Abstract
Despite the decreased incidence of human immunodeficiency virus (HIV)-associated nephropathy due to the widespread use of combined active antiretroviral therapy, it remains one of the leading causes of end-stage renal disease (ESRD) in HIV-1 seropositive patients. Patients usually present with low CD4 count, high viral load and heavy proteinuria, with the pathologic findings of collapsing focal segmental glomerulosclerosis. Increased susceptibility exists in individuals with African descent, largely due to polymorphism in APOL1 gene. Other clinical risk factors include high viral load and low CD4 count. Advanced kidney disease and nephrotic range proteinuria have been associated with progression to ESRD. Improvement in kidney function has been observed after initiation of combined active antiretroviral therapy. Other treatment options, when clinically indicated, are inhibition of the renin–angiotensin system and corticosteroids. Further routine management approaches for patients with chronic kidney disease should be implemented. In patients with progression to ESRD, kidney transplant should be pursued, provided that viral load control is adequate. Screening for the presence of kidney disease upon detection of HIV-1 seropositivity in high-risk populations is recommended.
Introduction
Kidney disease is among the major causes of morbidity and mortality in human immunodeficiency virus (HIV)-1 positive individuals.Citation1 HIV-associated nephropathy (HIVAN) is one of the most important causes of end-stage renal disease (ESRD) in this population. Factors such as African American ancestry, APOL1 polymorphisms, comorbidities, high viral load, low CD4 count, advanced kidney disease and nephrotic range proteinuria have been associated as risk factors for the development of HIVAN and its progression to ESRD.Citation2 Concurrent with the widespread use of combined anti-retroviral therapy (cART), prevalence of kidney disease in HIV-1 positive individuals has been increasing and is expected to rise further as a result of aging population and improved patients’ survival. Existing management options for HIVAN range from cART, blockade of the renin–angiotensin system and steroids to renal replacement therapy with dialysis and, most recently, kidney transplant. In this review, we describe the trends in development of HIVAN and its currently accepted pathophysiology. An extensive PubMed review of the literature of HIV and associated kidney disease was performed. We will explore how the understanding of HIVAN has developed since its first description to the latest one.
Pathophysiology
HIVAN is pathologically characterized by a collapsing glomerulopathy with active tubulointerstitial inflammation. The collapse of glomerular basement membranes is usually observed, along with hypertrophy and hyperplasia of the overlying glomerular epithelial cells, as well as active tubulointerstitial disease manifested by microcytic tubular dilatation, interstitial inflammation and tubular injury (). HIVAN presents clinically with a rapid rise in serum creatinine (sCr) and proteinuria.Citation3,Citation4 Other glomerular disorders can have a clinical presentation comparable to that of HIVAN and should be considered in this population ().
Figure 1 HIV-associated nephropathy.
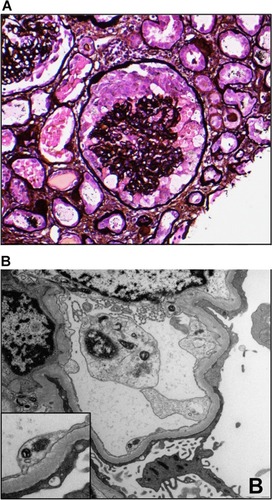
Table 1 Differential diagnosis of HIV-associated nephropathy
Links and risks
Host factors
African ancestry and genetic predisposition
HIVAN predominantly affects HIV-1 positive individuals of African ancestry and its prevalence has been estimated to be as high as 3.5% in clinical studies.Citation5 According to a cohort from 1989 to 2011 of the United States Renal Data System, 89% of patients with ESRD from HIVAN were African Americans.Citation6 While the incidence of ESRD in HIV-positive individuals has decreased over time due to the widespread use of combined antiretroviral therapy (cART), HIV-positive individuals are still more likely than HIV-negative individuals to develop ESRD.
The increased risk in west African and African American populations has been demonstrated to be driven by polymorphisms in the APOL1 gene, as described by Genovese et al.Citation7 Two APOL1 risk alleles, G1 (containing two missense mutations, rs73885319 and rs60910145) and G2 (a frameshift deletion rs71785313), at the serum resistance–associated interacting-domain-encoding region of APOL1 increase the susceptibility for the development of HIVAN.Citation8 These two alleles appear to have arisen adaptively, possibly as a result of their protective effects against trypanosomal infections. Trypanosoma brucei rhodesiense, the cause of human African sleeping sickness, carries a serum resistance–associated protein that binds and inactivates the wild-type APOL1, preventing this from lysing the trypanosome.Citation9 Individuals with two copies of the high-risk alleles have 29 times higher odds of developing HIVAN, as compared to those with zero risk alleles (odd ratio: 29, 95% CI, 13–68).Citation8 Until recently, the lack of animal models represented a major challenge to demonstrate the pathogenic effect of APOL1 risk alleles, since the APOL1 gene is only present in some primates and humans. The G1 allele was found in 52% of focal segmental glomerulosclerosis (FSGS) compared to 18% in geographically matched African American controls without kidney disease. The G2 allele, in contrast, was noted in 23% of FSGS cases compared to 15% in controls.Citation7 APOL1 risk variants were associated with progression of kidney disease in a cohort of non-diabetic African Americans with hypertension-attributable kidney disease, regardless of blood pressure control target or antihypertensive agent.Citation10
In vivo, mice with podocyte-specific expression of either APOL1 risk alleles develop albuminuria, foot-process effacement, glomerulosclerosis and gene expression changes reminiscent of human kidney disease.Citation11 Although the mechanism by which APOL1 variants cause kidney disease is not well defined, several studies have suggested several potential mechanisms.
In vitro studies by Granado et alCitation12 showed that APOL1 proteins localize intracellularly, predominantly in the endoplasmic reticulum and to a lesser extent in the mitochondria in podocyte cells. In comparison to the G0 wild-type variant, in vitro overexpression of G1 and G2 led to an increase in phosphorylation of stress-triggered kinases such as the p38 MAPK and AMP-activated protein kinase. This ultimately led to adenosine triphosphate depletion and increased cytotoxicity, indicating APOL1-associated cellular injury is induced by decreased functioning at these particular sites. In another study using T-Rex-293 cells lines, overexpression of G1 or G2 APOL1 risk variants led to an apparent cell swelling, potassium depletion and activation of stress-activated protein kinases, resulting in a dose-dependent cell injury.Citation13 Most recently, Ma et al demonstrated in vitro that overexpression of G1 and G2 alleles led to impaired mitochondrial function, resulting in cell injury and death characterized by intracellular potassium depletion.Citation14 The authors postulated that this could be due to disruption of the sodium–potassium ATPase activity and with changes in membrane potential, a cascade of events can ensue leading ultimately to cell injury and death.
Another novel mechanism for APOL1-induced kidney injury in vivo used a transgenic Drosophila fly line expressing G0 vs G1 variants, which resulted in nephrocyte hypertrophy and accelerated cell death.Citation15 The authors note that the Drosophila nephrocyte has a very similar structural and functional homology to mammalian podocytes, which has been the target of previous in vitro research. Finally, a group led by Kruzel-Davila showed another potential pathway to cell injury via impaired vacuole acidification and impaired endosomal trafficking using Drosophila melanogaster and the yeast Saccharomyces cerevisiae.Citation16
The strong associations of the APOL1 risk alleles have been described in multiple case–control and population studies of kidney disease in patients with ESRD, FSGS, HIVAN and hypertension-attributed nephropathy. This association has more recently been extended to certain glomerulopathies such as systemic lupus erythematosus, membranous nephropathy and diabetic kidney disease ().Citation17 However, currently, the possible association of APOL1 risk alleles with these glomerulopathies may not represent development of the specific glomerulopathy per se, but rather APOL1-associated glomerulopathies (e.g., FSGS in a patient with systemic lupus erythematosus) and requires further investigation.
Male sex and age
Previous studies have shown that individuals with HIVAN are predominantly male. Based on the data from United States Renal Data System, 70% of ESRD patients with HIVAN were male. While older age has been reportedly associated with HIVAN,Citation18,Citation19 median age among several other studies appears to be from 35 to 48 years.Citation6,Citation20,Citation21 However, studies have failed to show a direct correlation between gender or age and HIVAN.
Heavy proteinuria
Classically, HIVAN presents with rapidly declining glomerular filtration rate (GFR) and nephrotic range proteinuria. In one study characterizing 57 patients with HIVAN on kidney biopsy, mean proteinuria was 4.1 g/day and only 14% of the patients had proteinuria <1.5 g/day.Citation22 Atta et al established that the sensitivity and specificity of nephrotic range proteinuria for identifying HIVAN in HIV-positive individuals were 73% and 61%, respectively.Citation23 A summary of the risk factors and predictors of HIVAN and its progression is listed in .
Table 2 Risk factors and predictors of HIV-associated nephropathy
Comorbidities – diabetes mellitus and hypertension
Diabetes mellitus and hypertension have been reported as the most frequent causes of chronic kidney disease (CKD) in the US general population, increasing the CKD risk almost 10-fold and accounting for 71% of all cases of ESRD.Citation24,Citation25 Diabetes and hypertension are increasingly frequent, and are potential drivers for CKD progression in individuals with coexisting HIVAN. Several studies have shown increased odds of diabetes and hypertension in HIV-positive individuals compared to seronegative controls.Citation26–Citation30 Furthermore, in cross-sectional and cohort studies, the prevalence of hypertension and diabetes approaches 55% and 20%, respectively. A possible explanation of this is that the use of ART can lead to altered lipid metabolism, dysregulation of glucose control and lipodystrophy, which are traditional risk factors that contribute to the development of CKD.Citation31
Reduced kidney function or estimated GFR (eGFR) <90 mL/min/1.73 m2
Due to the rapid progressive course of HIVAN, kidney function may be severely reduced at the time of diagnosis. Bige et al reported severely impaired kidney function in their cART-treated patient population, with a median eGFR of 20 mL/min/1.73 m2.Citation22 Higher sCr at the time of presentation in HIV-positive patients is also associated with the diagnosis of HIVAN. In a study that included 87 patients, mean baseline sCr in patients with HIVAN was 7.6 mg/dL compared with 2.5 mg/dL in patients without HIVAN,Citation32 and can be a strong negative predictor for HIVAN diagnosis.Citation33
Viral factors
Advanced HIV disease (low CD4, high viral load)
Advanced HIV-1 infection is often found in patients with HIVAN. Based on the results of two studies, ~80%–90% of the patients who present with HIVAN have a CD4-positive T-cell count <200 cells/mm3.Citation31,Citation34 In a 57-patient study with biopsy-proven HIVAN, the mean HIV viral load was >30,000 copies/mL and the mean CD4 count was 127 cells/mm3.Citation22 HIV-1 RNA levels can help in suggesting the emergence of HIVAN in a patient with typical clinical presentation or otherwise its absence if HIV-1 RNA level is <400 copies/mL.Citation35 HIV-1 RNA level of ≥400 copies/mL resulted in a high sensitivity of 95.8%, but with a low specificity of 35.5% for the diagnosis of HIVAN. Moreover, intracellular HIV-1 proviral DNA level in peripheral blood mononuclear cells may also predict the likeliness of developing HIVAN. An intracellular HIV-1 proviral DNA level <10 copies/ml had a negative predictive value and sensitivity of 100% for the diagnosis of HIVAN.Citation36
HIV infection of renal cells
The pathogenesis of HIV-associated kidney disease is poorly understood. The proposed mechanisms include direct infection of renal parenchymal cells and indirect injury to the kidney by renal cellular uptake of circulating virally encoded molecules or indirect injury through the release of cytokines. Also, multiple mechanisms might be involved when different HIV variants are present.Citation37 However, the mechanism by which HIV-1 enters these cells has not been identified. Renal tubular cells do not express any of the known HIV-1 receptors (CD4, CCR5, CXCR4, DC-sign or mannose receptors).Citation37,Citation38 A previous study assessing the role of DEC-205 as an HIV-1 receptor in mediating internalization of the virus into renal tubular cells has been proposed.Citation39
Management
Currently, there are no randomized controlled trials that address the management of HIVAN. Most of the treatment options including cART, inhibition of renin–aldosterone system and corticosteroids are based on retrospective studies and small non-randomized trials ().
Table 3 Summary of previous studies regarding management
Combined active ART
Clinical practice guidelines recommend ART initiation in all HIV-positive individuals, regardless of their CD4 count. HIVAN generally affects patients with advanced HIV-1 infection who would require treatment regardless of renal involvement, making it difficult to perform randomized clinical trials to evaluate the efficiency of cART in this population. Thus, evidence-based recommendations for cART in patients with HIVAN come mainly from observational or retrospective studies. In a retrospective cohort study that included patients with HIVAN not on dialysis, renal survival was significantly better in the patients treated with cART compared to patients with no treatment.Citation40 In a cohort of 4000 patients with HIV, the difference in incidence rate of HIVAN in those receiving and not receiving cART was 19.6 per 1000 patients per year (26.4 vs 6.8 per 1000 patients per year). The HIVAN risk was reduced by 60% (95% CI, −30% to −80%) with cART.Citation41 Notably, Post et al followed a large cohort of HIV-positive patients from 1998 to 2004, of whom 61 were diagnosed with HIVAN (45 biopsy proven).Citation42 These authors showed that despite improvement in overall survival with the use of cART, renal survival remained poor, with 34 patients reaching ESRD, including even those who had achieved complete viral suppression. However, as pointed out by the authors, most of these patients already had advanced renal failure at the time of HIVAN diagnosis; also, there was no control untreated group in this study.
Renin–angiotensin–aldosterone system blockade
No randomized trials of renin–angiotensin–aldosterone system blockade in patients with HIV-related CKD have been conducted. Most studies concerning the use of angiotensin-converting enzyme (ACE) inhibitors/angiotensin receptor blockers (ARBs) have been extrapolated from studies in other glomerular diseases or from the pre-cART era. ACE inhibitors/ARBs have been shown to be effective in CKD.Citation43 Such protective effects are mainly due to decreased intraglomerular pressure, decrease in inflammatory mediators and reduction in proteinuria independent of blood pressure control.Citation44 From the few studies addressing ACE inhibitors/ARBs, Kimmel et al studied 18 patients with biopsy-proven HIVAN, of whom nine patients were treated. They showed that renal survival was significantly enhanced in patients treated with capto-pril vs controls (mean renal survival 156±71 vs 37±5 days, respectively; p<0.002).Citation45 A subsequent study demonstrated significantly longer renal survival for HIV-positive patients treated with fosinopril as compared to untreated patients (relative risk: 0.003; p<0.0001).Citation46
Treatment with ACE inhibitor/ARB is recommended, when clinically feasible, in HIV-positive individuals with confirmed or suspected HIVAN or clinically significant albuminuria (>30 mg/day in diabetic patients and >300 mg/day in non-diabetic patients).Citation47
Corticosteroids
Despite the lack of large randomized controlled trials to support steroid use in this population, the rationale behind steroid use in patients with HIVAN comes from the significant tubulointerstitial inflammation shown on renal biopsy in these individuals. After steroid treatment, the inflammation was found to improve. In a small retrospective study including 21 HIV-positive individuals of whom 13 received prednisone 60 mg/day for 1 month, 7 remained dialysis free at 6 months compared to only 1 of the 8 non steroid treated individuals.Citation48 In another retrospective cohort of 102 biopsy-proven HIVAN cases, delay in the initiation of hemodialysis was seen in individuals who received prednisone at 1 mg/kg for 2–6 weeks. The proportion of patients free of dialysis at 0.5, 1 and 3 years was 73%±5%, 60%±7% and 18%±10%, respectively.Citation49 HIV Medicine Association of the Infectious Diseases Society of America clinical practice guidelines suggest that clinicians can consider corticosteroids as an adjunct to ART and ACE inhibitors or ARBs in biopsy-confirmed HIVAN.Citation47
Renal replacement therapy in HIVAN
Due to the rapid disease progression, HIVAN often advances to ESRD requiring renal replacement therapy, despite HIV treatment. Risk factors for the development of ESRD in HIV-positive individuals include traditional risk factors such as hypertension, diabetes mellitus, cardiovascular disease and HIV-associated factors such as low CD4 counts and high HIV RNA levels.Citation50 In a cohort study performed in Denmark, individuals with HIVAN showed a 4-fold increased requirement for any requirement for renal replacement therapy and a 3-fold increased requirement for chronic renal replacement therapy compared to the seronegative population, with no association to cART.Citation51 Although some studies have suggested that overall long-term survival is possible in patients with HIVAN on dialysis, older age, presence of lower serum albumin level, low CD4 count and the lack of cART are independent predictors of poor survival.Citation52,Citation53 Regarding the incidence of dialysis access infection, rates for prosthetic arteriovenous graft infection in HIV-positive patients and HIV-negative patients were 30% and 7%, respectively (p=0.04). Infection rates in autologous arteriovenous fistulas did not differ between the groups.Citation54 Patient to patient transmission of HIV has not been documented in the USA, and thus, the Center for Disease Control does not recommend routine isolation or dedicated machines for HIV-infected patients, unless there is a coinfection with hepatitis B.Citation55
Kidney transplantation
An increasing number of HIV-positive individuals with ESRD are seeking kidney transplantation. A multicenter, prospective, 3-year trial examined outcomes of kidney transplantation in 150 HIV-positive individuals who had CD4 counts of at least 200 cells/mL and undetectable HIV-1 RNA levels (<50 copies/mL) or <75 copies/mL on viral load while receiving cART in the 16 weeks prior to transplantation. Median follow-up was 1.7 years. Unexpectedly, the study reported a higher rejection rate by 2–3-fold increase in HIV-positive individuals compared with HIV-negative individuals. However, the 1- and 3-year allograft survival was reasonable at 90% and 74%, respectively.Citation56 Waheed et al examined transplant recipients with HIVAN and its impact on graft and patient survival in HIV-1 positive individuals who underwent kidney transplantation at a large tertiary care center from 2006 to 2014. They included 16 individuals with HIV-1 seropositivity who underwent kidney transplantation. Of these, 11 patients were identified to have biopsy-proven HIVAN as the primary cause of their ESRD. Seven (64%) individuals developed delayed graft function, of whom six (54%) required postoperative dialysis within 1 week of transplant. Graft survival rates at 1 and 3 years were 100% and 81%, respectively, and acute rejection rates at 1 and 3 years were 18% and 27%, respectively.Citation57
In recent years, kidney transplantation has been successfully implemented from HIV-positive donors to HIV-positive recipients, both in South Africa and in the USA.Citation58,Citation59 While HIV-negative donor to HIV-positive recipient kidney transplantation has been carried out since 2001, the concerns regarding HIV-positive to HIV-positive transplantation include HIV superinfection, resistance to cART and potential recurrence of HIVAN, in addition to inferior outcomes in general.Citation60 While short-term end points have shown promise, long-term outcome data have been somewhat lacking. A more recent study detailed the outcomes of kidney transplantation from a HIV-positive donor to a HIV-positive recipient from 2008 to 2017,Citation60 totaling 43 kidneys transplanted from 25 HIV-positive deceased donors to HIV-positive recipients. Notably, 23 of the 25 deceased donors had not received any type of cART. Among the 43 recipients, 6 had protocol biopsies that were concerning for recurrent HIVAN. However, the authors argue that in certain areas of the world, including South Africa, the risk of shortened transplant kidney survival using HIV-positive donor kidneys far outweighs the risk of advanced CKD, given the lack of access to dialysis.
Routine CKD care – blood pressure control, vaccinations
Screening and early diagnosis of CKD will improve outcomes in patients who are HIV positive. From the renal standpoint, strategies for care in HIV-positive individuals should include measurement of blood pressure, kidney function (sCr, eGFR), urine examination of proteinuria, administration and monitoring of cART and/or ACE inhibitors/ARBs. Patient should be referred to a nephrology service for early CKD management, predominantly to distinguish cART nephrotoxicity from other non-HIVAN–related kidney disorders, monitoring disease progression and complications and ultimately for timely preparation for dialysis and/or kidney transplantation. Establishing the level of kidney function is important in minimizing nephrotoxicity of cART, considering that the majority of patients worldwide receive tenofovir-based regimens as first-line therapy. Moreover, given the broad differential diagnosis in HIV-1-positive individuals, a kidney biopsy may be an essential tool for evaluation.Citation61 The high incidence of hypertension and diabetes mellitus in HIVAN underscores the importance of optimizing blood pressure and achieving glycemic control as a means of minimizing the rate of CKD progression. Thus, the The Eighth Joint National Committee (JNC8) Guidelines for Hypertension in adults have recommended target blood pressure of <140/90 mmHg,Citation62 whereas the American Heart Association 2017 guidelines have recently recommended target blood pressure of <130/80 mmHg and suggest the use of ACE inhibitors if albuminuria is >300 mg/day.Citation63
Conclusion
HIVAN is a rapidly progressing kidney disorder that affects HIV-positive individuals predominantly of African American ancestry. Clinical and genetic risk factors such as low CD4 count, high viral load, high-grade proteinuria, presence of comorbidities such as hypertension and diabetes, and APOL1 polymorphism contribute to greater progression of kidney disease in this population. We now have a greater understanding of the cellular mechanisms by which APOL1 risk variants may affect cellular function through dysregulation of cellular signaling and mitochondrial function, though more work is needed in these areas.
This review has a number of limitations. First, this was not a meta-analysis of multiple previous studies, but rather an overview of the general understanding of HIVAN pathophysiology. Many studies described are small retrospective observational studies which have inherent limitations. This review serves to provide the evidence behind the current, guideline-based strategies for HIVAN management. Further, we wish to highlight active areas of research on the pathophysiology of HIVAN and optimization of graft survival outcomes of HIV-positive kidney transplantation recipients.
Acknowledgments
MGA was supported by The National Institute of Diabetes and Digestive and Kidney Diseases 5P01DK056492-13.
Disclosure
The authors report no conflicts of interest in this work.
References
- MocroftAReissPGasiorowskiJSerious fatal and nonfatal non-AIDS-defining illnesses in EuropeJ Acquir Immune Defic Syndr201055226227020700060
- WaheedSAttaMGPredictors of HIV-associated nephropathyExpert Rev Anti Infect Ther201412555556324655211
- FogoABLuscoMANajafianBAlpersCEAJKD atlas of renal pathology: HIV-associated nephropathy (HIVAN)Am J Kidney Dis2016682e13e1427477363
- SwanepoelCRAttaMGD’AgatiVDKidney disease in the setting of HIV infection: conclusions from a kidney disease: improving global outcomes (KDIGO) controversies conferenceKidney Int201893354555929398134
- AhujaTSBoruckiMFuntanillaMShahinianVHollanderMRajaramanSIs the prevalence of HIV-associated nephropathy decreasing?Am J Nephrol199919665565910592359
- Razzak ChaudharySWorkenehBTMontez-RathMEZolopaARKlotmanPEWinkelmayerWCTrends in the outcomes of end-stage renal disease secondary to human immunodeficiency virus-associated nephropathyNephrol Dial Transplantat2015301017341740
- GenoveseGFriedmanDJRossMDAssociation of trypanolytic ApoL1 variants with kidney disease in African AmericansScience2010329599384184520647424
- KoW-YRajanPGomezFIdentifying darwinian selection acting on different human APOL1 variants among diverse African populationsAm J Hum Genet2013931546623768513
- KoppJBNelsonGWSampathKAPOL1 Genetic variants in focal segmental glomerulosclerosis and HIV-associated nephropathyJ Am Soc Nephrol201122112129213721997394
- LipkowitzMSFreedmanBILangefeldCDApolipoprotein L1 gene variants associate with hypertension-attributed nephropathy and the rate of kidney function decline in African AmericansKidney Int201383111412022832513
- BeckermanPBi-KarchinJParkATransgenic expression of human APOL1 risk variants in podocytes induces kidney disease in miceNat Med234429438
- GranadoDMullerDKrauselVIntracellular APOL1 risk variants cause cytotoxicity accompanied by energy depletionJ Am Soc Nephrol201728113227323828696248
- OlabisiOAZhangJYVerPlankLAPOL1 kidney disease risk variants cause cytotoxicity by depleting cellular potassium and inducing stress-activated protein kinasesProc Natl Acad Sci USA2016113483083726699492
- MaLChouJWSnipesJAAPOL1 renal-risk variants induce mitochondrial dysfunctionJ Am Soc Nephrol20172841093110527821631
- FuYZhuJRichmanAAPOL1-G1 Nephrocytes induces hypertrophy and accelerates cell deathJ Am Soc Nephrol20172841106111627864430
- Kruzel-DavilaEShemerROfirAAPOL1-mediated cell injury involves disruption of conserved trafficking processesJ Am Soc Nephrol20172841117113027864431
- Kruzel-DavilaEWasserWGAviramSSkoreckiKAPOL1 nephropathy: from gene to mechanisms of kidney injuryNephrol Dial Transplant201631334935825561578
- RyomLKirkOLundgrenJDAdvanced chronic kidney disease, end-stage renal disease and renal death among HIV-positive individuals in EuropeHIV Med201314850350823590641
- NadkarniGNKonstantinidisIWyattCMHIV and the aging kidneyCurr Opin HIV AIDS20149434034524824884
- CheungCYWongKMLeeMPPrevalence of chronic kidney disease in Chinese HIV-infected patientsNephrol Dial Transplant200722113186319017575315
- YanagisawaNAndoMAjisawaAClinical characteristics of kidney disease in Japanese HIV-infected patientsNephron Clin Pract20111183c285c29121212692
- BigeNLanternierFViardJ-PPresentation of HIV-associated nephropathy and outcome in HAART-treated patientsNephrol Dial Transplant20112731114112121745806
- AttaMGChoiMJLongeneckerJCNephrotic range proteinuria and CD4 count as noninvasive indicators of HIV-associated nephropathyAm J Med2005118111288
- U.S. Renal Data SystemUSRDS 2007 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United StatesBethesda, MDNational Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases2007
- CoreshJByrd-HoltDAstorBCChronic kidney disease awareness, prevalence, and trends among U.S. adults, 1999 to 2000J Am Soc Nephrol200516118018815563563
- BrownTTColeSRLiXAntiretroviral therapy and the prevalence and incidence of diabetes mellitus in the multicenter AIDS cohort studyArch Intern Med2005165101179118415911733
- BrownTTLiXColeSRCumulative exposure to nucleoside analogue reverse transcriptase inhibitors is associated with insulin resistance markers in the Multicenter AIDS Cohort StudyAIDS200519131375138316103768
- TienPCSchneiderMFColeSRAntiretroviral therapy exposure and incidence of diabetes mellitus in the Women’s Interagency HIV StudyAIDS200721131739174517690572
- GazzarusoCBrunoRGarzanitiAHypertension among HIV patients: prevalence and relationships to insulin resistance and metabolic syndromeJ Hypertens20032171377138212817187
- JungOBickelMDittingTHypertension in HIV-1-infected patients and its impact on renal and cardiovascular integrityNephrol Dial Transplant20041992250225815238630
- LescureFXFlateauCPacanowskiJHIV-associated kidney glomerular diseases: changes with time and HAARTNephrol Dial Transplant20122762349235522248510
- AttaMGLongeneckerJCFineDMSonography as a predictor of human immunodeficiency virus–associated nephropathyJ Ultrasound Med200423560361015154526
- BerlinerARFineDMLucasGMObservations on a cohort of HIV-infected patients undergoing native renal biopsyAm J Nephrol20082847848618176076
- WilliamsDIWilliamsDJWilliamsIGUnwinRJGriffithsMHMillerRFPresentation, pathology, and outcome of HIV associated renal disease in a specialist centre for HIV/AIDSSex Transm Infect19987431791849849552
- EstrellaMFineDMGallantJEHIV type 1 RNA level as a clinical indicator of renal pathology in HIV-infected patientsClin Infect Dis200643337738016804855
- IzzedineHAcharyaVWirdenMRole of HIV-1 DNA levels as clinical marker of HIV-1-associated nephropathiesNephrol Dial Transplant201026258058320624771
- EitnerFCuiYHudkinsKLChemokine receptor (CCR5) expression in human kidneys and in the HIV infected macaqueKidney Int1998546194519549853259
- DianaNENaickerSUpdate on current management of chronic kidney disease in patients with HIV infectionInt J Nephrol Renovasc Dis2016922323427695357
- HatsukariISinghPHitosugiNDEC-205-Mediated internalization of HIV-1 results in the establishment of silent infection in renal tubular cellsJ Am Soc Nephrol200718378078717287423
- AttaMGGallantJERahmanMHAntiretroviral therapy in the treatment of HIV-associated nephropathyNephrol Dial Transplantat2006211028092813
- LucasGMEustaceJASozioSMentariEKAppiahKAMooreRDHighly active antiretroviral therapy and the incidence of HIV-1-associated nephropathyAIDS200418354154615090808
- PostFACampbellLJHamzahLPredictors of renal outcome in HIV-associated nephropathyClin Infect Dis20084681282128918444868
- CasasJPChuaWLoukogeorgakisSEffect of inhibitors of the renin-angiotensin system and other antihypertensive drugs on renal outcomes: systematic review and meta-analysisLancet200536695022026203316338452
- AlsauskasZCMedapalliRKRossMJExpert opinion on pharmacotherapy of kidney disease in HIV-infected patientsExpert Opin Pharmacother201112569170421250871
- KimmelPLMishkinGJUmanaWOCaptopril and renal survival in patients with human immunodeficiency virus nephropathyAm J Kidney Dis19962822022088768914
- WeiABurnsGCWilliamsBAMohammedNBVisintainerPSivakSLLong-term renal survival in HIV-associated nephropathy with angiotensin-converting enzyme inhibitionKidney Int20036441462147112969167
- LucasGMRossMJStockPGHIV Medicine Association of the Infectious Diseases Society of America Clinical practice guideline for the management of chronic kidney disease in patients infected with HIV: 2014 update by the HIV Medicine Association of the Infectious Diseases Society of AmericaClin Infect Dis2014599e96e13825234519
- EustaceJANuermbergerEChoiMScheelPJJrMooreRBriggsWACohort study of the treatment of severe HIV-associated nephropathy with corticosteroidsKidney Int20005831253126010972688
- LaradiAMalletABeaufilsHAllouacheMMartinezFHIV-associated nephropathy: outcome and prognosis factorsJ Am Soc Nephrol1998912232723359848787
- JotwaniVLiYGrunfeldCChoiAIShlipakMGRisk factors for ESRD in HIV-infected individuals: traditional and HIV-related factorsAm J Kidney Dis201259562863522206742
- RaschMGHellebergMFeldt-RasmussenBIncreased risk of dialysis and end-stage renal disease among HIV patients in Denmark compared with the background populationNephrol Dial Transplant20142961232123823975841
- DaveMBShabihKBlumSMaintenance hemodialysis in patients with HIV-associated nephropathyClin Nephrol1998506367749877110
- AttaMGFineDMKirkGDMehtaSHMooreRDLucasGMSurvival during renal replacement therapy among African Americans infected with HIV type 1 in urban Baltimore, MarylandClin Infect Dis200745121625163218190325
- CuriMAPappasPJSilvaMBJrHemodialysis access: influence of the human immunodeficiency virus on patency and infection ratesJ Vasc Surg199929460861610194487
- Recommendations for preventing transmission of infections among chronic hemodialysis patientsMMWR Recomm Rep200150RR-5143
- StockPGBarinBMurphyBOutcomes of kidney transplantation in HIV-infected recipientsN Engl J Med2010363212004201421083386
- WaheedSSakrAChhedaNDOutcomes of Renal Transplantation in HIV-1 Associated NephropathyPLoS One2015106e012970226061701
- MullerEBardayZMendelsonMKahnDHIV-positive-to-HIV-positive kidney transplantation–results at 3 to 5 yearsN Engl J Med201537261362025671253
- DurandCMSegevDSugarmanJRealizing HOPE: the ethics of organ transplantation from HIV-positive donorsAnn Intern Med201616513814227043422
- MullerEBardayZHIV-positive donor selection for HIV-positive transplant recipientsJ Am Soc Nephrol Epub2018112
- FineDMPerazellaMALucasGMAttaMGKidney biopsy in HIV: beyond HIV-associated nephropathyAm J Kidney Dis200851350451418295067
- JamesPAOrtizECarterBL2014 evidence-based guideline for the management of high blood pressure in adults: (JNC8)JAMA2014311550752024352797
- PhilipGreenlandEricPetersonThe New 2017 ACC/AHA guidelines “up the pressure” on diagnosis and treatment of hypertensionJAMA2017318212083208429159417