in India affects “heavier” and “larger” babies compared to their western counterparts.Citation32,Citation33,Citation36,Citation37 Shah et al reported the similarity of the APROP seen in India with the oxygen-induced retinopathy models in animals. This was due to the injudicious use of unblended, high-volume oxygen in these babies.Citation32 Interestingly, the 21 cases of APROP seen in the first 2 years of this study resembled the cases described by Shah et al in location and severity. Indeed, site visits to these “APROP centers” revealed poor understanding and practice of oxygen delivery and general neonatal risk factor management. In one such center, the nurse had been discharged from duty and an unqualified paramedic who would switch off the pulse oxymetry alarms at night and administer 100% oxygen to babies admitted in the NICU took her place. It became imperative for us to address neonatal care practices besides providing regular ROP screening services at these centers.
The guidelines for improved neonatal care that are described in this study were evolved with the purpose of providing simple recommendations that were aimed at addressing this lacunae in neonatal care practice.Citation11 They were developed by the ROP subcommittee of the state and also addressed the pediatrician, given the reality that pediatricians and not formally trained neonatologists, owing to the scarcity of the latter in the country, manage most rural centers. By comparing the incidence and profile of ROP in these centers before and after the introduction of these guidelines, we aimed to study the impact of improved neonatal care practices in a rural zone, which had very severe ROP.
The study results bring out some interesting aspects. First, the number of babies enrolled for ROP screening increased between the two periods from 1,825 to 2,342 babies, respectively (P<0.001). This occurred despite the number of centers and districts remaining constant. This could be attributed to more diligent referral from the study center pediatricians and by local pediatricians from smaller hospitals, which were not visited by our team. It must be emphasized that ROP screening was nonexistent in these centers before our program in 2011, and enhancing awareness resulted in an enhanced participation. Second, the incidence of any stage ROP decreased between the two periods (26.8% vs 22.4%, P<0.001). This occurred despite the fact that more babies were enrolled between the two periods. This positive trend could be attributed to an overall improvement in neonatal care, stricter adherence to the ROP screening guidelines,Citation8 and better control of risk factors. Third, the incidence of treatment-requiring ROP (101 vs 84 babies) and APROP (21 vs 11 babies) showed a decline in the later 2 years. Although this was not significant statistically, this decline is of clinical importance. Fourth, the type of severe disease showed a change in the morphology from a more severe form to a less severe one. All cases of APROP in the first period were in zone 1 and resembled the oxygen-induced retinopathy model described by Shah et al,Citation32 with occluded vessels, posterior extension of the ischemic tongues, and severe plus disease ( and ). In the second period, four of eleven babies (36%) were in zone 1 and the remaining cases of APROP showed a less severe form ( and ). This change in morphology highlights the importance of better oxygen delivery management, which was an important aspect of the guidelines. Finally, these changes, that is, the decline of incidence and severity of ROP occurred in a relatively short period of 4 years.
This report differs from others in the study duration, number of infants screened, and the disease trends. While most previous reports have shown an increase in the disease burden over an 8- to 10-year period,Citation38–Citation41 one study has found a significant decrease in ROP during the second period.Citation42 We found a decline in the disease incidence over a shorter time period of 2 years, despite a larger number of infants screened. This could be attributed to the impact this program has had in an area where no ROP screening previously existed. The critical level of change in risk factor modifications required to create a significant difference in outcome would appear to be less, contrasted to an area where ROP screening was already in practice. In the next few years, it is likely that a further reduction in the disease profile will take longer and with more extensive intervention-based modifications.
The limitation of the study lies in the fact that we do not have a quantitative estimate on which neonatal practice contributed in what measure. We did not undertake objective measurements of the level of knowledge or skill before and after the guidelines were introduced. In addition, we did not assess the knowledge, attitude, and practice among the pediatricians or neonatologists who were introduced to these practices. However, subsequent site visits have documented an improvement in equipment and protocols being followed in these centers. Indeed, not all centers are equally amenable to change, and we have attempted to classify “supportive” and “nonsupportive” neonatal centers in these rural areas.Citation43 A formal test of knowledge and assessment of the extent of practices adopted needs to be evaluated. Finally, given the occurrence of high variability in the infant mortality rates and neonatal care practices in different states and zones of the country, our results in rural Karnataka cannot be extrapolated to other states without considering regional demographics and further operational research.
Conclusion
The experience from our rural work has become the subject of the national task force for ROP constituted by the federal government (National Health Mission, Government of India). In the ROP management guidelines that are being prepared, neonatal care practices that promote the prevention of severe ROP are being actively considered. This includes training of nursing and paramedical staff of the district-based, special newborn care units. The role of the treating pediatrician or neonatologist has also been highlighted. Observing the cohort prospectively will address this concern.
With the expansion of neonatal care services throughout the country, the special newborn care units envisaged by the federal government to be setup in each district of the country are going to add a very large load of at-risk babies for ROP screening.Citation44 The role of the pediatrician, neonatologist, and the obstetrician has become paramount. The current infrastructure is equipped neither with trained manpower nor with essential technology to tackle this challenge. Integrating training of the neonatal staff and physicians with best care practices would help reduce the incidence of severe ROP. The National Task Force for ROP in India in collaboration with the National Neonatology Foundation of India is preparing training modules for pediatricians and nurses to address these lacunae.
Acknowledgments
We acknowledge the contributions of the National Neonatology Foundation, Karnataka state chapter, subcommittee for ROP members, Dr Archana Bilagi, Dr Ranjan Pejaver, Dr Sangappa Dhaded, and Dr Ravindra Battu for their help in framing the guidelines and the KIDROP team members of North Karnataka, Mr Muralidhar Gayakwad, Mr Ravishankar Kandagal and Bangalore team members Mr Praveen Sharma, Mr Sivakumar Munusamy, and Mr Krishnan for their support.
Disclosure
The authors report no conflicts of interest in this work.
References
- GilbertCRahiJEcksteinMO’SullivanJFosterARetinopathy of prematurity in middle-income countriesLancet1997350907012149217713
- GilbertCFielderAGordilloLInternational NO-ROP GroupCharacteristics of infants with severe retinopathy of prematurity in countries with low, moderate, and high levels of development: implications for screening programsPediatrics20051155e518e52515805336
- The Global Action Report on Preterm Birth [webpage on the Internet]. United Nations 2012. Born Too Soon135393396 Available from: http://www.who.int/pmnch/media/news/2012/201204_borntoosoon-report.pdfAccessed June 26, 2015
- VinekarAIT – enabled innovation to prevent infant blindness in rural India: the KIDROP experienceJ Indian Bus Res20113298102
- VinekarAGilbertCDograMThe KIDROP model of combining strategies for providing retinopathy of prematurity screening in underserved areas in India using wide-field imaging, tele-medicine, non-physician graders and smart phone reportingIndian J Ophthalmol2014621414924492500
- VinekarAJayadevCMangaleshSShettyBVidyasagarDRole of tele-medicine in retinopathy of prematurity screening in rural outreach centers in India – a report of 20,214 imaging sessions in the KIDROP programSemin Fetal Neonatal Med201520533534526092301
- VinekarAJayadevCBauerNNeed for telemedicine in retinopathy of prematurity in middle-income countries: e-ROP vs KIDROPJAMA Ophthalmol2015133336036125474398
- PejaverRKVinekarABilagiA webpage on the InternetNational Neonatology Foundation’s Evidence Based Clinical Practice Guidelines 2010 Retinopathy of Prematurity (NNF India, Guidelines)2010253262 Available from: http://aimaonline.org/iap-neochap-2013/uploads/acd-corner/nnf_guidelines-2011.pdfAccessed January 23, 2016
- VinekarAJayadevCMangaleshSComparing the outcome of single versus multiple session laser photoablation of flat neovascularization in zone 1 aggressive posterior retinopathy of prematurity: a prospective randomized studyRetina201535102130213625996425
- Early Treatment For Retinopathy Of Prematurity Cooperative GroupRevised indications for the treatment of retinopathy of prematurity: results of the early treatment for retinopathy of prematurity randomized trialArch Ophthalmol2003121121684169414662586
- VinekarAJayadevCBilagiAPejaverRDhadedSMBattuRScreening and prevention of ROP: practical pearls for the ophthalmologist and the pediatricianPerinatology2014159799
- TavosnanskaJMorbimortalidad de recién nacidos con menos de 1500 gramos asistidos en hospitales públicos de la ciudad de Buenos Aires. [Mortality and morbidity of very low birth weight newborn infants assisted in Buenos Aires public hospitals]Arch Argent Pediatr20121105394403 Spanish23070181
- ZinAAMoreiraMELBunceCDarlowBAGilbertCERetinopathy of prematurity in 7 neonatal units in Rio de Janeiro: screening criteria and workload implicationsPediatrics20101262e410e41720660549
- LiQWangZLiYRetinopathy of prematurity screening in 2185 premature infantsZhonghua Yan Ke Za Zhi2012481090390723302245
- ZuluagaCLlanosGTorresJEffects of the screening program on ROP in Cali, ColumbiaActa Med Litu2006133176178
- SaeidiRAhmadHAhmadiSRahmaniSPrevalence and predisposing factors of ROP in very low-birth-weight infants discharged from NICUIran J Pediatr20091915963
- JakuskieneRVollmerBSaferisVDaugelieneDNeonatal outcomes of very preterm infants admitted to a tertiary center in Lithuania between the years 2003 and 2005Eur J Pediatr2011170101293130321404102
- VǎtavuINascutzyCCiomârtanTBrezanFAncaIStoicescuSRetinopathy of prematurity–screening resultsOftalmologia2010541110117
- KnezevicSStojanovicNOrosASavicDSimovicAKnezeviJAnalysis of risk factors in the development of retinopathy of prematuritySrp Arh Celok Lek20111397–843343821980650
- DelportSDSwanepoelJCOdendaalPJLRouxPIncidence of retinopathy of prematurity in very-low-birth-weight infants born at Kalafong Hospital, PretoriaS Afr Med J2002921298699012561416
- AkkoyunIOtoSYilmazGRisk factors in the development of mild and severe retinopathy of prematurityJ AAPOS200610544945317070481
- CharanRDograMRGuptaANarangAThe incidence of retinopathy of prematurity in a neonatal care unitIndian J Ophthalmol19954331231268822486
- GopalLSharmaTRamachandranSShanmugasundaramRAshaVRetinopathy of prematurity: a studyIndian J Ophthalmol199543259618818311
- VinekarADograMRSangtamTNarangAGuptaARetinopathy of prematurity in Asian Indian babies weighing greater than 1250 grams at birth: ten year data from a tertiary care center in a developing countryIndian J Ophthalmol200755533133617699940
- VarugheseSJainSGuptaNSinghSTyagiVPuliyelJMMagnitude of the problem of retinopathy of prematurity. Experience in a large maternity unit with a medium size level-3 nurseryIndian J Ophthalmol200149318718815887728
- JalaliSMataliaJHussainAAnandRModification of screening criteria for retinopathy of prematurity in India and other middle-income countriesAm J Ophthalmol2006141596696816678524
- HungiBVinekarADattiNRetinopathy of prematurity in a rural neonatal intensive care unit in south India-a prospective studyIndian J Pediatr201279791191522359197
- TaquiAMSyedRChaudhryTAAhmadKSalatMSRetinopathy of prematurity: frequency and risk factors in a tertiary care hospital in Karachi, PakistanJ Pak Med Assoc200858418619018655427
- PhanMHNguyenPNReynoldsJDIncidence and severity of retinopathy of prematurity in Vietnam, a developing middle-income countryJ Pediatr Ophthalmol Strabismus200340420821212908532
- AhmedASMuslimaHAnwarKSRetinopathy of prematurity in Bangladeshi neonatesJ Trop Pediatr200854533333918503093
- MaheshwariRKumarHPaulVKSinghMDeorariAKTiwariHKIncidence and risk factors of retinopathy of prematurity in a tertiary care newborn unit in New DelhiNatl Med J India1996952112148937058
- ShahPKNarendranVKalpanaNAggressive posterior retinopathy of prematurity in large preterm babies in south IndiaArch Dis Child Fetal Neonatal Ed2012975F371F37522611114
- JalaliSKesarwaniSHussainAOutcomes of a protocol-based management for zone 1 retinopathy of prematurity: the Indian Twin Cities ROP Screening Program report number 2Am J Ophthalmol2011151471972421257156
- AzadRPrevention of blindness due to retinopathy of prematurity: a national movementIndian J Pediatr201481121373137524794325
- KeerthiBJBabuSVinekarAGoudNBullappaARetinopathy of prematurity screening of 500 infants in a level II neonatal intensive care unit at a medical college hospital in southern KarnatakaJ Evol Med Dent Sci201431066510672
- SanghiGDograMRDasPVinekarAGuptaADuttaSAggressive posterior retinopathy of prematurity in Asian Indian babies: spectrum of disease and outcome after laser treatmentRetina20092991335133919574949
- VinekarAAvadhaniKBraganzaSShettyBDograMGilbertCOutcomes of a protocol-based management for zone 1 retinopathy of prematurity: the Indian twin cities ROP screening program report number 2Am J Ophthalmol20111524712 author reply 71321961849
- Schalij-DelfosNECatsBPRetinopathy of prematurity: the continuing threat to vision in preterm infants. Dutch survey from 1986 to 1994Acta Ophthalmol Scand199775172759088406
- HameedBShyamanurKKotechaSTrends in the incidence of severe retinopathy of prematurity in a geographically defined population over a 10-year periodPediatrics200411361653165715173486
- SchiaritiVMatsubaCHoubéJSSynnesARSevere retinopathy of prematurity and visual outcomes in British Columbia: a 10-year analysisJ Perinatol200828856657218368058
- DemirSYücelÖENiyazLKarakuGArıtürkNIncidence of retinopathy of prematurity in the middle Black Sea region of Turkey over a 10-year periodJ AAPOS2015191121525727579
- GauglerCBeladdaleJAstrucDRetinopathy of prematurity: 10-year retrospective study at the University Hospital of StrasbourgArch Pediatr20029435035711998419
- HosamaniSKumarSVAVallabhaKBiradarSWVComparison of tele screening for retinopathy of prematurity screening and impact of neonatal team support in government and private centers in rural Bijapur district of Southern IndiaJ Vis Sci2015121520
- Ministry of Health and Family Welfare [webpage on the Internet]Operational Guidelines for Rashtriya Bal Swasthya Karyakram. Child Health Screening and Early Intervention Services Under NRHM2013 Available from: http://www.pbnrhm.org/docs/rbsk_guidelines.pdfAccessed July 12, 2015
