Abstract
Background
AIDS, caused by HIV, is a multisystem disease that affects hematopoiesis. The aim of this study was to assess cytopenias among HIV-infected children who had a follow-up at Felege Hiwot Referral Hospital, Bahir Dar, northwest Ethiopia.
Methods
An institution-based cross-sectional study was conducted between April and May 2013. Systematic random sampling method was used to select the study participants. Descriptive statistics, independent t-test as well as chi-square and logistic regression were used for analysis. A p-value <0.05 was considered as statistically significant.
Results
A total of 224 children (112 highly active antiretroviral therapy [HAART]-naïve and 112 HAART-experienced) participated in the study. The magnitude of anemia, thrombocytopenia, neutropenia, leukopenia and pancytopenia among HAART-naïve HIV-infected children were 30.4%, 9.8%, 8%, 4.5% and 1.8%, respectively. The overall prevalence of anemia, neutropenia, thrombocytopenia, leukopenia and pancytopenia were 29.5%, 8.9%, 8%, 4.5% and 1.4%, respectively. Cluster of differentiation-4 percentage and mean corpuscular volume were significantly different between HAART-experienced and HAART-naïve children. Being of younger age and severely immunosuppressed were risk factors of anemia.
Conclusion
Anemia was the most common cytopenia, followed by neutropenia. Severe immunosuppression and younger age were significantly associated with anemia. Therefore, emphasis should be given for investigation and management of cytopenias in HIV-infected children, particularly for those who are immunosuppressed and of younger age.
Background
AIDS is caused by HIV and is characterized by progressive damage to the body’s immune system, which results in a number of OIs, immunological and hematological complications.Citation1,Citation2 Immunological complication due to CD4+ T-lymphocyte depletion is a hallmark of HIV infection.Citation3 Hematological manifestations are among the most common clinicopathological manifestations of HIV infection, and they have been documented as the second most common cause of morbidity and mortality in HIV patients.Citation4–Citation6 These complications are generally marked with cytopenias and dysplasias of all major blood cell lines, leading to anemia, leukopenia, thrombocytopenia and neoplasms.Citation7–Citation9
Despite the attempts made to clearly understand the hematopoiesis impairment mechanism(s), it remained an intractable problem because of the paucity of studies using a suitable experimental animal model that closely replicates human hematopoiesis during an ongoing HIV infection in vivo.Citation10 It has been evidenced that HIV-associated cytopenias seem to be dependent on the level of viral replication, OIs, liver cirrhosis, malignancies and the effects of the HAART used.Citation11,Citation12 Involvement of the hematopoietic system tends to be more severe in advanced stages of the disease.Citation7,Citation13 The incidence and severity of cytopenias are generally correlated to the stage of the disease. In addition, cytopenias can adversely affect ART outcomes and result in higher mortality.Citation12,Citation14–Citation16 In HIV-infected children, cytopenias are the common problems.Citation3,Citation17–Citation21 The pathophysiology of HIV-related cytopenias in childhood is not well understood, which may be due to the complicated and dynamic changes associated with normal hematological development in early life.Citation18 The frequency and severity of cytopenias vary, while the disease progresses from the asymptomatic carrier state to advanced symptomatic stages. The frequencies of anemia, leukopenia and thrombocytopenia in asymptomatic HIV-infected children were 20%, 10% and 15%, respectively, while in HIV-infected children at the AIDS stage, their proportions were 70%, 65% and 40%, respectively.Citation22
Even though the use of HAART reduces the rate of mortality, therapy-related potential adverse events are becoming the major concern in the era of HAART, particularly in resource-limited counties where undernutrition is common.Citation23–Citation26 Both HIV/AIDS and undernutrition affect immune function; HIV/AIDS, together with lack of essential micronutrients, leads to severe immune dysfunction. Furthermore, compromised immune status increases susceptibility to infectious diseases and profoundly complicates cytopenias and their management.Citation27,Citation28 A number of studies have been conducted on cytopenias among adult HIV-infected patients before and after the initiation of HAART. However, in HIV-infected children, limited data are available, and these are not much well elucidated, especially in developing countries. Moreover, there are controversial reports regarding the efficacy and impact of HAART in resolving immunological and hematological complication in HIV patients.Citation29–Citation31 Thus, this study was aimed to assess the cytopenias among HAART-naïve and HAART-experienced HIV-infected children.
Patients and methods
Study setting, population, sample size and sampling procedure
A cross-sectional study was conducted at Bahir Dar Felege Hiwot Referral Hospital, northwest Ethiopia, between April and May 2013. Felege Hiwot Referral Hospital is found in Bahir Dar, which is located 565 km away from Addis Ababa. Geographically, the city is located between 9°20′ and 14°20′ north latitudes and between 30°20′ and 40°20′ east longitudes and is at an altitude of 1,830 m above sea level. The hospital serves >5 million people and provides comprehensive health care services, including ART treatment and monitoring for both pediatric and adult people living with HIV/AIDS.
The study population comprised HIV-infected children who had been followed up at the Pediatric ART Clinic of Felege Hiwot Referral Hospital during the study period. HIV-infected children who were HAART-naïve and HAART-experienced for at least 6 months were eligible to be included in the study. Children who had been previously confirmed as having chronic renal failure and liver disease prior to HIV infection, as well as those who underwent radiation therapy and/or immunosuppressive chemotherapy in the previous 45 days, were excluded from the study due to the fact that these may unambiguously affect the hematological values.
For sample size determination, double population proportional formula was used by considering the following assumptions: 2-sided confidence level at 95%, power of 80% and 1:1 ratio of HAART-experienced:HAART-naïve children. We used a 21.9% prevalence rate of anemia for HAART-experienced children, as per a study conducted in Jimma, Ethiopia,Citation32 and 40% for HAART-naïve children (a default value of OpenEpi) to get the maximum sample size. Then, a total of 224 HIV-infected children (112 HAART-naïve and 112 HAART-experienced for at least 6 months) were included in the study.
A systematic random sampling technique was used. On a daily basis, an average of 9 HAART-naïve and 12 HAART-experienced children were getting health care service in the Pediatric ART Clinic of Felege Hiwot Referral Hospital. A total of 840 HIV-infected children (360 HAART-naïve and 480 HAART-experienced) visited the ART clinic during the study period. Every third HAART-naïve HIV-infected child from the sequence of ART visitors was included in the study. Similarly, every fourth HAART-experienced HIV-infected child was included.
Data collection and laboratory analysis
Sociodemographic and socioeconomic characteristic of children and their caregivers were collected using a structured questionnaire via a face-to-face interview technique. Clinical data were collected by reviewing the medical records of HIV-infected children. The aforementioned data were collected by trained clinical nurses working in the Pediatric ART Clinic. Weight and height were measured, and the weight-for-age status and height-for-age status were scored from the child’s growth monitoring chart.
Venous blood (4 mL) was collected from each study participant using test tubes containing ethylenediaminetetraacetic acid following aseptic procedures. Part of the blood sample was analyzed using Cell DYN 1800 (Abbott Laboratories, Abbott Park, IL, USA) for the determination of hematological parameters, which include RBC parameters (RBC count, Hg level, MCH, MCHC, MCV and RDW%); WBC parameters (total WBC count, ANC, neutrophil percentage, lymphocyte count, lymphocyte percentage, mid count that encompasses eosinophil, basophil and monocyte and mid cell percentage) and platelet parameter (platelet count and MPV). The remaining blood sample was analyzed for the determination of CD4+ T-cell value using fluorescence-activated cell sorter counter (BD, San Jose, CA, USA). While doing all laboratory analyses, the standard operating procedure, daily maintenance, weekly maintenance and internal quality control procedure were strictly followed throughout the research process.
Assessment of cytopenias and immunological status
HIV-associated immunodeficiency was defined using the World Health Organization (WHO) age-related CD4 value stratification for HIV-infected infants and children.Citation33 Mild immunodeficiency was defined as CD4% of 30% to <35% for infants <11 months, CD4% of 25% to <30% for children of age 12–35 months, CD4% of 20% to <25% for children aged 36–59 months and CD4 count of 350–499 cells/mm3 for children aged >5 years. Advanced immunodeficiency was defined as follows: CD4% of 25% to <30% for infants <11 months, CD4% of 20% to <25% for children aged 12–35 months, CD4% of 15% to <20% for children aged 36–59 months and CD4 count of 200–349 cells/mm3 for children aged >5 years. Severe immunodeficiency was also defined as follows: CD4% <25% for infants <11 months, 15% to <20% for children aged 12−35 months and <15% for children aged >3 years.Citation33
Anemia was defined based on the WHO criteria after Hg has been adjusted for altitude and was stratified based on age (Hg <11.0 g/dL for children aged 6–59 months, Hg <11.5 g/dL for children aged 5–11 years and Hg <12.0 g/dL for children aged ≥12 years). Mild anemia was defined as follows: Hg 10.0–10.9 g/dL for children aged 5–59 months, 11.0–11.4 g/dL for children aged 5–11 years and 11.0–11.9 g/dL for children aged 12–14 years. Moderate anemia was defined as Hg 7.0–9.9 g/dL for children aged 5–59 months and 8–10.9 g/dL for children aged 5–14 years. Severe anemia was also defined as Hg <7.0 g/dL for children aged 6–59 months and <8.0 g/dL for those aged 5–14 years.Citation34
Leukopenia was defined as a total WBC count <3,000 cells/mm3.Citation9 Thrombocytopenia and thrombocytosis were defined as a platelet count <150,000/mm3 and platelet count >450 × 103 cells/mm3, respectively.Citation32 Neutropenia was also defined as absolute neutrophil count of <1,000/mm3, and the severity has also been classified as mild, moderate and severe.Citation32
Statistical analysis
Data were cleaned, sorted, categorized, coded and entered into Epi Info version 3.5.1. The data were transferred to SPSS version 20 for analysis. Descriptive statistics were obtained and the results are presented in – and . Normality of data was checked; and chi-square and independent t-tests were used to compare the mean hematological values between the HAART-naïve and HAART-experienced HIV-infected children. Bivariate logistic regression analyses were carried out for the cytopenias, and variables having p-value <0.2 in bivariate logistic analysis were included in the multivariable logistic analysis model to assess the association between cytopenias and explanatory variables. Odds ratios (ORs) with 95% CIs were used to measure the strength of the statistical associations. A p-value <0.05 was considered statistically significant.
Figure 1 Frequency of cytopenias in HIV-infected children at Pediatric ART Clinic, Felege Hiwot Referral Hospital, Bahir Dar, northwest Ethiopia.
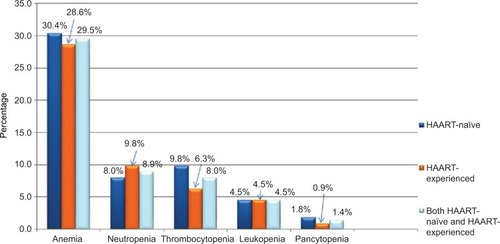
Table 1 Sociodemographic characteristics of caregivers/guardians and HIV-infected children at the Pediatric ART Clinic, Felege Hiwot Referral Hospital, Bahir Dar, northwest Ethiopia, 2013
Table 6 Factors associated with anemia among HAART-naïve and HAART-experienced children at Pediatric ART Clinic, Felege Hiwot Referral Hospital, northwest Ethiopia, April–May 2013 (N=224)
Ethical considerations
This study was approved by the College of Medicine and Health Sciences Research Ethical Committee and the Institutional Review Board of the University of Gondar. The purpose and importance of the study was explained to each caregiver. Informed written consent was taken from the caregivers, and in addition, assent was obtained from children aged >7 years before the commencement of the study. To ensure confidentiality of participants and their information, anonymous typing was used whereby the name of the participants and any participants’ identifiers were not written on the questionnaire. The participants were interviewed alone to maintain their privacy. Laboratory findings of study participants were communicated with the responsible clinicians assigned at the Pediatric ART Clinic.
Results
Sociodemographic characteristics
A total of 224 study participants were enrolled in this study. The median age of the study participant was 8 years (interquartile range: 6 years). More than half of the study participants, 126 (56.3%), were males. Among the study participants, 180 (80.4%) were from urban setting and 96 (42.9%) were attending primary school. A majority, 157 (70.1%), of caregivers earned monthly income <1,400 ETB ().
Medical characteristics of the study participants
Among the participants, 64 (57.2%) of HAART-naïve and 47 (41.9%) of HAART-experienced children were in WHO clinical stage I. Thirty (26.8%) and 39 (34.8%) of HAART-naïve children were underweight and stunted, respectively, whereas 44 (39.3%) and 31 (27.7%) HAART-experienced children were underweight and stunted, respectively. Moreover, 19 (17%), 16 (14.3%), 15 (13.4%), 15 (13.4%), 14 (12.5%) and 9 (8%) of HAART-naïve children presented with fever, skin rash, diarrhea, OIs, pneumonia and oral thrush, respectively. Likewise, 14 (12.5%), 13 (11.6%), 10 (9%), 10 (9%), 9 (8%) and 8 (7.1%) of HAART-experienced children presented with skin rash, fever, pneumonia, OIs, gastroenteritis and diarrhea, respectively. Severe, advanced and moderate immunosuppression was observed among 17.9%, 12.9% and 32.1% of the study participants, respectively ().
Table 2 Medical characteristics of HIV-infected children at the Pediatric ART Clinic, Felege Hiwot Referral Hospital, Bahir Dar, northwest Ethiopia, 2013 (N=224)
Cytopenias
Of the total study participants, 66 (29.5%), 20 (8.9%), 18 (8%), 10 (4.5%) and 3 (1.4%) were anemic, neutropenic, thrombocytopenic, leukopenic and pancytopenic, respectively. The occurrence of anemia, thrombocytopenia, neutropenia, leukopenia and pancytopenia among HAART-naïve HIV-infected children was reported to be 30.4% (n=34), 9.8% (n=11), 8% (n=9), 4.5% (n=5) and 1.8% (n=2), respectively ().
Comparison of hematological profile
The mean values (±SD) of RBC, Hg, WBC, ANC, lymphocyte count, platelet count and absolute CD4 count in HAART-naïve children were 4.48±0.58 × 106/µL, 13.25±3.03 g/dL, 8.18±3.32 × 103/µL, 3.94±2.26 × 103/µL, 3.46±2.2 × 103/µL, 297.91±107.67 × 103/µL and 873.16±446.57/µL respectively. In children who were HAART-experienced, the mean values (±SD) were 4.94±4.75 × 106/µL, 13.21±1.6 g/dL, 8.01±3.26 × 103/µL, 3.84±2.16 × 103/µL, 3.75±5.17 × 103/µL, 300.99±106.16 × 103/µL and 767.86±486.6/µL, respectively.
On the basis of the mean values, the data indicated that Hg, RBC count, WBC count and platelet count did not show statistically significant differences between HAART-naïve and HAART-experienced HIV-infected children. However, there was a statistically significant difference in CD4% and MCV values between HAART-naïve and HAART-experienced children (p<0.05) ().
Table 3 Comparison of hematological profiles between HAART-naïve and HAART-experienced HIV-infected children at Pediatric ART Clinic of Felege Hiwot Referral Hospital in Bahir Dar, northwest Ethiopia, 2013 (N=224)
Immune status and cytopenias
In this study, the prevalence of anemia among cases with severe, advanced and mild immunosuppression was 19 (47.5%), 5 (17.2%) and 19 (26.4%), respectively. Furthermore, leukopenia and thrombocytopenia were found in 7 (17.5%) and 4 (10%) of cases with severe immune suppression ().
Table 4 Cytopenias with regard to immune status of HIV-infected children at Pediatric ART Clinic, Felege Hiwot Referral Hospital, Bahir Dar, northwest Ethiopia, 2013 (N=224)
Comparison of cytopenias
The overall prevalence of anemia among HIV-infected children was 66 (29.5%). Anemia was found in 34 (30.5%) and 32 (28.6%) of HAART-naïve and HAART-experienced children, respectively. Among anemic cases, 1 (2.9%) of HAART-naïve and 2 (6.3%) of HAART-experienced children had severe anemia. About 11 (9.8%), 9 (8%) and 5 (4.5%) of HAART-naïve HIV-infected children were thrombocytopenic, neutropenic and leukopenic, respectively. Moreover, 11 (9.8%), 7 (6.2%) and 5 (4.5%) of HAART-experienced HIV-infected children were neutropenic, thrombocytopenic and leukopenic, respectively ().
Table 5 Cytopenias and other hematologic abnormalities in HIV-infected children with respect to HAART status at Pediatric ART Clinic, Felege Hiwot Referral Hospital, Bahir Dar, northwest Ethiopia, 2013 (N=224)
Anemia and associated factors
In bivariate analysis, age, severe immunosuppression and presence of bleeding were significantly associated with anemia. But in multivariate logistic regression analysis, controlling the possible cofounders, age of ≤5 years (adjusted odds ratio [AOR] =4.3, 95% CI: 1.7–10.9), age of 6–10 years (AOR =3.1, 95% CI: 1.3–7.2) and severe immunosuppression (AOR =2.95, 95%CI: 1.26–6.9) remained risk factors of anemia in HIV-infected children ().
Discussion
Cytopenia is the most common manifestation of advanced HIV infection.Citation13,Citation17,Citation34 It is proposed that they are caused by the impaired growth and development of hematopoietic progenitor cells in the bone marrow due to the presence of HIV proteins and changes in the cytokine expression, which potentially lead to an altered maturation process and increased cell death of ≥1 bone marrow cell lineages.Citation13,Citation35 These abnormalities have been directly correlated with the degree of immunosuppression and disease progression.Citation15,Citation36–Citation38 It has also been documented that they potentially limit the efficacy of HAART treatment and strongly predict morbidity and mortality in HIV-infected individuals.Citation39–Citation43
In this study, the prevalence of anemia was found to be 29.5%, making it more common than neutropenia, thrombocytopenia, leukopenia and cytopenia. This is in agreement with most of the literatures.Citation9,Citation32,Citation44 Experimental studies suggest that dyserythropoiesis and early apoptosis of red cell precursors are common features in HIV infection.Citation45 In addition, HIV infection causes deficiency and defects in metabolism of iron, vitamin B12 and other micronutrients, which may lead to anemia.Citation46,Citation47 Moreover, immune suppression associated with HIV infection can be a cause for the onset of chronic inflammation and/or chronic disease, such as tuberculosis, recurrent diarrheal diseases, recurrent bacterial pneumonia and viral infections, which can result in anemia as well as other cytopenias. The prevalence of anemia (29.5%) is lower than that reported in Lagos (77.9%),Citation9 Nepal (74.4%),Citation48 West Bengal, India (69%),Citation37 Uganda (57.6%)Citation49 and Dar es Salaam, Tanzania (44%),Citation18 and it is higher than that in Addis Ababa, Ethiopia (22.2%),Citation50 Jimma, Ethiopia (21.9%),Citation32 Gondar, Ethiopia (16.2%)Citation51 and Enugu, Nigeria (3%).Citation52 This variation might be attributed to the differences in ethnicity, study designs and time of study. In addition, variation in age of the study participants, HAART status and cutoff value in defining anemia, local prevalence of parasitic infections such as malaria or hookworms, as well as local nutritional patterns might contribute to the variation in magnitude of anemia.
In this study, the number of cases with mild, moderate and severe anemia was 37 (56.1%), 26 (39.4%) and 3 (4.5%), respectively. This is comparable with the results of another study done in Gondar, Ethiopia,Citation51 where occurrence of mild, moderate and severe anemia was 60.5%, 37.2% and 2.3%, respectively. Similarly, it is in agreement with a study done in Addis Ababa, Ethiopia,Citation50 where mild, moderate and severe anemia were reported to be 52.2%, 42.5% and 5%, respectively. The proportion of severe anemia in our studies is lower than that in studies conducted in Jimma, Ethiopia,Citation32 and Dar es Salaam, Tanzania,Citation18 which were reported to be 14.3% and 15%, respectively. The low magnitude of severe anemia in the current study might be related to the improved level of societal awareness about the positive implication of HIV monitoring and treatment, improved access to HIV monitoring and treatment facilities as well as updating of HIV monitoring and therapeutic modalities over time.
In the current study, the second most common cytopenia was neutropenia, which was observed in 8.9% HIV-infected children. The possible explanation may be the fact that HIV directly infects bone marrow and bone marrow stromal cell, which may reduce hematopoiesis. In addition, it is speculated that the decline in vitamin B12 and the presence of antibodies to HIV envelope glycoprotein 120 suppress bone marrow progenitor cells, in addition to being implicated as a causal factor of cytopenia, including neutropenia.Citation20 Furthermore, ART used to suppress the viral load may adversely affect the hematopoietic capacity of bone marrow.Citation12 Compared to studies done in Lagos, Nigeria, (17.5%)Citation9 and West Bengal, India (19%),Citation37 the prevalence of neutropenia is lower. The possible difference might be the difference in immunological status of the study participants and sample size. In the study from Lagos, Nigeria, of the total 68 children who participated in the study, about 75% were in the Centers for Disease Control and Prevention, USA (CDC) clinical stages B and C. Likewise, in the West Bengal study, of the total 100 children participating in the study, 50% were in WHO stages 3 and 4. But, in the current study, 20% of the study participants were in WHO stages 3 and 4.
In this study, thrombocytopenia was found to be 8%, which is the third most common cytopenia in HIV-infected children. The possible biological explanations for why thrombocytopenia is common in HIV infection might be due to an increased platelet destruction either caused by the nonspecific deposition of circulating immune complexes on platelets or the presence of specific antiplatelet glycoprotein antibodies, leading to immune-mediated thrombocytopenia, as well as direct HIV infection of megakaryocytes and their precursors, resulting in higher thrombocytopeniaCitation15,Citation53 The result is comparable to studies done in Mumbai, India (10%)Citation54 and West Bengal, India (11%).Citation37 However, it is higher than that in the report from Lagos (2.5%);Citation9 and lower than studies done in Nepal (17.9%),Citation48 Kenya (21%)Citation44 and Uttar Pradesh, India (29.78%).Citation55 The variations in prevalence could be attributed to the difference in the reference range used to define thrombocytopenia as Adetifa et alCitation9 used platelet count <100 × 103/mm3; HAART status, as Kibaru et alCitation44 included children who were HAART-naïve; and sample size, as Poudel et alCitation48 and Kumar et alCitation55 used small sample size to determine the magnitude of thrombocytopenia.
In the current study, 4.5% of study participants were leukopenic. This is comparable with a study done in Lagos, Nigeria (6%).Citation9 However, it is lower than that in studies done at Kenyatta hospital, Kenya (10%)Citation44 and West Bengal, India (34%),Citation37 and higher than that in the study done in Mumbai, India (2%).Citation54 The possible reason for the variation in magnitude of leukopenia might be due to the differences in ethnicity, age of study participants included in study, HAART status as well as prevalence of infectious and noninfectious diseases.
In this study, severe immunosuppression (AOR =2.95, 95% CI: 1.26–6.9) was significantly associated with anemia. Similar findings had been reported previously in Addis Ababa, Ethiopia,Citation50 Western Uganda,Citation49 and South India,Citation46 revealing that there was statistically significant association between immune suppression and anemia. This could be explained by the fact that immune suppression potentially leads to viral replication, which may cause anemia through increased cytokine-mediated myelosuppression and higher burden of OIs.Citation13,Citation56 Furthermore, age <5 years (AOR =4.3, 95% CI: 1.7–10.9) and age of 6–10 years (AOR =3.1, 95% CI: 1.3–7.2) also remained risk factors of anemia. This is also similar with other studies.Citation18,Citation49,Citation57 The possible reason for increased risk of anemia in younger children may be related with the high nutritional requirement for production of RBCs and the high frequency of comorbidity because their immune status is not well developed. Unlike anemia, neutropenia, thrombocytopenia and leukopenia were not significantly associated with independent variables. The possible explanation could be the small number of cases of children who had these cytopenias and the small sample size; therefore, the number of observations in each category of independent variables would be small and these observations would have low power to predict association.
In the current study, the mean MCV value in HAART-experienced HIV-infected children was significantly higher than in children who were HAART naïve. The possible reason for the high MCV value in HAART-experienced HIV-infected children might be related with the use of zidovudine-based first-line HAART. In this study, of the total 18 children who were on first-line HAART having MCV value >100 fL, 11 (61%) were taking zidovudine-based ART regimen. Evidence suggests that zidovudine causes marrow erythroid hypoplasia, aplasia and megaloblastic maturation, which can be accompanied by a progressive raise in erythrocyte MCV.Citation58,Citation59 The other possible reason for the difference would be related to the nutritional deficiencies, particularly iron deficiency, the most common nutritional deficiency in developing countries,Citation60 which causes microcytic–hypochromic RBC morphology in HAART-naïve children.
The MCV could show an increment due to iron supplements and nutritional modification provided after HAART initiation. Together with the macrocytosis that appears to be overt after they are initiated into zidovudine-containing regimens, the MCV value shows significant elevation. Our data also supported that microcytosis is significantly higher in HAART-naïve (33.9%), compared to HAART-experienced, HIV-infected children (16.1%). A retrospective study done in Kenya has shown that, compared with the baseline value, the mean MCV value of HIV-infected children was significantly raised at 6 months after initiation of HAART.Citation61
However, the mean CD4% of HAART-experienced HIV-infected children was significantly lower than that of HAART-naïve ones. The possible reason for the low value of mean CD4% in HAART-experienced children might be delayed diagnosis and ART initiation, poor adherence to ART and delayed response to ART. These may limit the success of HAART and may be related with continued viral replication and immunological failure. Evidence also demonstrated that initiation of HAART in children with a low CD4 value is less likely to result in a robust increase in CD4 cells, thereby being less likely to achieve a successful treatment outcome.Citation62 Moreover, poor adherence to ART in children is one of the challenges in resource-limited settings, including Ethiopia.Citation63–Citation65
Limitations
The main limitation of this study is the cross-sectional nature of its design, which makes relationships between cytopenias and associated factors difficult, as it is temporal association. In addition, we were unable to analyze serum ferritin, vitamin B12 and folate levels and unable to perform bone marrow examination, which potentially limit this study. Another limitation of this study is that we did not assess all modifiable risk factors, such as intestinal parasitic infection, malaria, viral infections and fungal infections, which could potentially influence the magnitude and severity of cytopenias. We did not include HIV-negative children as a control to compare the magnitude of cytopenias between HIV-infected and noninfected children. Furthermore, the study is a single-center institutional study that could not be generalized for HIV-infected children in the study area.
Conclusion
The prevalence of anemia was higher, meeting the WHO criteria for a moderate public health problem. Neutropenia was the second most common cytopenia among HIV-infected children in the study area. Severe immunosuppression and younger age were significantly associated with anemia. Moreover, the proportion of cytopenias did not significantly vary between HAART-naïve and HAART-experienced HIV-infected children. Therefore, emphasis should be given for investigation and management of hematological abnormalities in HIV-infected children, particularly those who are immunosuppressed and of younger age. Furthermore, multicentered prospective studies need to be conducted to explore modifiable associated factors of cytopenias, patterns of cytopenias over time, and the impact of HAART on cytopenia among HIV-infected children in resource-limited settings such as Ethiopia.
Author contributions
YGT participated in conceiving and designing the study; collecting, analyzing and interpreting the data as well as drafting the manuscript. MM participated in conceiving and designing the study; analyzing and interpreting the data and drafting the manuscript, in addition to being the lead author of the manuscript. ZA, AT and AA participated in study design and data analysis, as well as contributing toward drafting and review of the manuscript. All authors have read and approved the final manuscript.
Abbreviations
| AIDS | = | acquired immune deficiency syndrome |
| ANC | = | absolute neutrophil count |
| ART | = | antiretroviral treatment |
| CI | = | confidence interval |
| CD4 | = | cluster of differentiation-4 |
| ETB | = | Ethiopian birr |
| HAART | = | highly active antiretroviral therapy |
| HIV | = | human immunodeficiency virus |
| Hg | = | hemoglobin |
| MCH | = | mean corpuscular hemoglobin |
| MCHC | = | mean corpuscular hemoglobin concentration |
| MCV | = | mean corpuscular volume |
| mid | = | mixed cell |
| MPV | = | mean platelet volume |
| OIs | = | opportunistic infections |
| RBC | = | red blood cell |
| RDW | = | red cell distribution width |
| WBC | = | white blood cell |
Acknowledgments
The authors thank the staff of the Pediatric ART Clinic, Felege Hiwot Referral Hospital, who actively participated during the data collection process. They are also grateful to and wish to thank the study participants and caregivers for their voluntary participation in this study. Finally, they thank the University of Gondar, Amhara Regional Health Bureau, Felege Hiwot Referral Hospital and Bahir Dar Regional Health Research Laboratory Center for financial and logistical support.
Disclosure
The authors report no conflicts of interest in this work.
References
- OkolieMNEghafonaNOOmoregieRAnti-human immunodeficiency virus (HIV) agentsJ Med Lab Sci200312114
- EnawgawBAlemMAddisZMelkuMDetermination of hematological and immunological parameters among HIV positive patients taking highly active antiretroviral treatment and treatment naïve in the antiretroviral therapy clinic of Gondar University Hospital, Gondar, Northwest Ethiopia: a comparative cross-sectional studyBMC Hematol201414824666771
- KamKMLeungWLWongKHLeeSSHungMYKwokMYMaturational changes in peripheral lymphocyte subsets pertinent to monitoring human immunodeficiency virus-infected Chinese pediatric patientsClin Diagn Lab Immunol20018592693111527805
- MosesANelsonJBagbyGCThe influence of human immunodeficiency virus-1 on hematopoiesisBlood1998915147914959473211
- SloandEHematologic complications of HIV infectionAIDS Rev200474187196
- CosbyCDHematologic disorders associated with human immunodeficiency virus and AIDSJ Infus Nurs2007301223217228196
- CoyleTEHematologic complications of human immunodeficiency virus infection and the acquired immunodeficiency syndromeMed Clin North Am19978124494709093237
- SullivanAKRabenDReekieJFeasibility and effectiveness of indicator condition-guided testing for HIV: results from HIDES I (HIV indicator diseases across Europe study)PLoS One201381e5284523341910
- AdetifaIMOTemiyeEAkinsulieAEzeakaVIrohaEHaematological abnormalities associated with pediatric HIV/AIDS in LagosAnn Trop Paediatr20062612112516709330
- AkkinaRNew insights into HIV impact on hematopoiesisBlood2013122132144214624072846
- KirchhoffFSilvestriGIs Nef the elusive cause of HIV-associated hematopoietic dysfunction?J Clin Invest200811851622162518431512
- ChoiSYKimIKimNJHematological manifestations of human immunodeficiency virus infection and the effect of highly active anti-retroviral therapy on cytopeniaKorean J Hematol201146425325722259631
- AlexakiAWigdahlBHIV-1 infection of bone marrow hematopoietic progenitor cells and their role in trafficking and viral disseminationPLoS Pathog2008412e100021519112504
- QuayeWKQuayeLAmiduNAddai-MensahANPrevalence of anaemia and immunological markers among Ghanaian HAART-naïve HIV-patients and those on HAARTAfr Health Sci201111121521572851
- AttiliSVSinghVPRaiMVarmaDVGulatiAKSundarSHematological profile of HIV patients in relation to immune status – a hospital-based cohort from Varanasi, North IndiaTurk J Hematol20082511319
- EleyBSSiveAAShuttleworthMHusseyGDA prospective, cross-sectional study of anaemia and peripheral iron status in antiretroviral naïve, HIV-1 infected children in Cape Town, South AfricaBMC Infect Dis20022311866864
- LiebmanHAViral-associated immune thrombocytopenic purpuraHematology Am Soc Hematol Educ Program200821221819074085
- MakubiANMugusiFMagesaPMRobertsDQuareshARisk factors for anaemia among HIV infected children attending care and treatment clinic at Muhimbili National Hospital in Dar es Salaam, TanzaniaTanzan J Health Res20121411926591739
- ClasterSBiology of anemia, differential diagnosis, and treatment options in human immunodeficiency virus infectionJ Infect Dis2002185suppl 2S105S10912001030
- KuritzkesDRNeutropenia, neutrophil dysfunction, and bacterial infection in patients with human immunodeficiency virus disease: the role of granulocyte colony-stimulating factorClin Infect Dis200030225626010671324
- SiegSFBazdarDAHardingCVLedermanMMDifferential expression of interleukin-2 and gamma interferon in human immunodeficiency virus diseaseJ Virol200175209983998511559831
- ZonLIGroopmanJEHematologic manifestations of human immune deficiency virus (HIV)Semin Hematol19882532082183043675
- OshikoyaKALawalSOreagbaIAAdverse events in HIV-infected children on antiretroviral therapy at a teaching hospital in Lagos, Nigeria: a retrospective studyAdv Pharmacoepidem Drug Safety2012141000117
- SutcliffeCGVan DijkJDBoltonCPersaudDMossWJEffectiveness of antiretroviral therapy among HIV-infected children in sub-Saharan AfricaLancet Infect Dis20088847748918652994
- TayeBShiferawSEnquselassieFThe impact of malnutrition in survival of HIV infected children after initiation of antiretroviral treatment (ART)Ethiop Med J201048111020607992
- SunguyaBFPoudelKCOtsukaKUndernutrition among HIV-positive children in Dar es Salaam, Tanzania: antiretroviral therapy alone is not enoughBMC Public Health20111186922087543
- BachouHTylleskärTDowningRTumwineJSevere malnutrition with and without HIV-1 infection in hospitalised children in Kampala, Uganda: differences in clinical features, haematological findings and CD4+ cell countsNutr J200652717042940
- HeikensGTBunnJAmadiBCase management of HIV-infected severely malnourished children: challenges in the area of highest prevalenceLancet200837196201305130718406865
- NielsenSDErsbollAKMathiesenLNielsenJOHansenJESHighly active antiretroviral therapy normalizes the function of progenitor cells in human immunodeficiency virus–infected patientsJ Infect Dis1998178129913059780249
- AurpibulLPuthanakitTSirisanthanaTSirisanthanaVHaematological changes after switching from Stavudine to Zidovudine in HIV-infected children receiving highly active antiretroviral therapyHIV Med2008931732118331562
- MolyeGSawyerWLawMAminJHilAChanges in hematologic parameters and efficacy of thymidine analogue-based, highly active antiretroviral therapy: a meta-analysis of six prospective, randomized, comparative studiesClin Ther2004261929714996521
- AbebeMAlemsegedFHematologic abnormalities among children on HAART, in Jimma university specialized hospital, Southwestern EthiopiaEthiop J Health Sci20091928389
- WHOAntiretroviral Therapy of HIV Infection in Infants and Children: Towards Universal Access. Recommendations for a Public Health Approach. Strengthening Health Services to Fight HIV/AIDS, WHO HIV/AIDS ProgramGenevaWHO2006 Available from: http://www.who.int/hiv/pub/guidelines/paediatric020907.pdfAccessed November 18, 2015
- WHO [webpage on the Internet]Haemoglobin Concentrations for the Diagnosis of Anaemia and Assessment of Severity, Vitamin and Mineral Nutrition information SystemGenevaWHO2011 Available from: http://www.who.int/vmnis/indicators/haemoglobin/en/Accessed November 20, 2015
- KokaPSReddySTCytopenias in HIV infection: mechanisms and alleviation of hematopoietic inhibitionCurr HIV Res20042327528215279591
- DenueBAGashauWBelloHSKidaIMBakkiBAjayiBRelation between some haematological abnormalities, degree of immunosuppression and viral load in treatment-naïve HIV-infected patientsEast Mediterr Health J201319436236823882962
- BhowmikABanerjeePHematological manifestation in HIV infected childrenJ Coll Physicians Surg Pak201525211912325703756
- EllaurieMBurnsERRubinsteinAHematological manifestations in pediatric HIV infection: severe anemia as a prognostic factorAm J Pediatr Hematol Oncol19901244494532285125
- BerhaneKKarimRCohenMHImpact of highly active antiretroviral therapy on anaemia and relationship between anemia and survival in a large cohort of HIV-infected women: women’s Interagency HIV StudyJ Acquir Immune Defic Syndr20043721245125215385731
- AnastosKShiQFrenchALTotal lymphocyte count, hemoglobin and delayed-type hypersensitivity as predictors of death and AIDS illness in HIV-1 infected women receiving highly active antiretroviral therapyJ Acquir Immune Defic Syndr20043538339215097155
- ChatterjeeABoschRJKupkaRHunterDJMsamangaGIFawziWWPredictors and consequences of anaemia among antiretroviral-naïve HIV-infected and HIV-uninfected children in TanzaniaPublic Health Nutr200913228929619650963
- GebremedhinAGebremariamSHaileFWeldearegawiBDecotelliCPredictors of mortality among HIV infected children on anti-retroviral therapy in Mekelle Hospital, Northern Ethiopia: a retrospective cohort studyBMC Public Health201313104724517533
- De SantisGCBrunettaDMVilarFCHematological abnormalities in HIV-infected patientsInt J Infect Dis201115e808e81121880530
- KibaruEGNduatiRWamalwaDKariukiNBaseline hematological indices among HIV-1 infected children at Kenyatta National HospitalInt J Novel Res Healthcare Nursing2014112126
- CalisJCPhiriKVetRJErythropoiesis in HIV-infected and uninfected Malawian children with severe anemiaAIDS201024182883288720871386
- ShetABhavaniPKKumarasamyNAnemia, diet and therapeutic iron among children living with HIV: a prospective cohort studyBMC Pediatr20151516426482352
- AnyaboluHCAdejuyigbeEAAdeoduOOSerum micronutrient status of Haart-naive, HIV infected children in South Western Nigeria: a case controlled studyAIDS Res Treat201420148
- PoudelPPokharelRChitlangiaMChaudharySProfile of HIV infected children: a hospital based study at Eastern NepalAsian Pac J Trop Dis201443169175
- Nyesigire RuhindaEBajunirweFKiwanukaJAnaemia in HIV-infected children: severity, types and effect on response to HAARTBMC Pediatr20121217023114115
- MihiretieHTayeBTsegayeAMagnitude of anemia and associated factors among pediatric HIV/AIDS patients attending Zewditu Memorial Hospital ART Clinic, Addis Ababa, EthiopiaAnemia2015201547932916
- EnawgawBAlemMMelkuMAddisZTerefeBYitayewGPrevalence and associated risk factors of anemia among HIV infected children attending Gondar university hospital, Northwest Ethiopia: a cross sectional studyBMC Hematol2015151226413303
- EzeonwuBUIkefunaANOguonuTOkaforHUPrevalence of hematological abnormalities and malnutrition in HIV-infected under five children in EnuguNiger J Clin Pract201417330330824714007
- LiZNardiMAKarpatkinSRole of molecular mimicry to HIV-1 peptides in HIV-1-related immunologic thrombocytopeniaBlood200510657257615774614
- ShahIKatiraBHematological manifestation in HAART naïve HIV-1 infected children in India in a resource limited settingPediatric Oncall J20118535
- KumarDKumarDSinghDKRaiRPrevalence of thrombocytopenia and its relation with WHO clinical and immunological staging among human immunodeficiency virus-infected childrenIndian J Child Health201413140142
- YadavJNandaSSharmaDOpportunistic infections and complications in human immunodeficiency virus-1-infected children: correlation with immune statusSultan Qaboos Univ Med J2014144e513e52125364555
- ShetAMehtaSRajagopalanNAnemia and growth failure among HIV-infected children in India: a retrospective analysisBMC Pediatr200993719531242
- SsaliFStohrWMunderiPPrevalence, incidence and predictors of severe anaemia with zidovudine-containing regimens in African adults with HIV infection within the DART trialAntivir Ther20061174117310818
- PryceCPierreRBSteel-DuncanJSafety of antiretroviral drugs in Jamaican children with HIVWest Indian Med J200857323824519583122
- WHO [webpage on the Internet]Iron Deficiency Anaemia: Assessment, Prevention and Control A Guide for Programme ManagersGenevaWorld Health Organization2001 Available from: http://www.who.int/nutrition/publications/micronutrients/anaemia_iron_deficiency/WHO_NHD_01.3/en/Accessed January 10, 2016
- KibaruEGNduatiRWamalwaDKariukiNImpact of highly active antiretroviral therapy on hematological indices among HIV-1 infected children at Kenyatta National Hospital-Kenya: retrospective studyAIDS Res Ther2015122626279668
- MusokePMMudiopePBarlow-MoshaLNGrowth, immune and viral responses in HIV infected African children receiving highly active antiretroviral therapy: a prospective cohort studyBMC Pediatr2010105620691045
- BiressawSAbegazWEAbebeMTayeWABelayMAdherence to antiretroviral therapy and associated factors among HIV infected children in Ethiopia: unannounced home-based pill count versus caregivers’ reportBMC Pediatr20131313224229394
- BiadgilignSDeribewAAmberbirADeribeKAdherence to highly active antiretroviral therapy and its correlates among HIV infected pediatric patients in EthiopiaBMC Pediatr200885319061515
- EtichaTBerhaneLCaregiver-reported adherence to antiretroviral therapy among HIV infected children in Mekelle, EthiopiaBMC Pediatr20141411424766911
