Abstract
Diarrhea is a common comorbidity present in patients with human immunodeficiency virus/acquired immune deficiency syndrome (HIV/AIDS) who are treated with highly active antiretroviral therapy. With a multifactorial etiology, this diarrhea often becomes difficult to manage. In addition, some antiretrovirals are associated with chronic diarrhea, which potentially creates an adherence barrier to antiretrovirals and may ultimately affect treatment outcomes and future therapeutic options for HIV. A predominant type of diarrhea that develops in HIV patients has secretory characteristics, including increased secretion of chloride ions and water into the intestinal lumen. One proposed mechanism that may lead to this type of secretory diarrhea is explained by the activation of the cystic fibrosis transmembrane conductance regulator and calcium-activated chloride channels. Crofelemer is a novel antidiarrheal agent that works by inhibiting both of these channels. The efficacy and safety of crofelemer has been evaluated in clinical trials for various types of secretory diarrhea, including cholera-related and acute infectious diarrhea. More recently, crofelemer was approved by the US Food and Drug Administration for the symptomatic relief of noninfectious diarrhea in adult patients with HIV/AIDS on antiretroviral therapy. Results from the ADVENT trial showed that crofelemer reduced symptoms of secretory diarrhea in HIV/AIDS patients. Because crofelemer is not systemically absorbed, this agent is well tolerated by patients, and in clinical trials it has been associated with minimal adverse events. Crofelemer has a unique mechanism of action, which may offer a more reliable treatment option for HIV patients who experience chronic secretory diarrhea from antiretroviral therapy.
Introduction
Secretory diarrhea is a common comorbidity found in patients infected with human immunodeficiency virus (HIV).Citation1–Citation3 Diarrhea can affect HIV patients at all stages of illness, with up to 60% of patients with HIV reporting symptoms of diarrhea.Citation1 In addition, one study showed that patients infected with HIV are significantly more likely to experience diarrhea than those without HIV infection (28% versus 7%, P < 0.001).Citation4 With the advent of highly active antiretroviral therapy (HAART), HIV patients are experiencing better clinical outcomes with improved survival. However, HIV-associated diarrhea remains common due to a multifactorial etiology that includes infection, malignancy, enteropathy, and antiretroviral treatment.Citation5 Because diarrhea is often intolerable for these patients, their quality of life can be negatively impacted, which can lead to possible nonadherence to antiretrovirals and medical care.Citation2 This could have clinical implications because nonadherence to antiretrovirals may result in drug resistance that can limit future antiretroviral options for HIV patients and cause poorer treatment outcomes. The impact on quality of life is illustrated in a national survey in which 40% of HIV patients reported that diarrhea negatively affected their social lives, causing them to alter their daily schedules and develop feelings of shame.Citation6
The type of diarrhea that develops in HIV patients generally has secretory properties. Secretory diarrhea results when there is an excess secretion of chloride ions followed by movement of sodium and water into the intestinal lumen. Increased secretion of chloride ions can occur when the cystic fibrosis transmembrane conductance regulator (CFTR) and calcium-activated chloride channels (CaCC) are overstimulated. HIV and various antiretroviral agents can activate these channels, leading to the development of secretory diarrhea.Citation7–Citation9 Severe complications of secretory diarrhea occur when the condition is left untreated. These complications include electrolyte abnormalities, acidosis, acute renal failure, hypovolemic shock, and even death.Citation10
Treatment of noninfectious or secretory diarrhea currently includes both pharmacological and nonpharmacological approaches. Pharmacological treatments comprise those listed in .Citation2,Citation11–Citation13 Most of these antidiarrheals are antimotility agents that cause unwanted side effects, such as constipation, bloating, and flatulence. Although these agents are commonly used, they do not target the causes of HIV-associated or HAART-associated diarrhea. Nonpharmacological supportive treatments may include dietary modifications consisting of fiber supplements, such as oat bran, concentrated vegetable powder, or psyllium.Citation11
Table 1 Pharmacologic agents available for the treatment of noninfectious diarrhea
Crofelemer is a novel agent that has been studied for the treatment of numerous types of diarrhea, including HIV-associated diarrhea, travelers’ diarrhea, infectious diarrhea, cholera-associated diarrhea, and diarrhea-predominant irritable bowel syndrome (IBS-D).Citation14–Citation17 provides a summary of the pivotal clinical trials that evaluated the use of crofelemer. Recently, crofelemer received approval from the US Food and Drug Administration (FDA) for the symptomatic relief of noninfectious diarrhea in adult patients with HIV/AIDS receiving antiretroviral therapy. Crofelemer’s unique mechanism of action makes this naturally occurring agent an appropriate and effective antidiarrheal for the HIV-infected population. This article reviews the mechanism of HIV-associated diarrhea and HAART-associated diarrhea, the pharmacology, efficacy, and safety of crofelemer in HIV patients, and important clinical considerations for HIV patients on antiretroviral therapy.
Table 2 Summary of clinical trials investigating the use of crofelemer for secretory diarrhea
HIV-associated diarrhea
Diarrhea is commonly experienced by the HIV/AIDS population and has either an infectious or noninfectious origin.Citation2 Opportunistic pathogens causing diarrhea include Cryptosporidium, Isospora belli, Microsporidia, and Mycobacterium avium-intracellulare. Other causative organisms include Salmonella, Shigella, and Campylobacter.Citation18 Diarrhea caused by opportunistic pathogens was more common prior to the advent of HAART. Noninfectious causes of diarrhea in HIV patients include HIV enteropathy, autonomic neuropathy, chronic pancreatitis and exocrine insufficiency, and the use of HAART.Citation2
HIV enteropathy is an idiopathic form of diarrhea that can occur during any stage of HIV infection. It comprises a variety of gastrointestinal (GI) illnesses including diarrhea, GI inflammation, increased intestinal permeability, and malabsorption. In particular, during the acute stage of HIV infection, gut-associated lymphoid tissue becomes one of the major sites for HIV replication, which can lead to a significant loss of CD4+ T-cells. When the GI tract is infiltrated by lymphocytes, inflammatory processes such as villous atrophy, crypt hyperplasia, and villous blunting can occur.Citation2,Citation19 HIV also causes local activation of immune cells, leading to the release in the GI tract of proinflammatory mediators such as interleukin-1, interleukin-4, interleukin-6, and interleukin-10, interferon-γ, tumor necrosis factor-α, β-chemokine RANTES (Regulated upon Activation, Normal T cell Expressed and Secreted), and macrophage inflammatory proteins 1α and 1β.Citation2 In addition, HIV infection can lead to structural deficiencies in the GI tract that result in the toxicity of enterocytes.Citation19 The HIV transactivating-factor protein can stimulate calcium-activated chloride ion secretion in enterocytes and colonic mucosa. This protein has also been shown to inhibit the proliferation of enterocytes and may actually induce apoptosis of these cells.Citation2 Another HIV protein involved in enteropathy is the envelope protein glycoprotein 120, which causes increased calcium concentration in enterocytes that leads to tubulin depolymerization and inadequate epithelial ion balance.Citation19 HIV protein R has also been shown to possess inflammatory properties. This protein also disrupts barrier function, which contributes to the development of HIV enteropathy. By these mechanisms, HIV itself can lead to GI dysfunction and possible secretory diarrhea.Citation2
HAART-associated diarrhea
Although HAART may attenuate the manifestation of HIV enteropathy by decreasing HIV viral replication and its possible effects on the GI system, it is also associated with increased incidence of diarrhea and other GI adverse effects.Citation2,Citation19,Citation20 One common class of antiretroviral drugs associated with diarrhea is protease inhibitors (PIs). Other antiretroviral classes include nucleoside reverse transcriptase inhibitors (NRTIs), nonnucleoside reverse transcriptase inhibitors, and integrase strand transfer inhibitors, but these tend to have lower incidences of diarrhea compared with PIs. In particular, the PI ritonavir, which is used concomitantly with other PIs to increase their concentrations, is known to cause diarrhea. Clinical studies have shown that lopinavir/ritonavir and fosamprenavir/ritonavir have the highest incidence of treatment-related grade 2–4 diarrhea (10%–15%) in comparison to other PI combinations such as atazanavir/ritonavir (2%–3%), darunavir/ritonavir (4%–8%), and saquinavir/ritonavir (6%–7%).Citation21–Citation32 A lower dose of ritonavir (100 mg total daily dose) is typically coadministered with atazanavir or darunavir than with lopinavir, fosamprenavir, or saquinavir (200 mg total daily dose), especially in HIV patients with no prior resistance to PIs – which may explain why a lower incidence of diarrhea is associated with atazanavir or darunavir.
A number of mechanisms that might explain the causes of HAART-induced diarrhea have been proposed. These include increased CaCC activity and apoptosis, necrosis, and blunted proliferation of the intestinal epithelium. Consequently, PIs may cause structural changes in the GI tract, altering water and electrolyte secretion and resulting in secretory diarrhea, also referred to in this case as leaky-flux diarrhea.Citation2,Citation33 One clinical study showed that HIV patients using the PI nelfinavir had increased concentrations of electrolytes and elevated pH in the feces, characteristic of secretory diarrhea.Citation34 Secretory diarrhea may also occur with PI use because PIs stimulate CaCC, leading to excess chloride secretion.Citation2
HAART-induced diarrhea remains a problem for the HIV population because it can negatively impact the quality of life for HIV patients and become an adherence barrier, which could cause potential resistance to antiretrovirals and poor treatment outcomes. The antimotility agents diphenoxylate/atropine or loperamide are commonly used to provide symptomatic relief of diarrhea in HIV patients. However, these therapeutic agents may not directly alleviate symptoms of diarrhea. In contrast, crofelemer has been shown to improve symptoms of diarrhea in patients with HIV/AIDS in clinical studies, where most of these patients were on PI-based antiretroviral regimens.Citation14,Citation35,Citation36 Crofelemer has a different mechanism of action compared with other antidiarrhea agents, and as a result it may provide a more effective treatment of chronic diarrhea for HIV patients and potentially increase adherence to antiretrovirals, leading to better treatment outcomes.
Clinical pharmacology
Chemistry
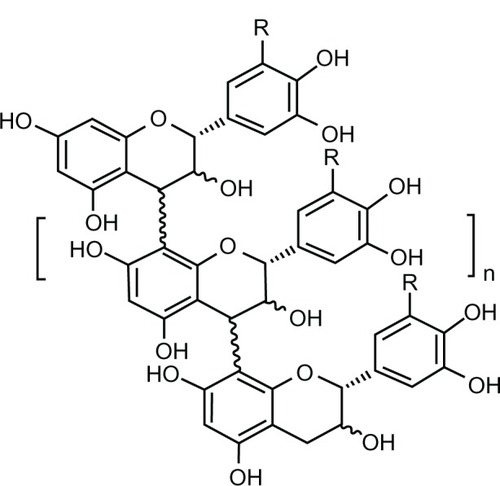
Crofelemer is a natural compound isolated from the stem bark latex of the Croton lechleri tree from the Euphorbiaceae family. This tree is commonly found in the western Amazonian region of South America.Citation37 Crofelemer is an acid-labile, proanthocyanidin oligomer with an average molecular weight of 2100 Da. The monomeric components of the polyphenolic molecule include (+)-catechin, (−)- epicatechin, (+)-gallocatechin, and (−)-galloepicatechin.Citation7,Citation38 depicts the chemical structure of crofelemer.Citation36
Figure 1 Chemical structure of crofelemer.Citation36
Mechanism of action
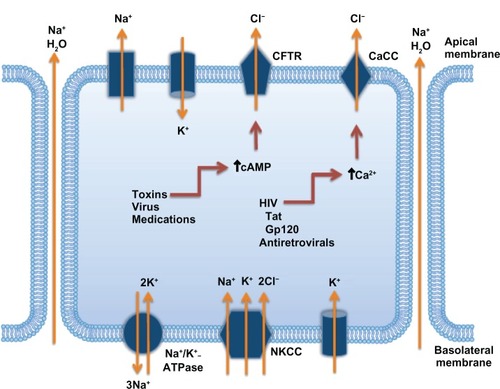
The exact mechanism of action of crofelemer remains unclear. However, studies have proposed plausible mechanisms by which crofelemer use causes decreased secretions from the intestinal membranes.Citation7,Citation37 One of these studies showed that crofelemer inhibited the cyclic adenosine monophosphate (cAMP)-stimulated CFTR chloride channel located on the intestinal apical membrane, as well as the CaCC located on the intestinal epithelial membrane.Citation7 Both of these chloride channels regulate chloride and fluid secretion in the intestine: activation of the CFTR and CaCC increase chloride and fluid secretions into the GI tract, contributing to secretory diarrhea. This mechanism is depicted in . Citation7–Citation9 Because crofelemer inhibits both of these channels, chloride secretion is decreased.Citation7 Thus, both stool weight and frequency are ultimately reduced, leading to relief of diarrhea, consistent with results from clinical studies using crofelemer for different types of secretory diarrhea.Citation14–Citation17,Citation35
Figure 2 Mechanisms involved in the development of secretory diarrhea.
Abbreviations: Ca2+, calcium; CaCC, calcium-activated chloride channels; cAMP, cyclic adenosine monophosphate; CFTR, cystic fibrosis transmembrane conductance regulator; Cl−, chloride; H2O, water; K+, potassium; Na+, sodium; NKCC, sodium potassium chloride cotransporter.
In addition, crofelemer seems to be highly active against diarrhea caused by particular bacterial species. Vibrio cholerae and Escherichia coli produce enterotoxins that cause an increase in cAMP production. The elevated levels of cAMP stimulate the CFTR chloride channel and, as a result, increase chloride and fluid secretion. Crofelemer is a useful agent in the treatment of diarrhea caused by these bacteria because of its inhibition of the CFTR chloride channel, as observed in several clinical studies.Citation15,Citation17 Crofelemer also exhibits antiviral activity against laboratory-identified strains, including respiratory syncytial virus, influenza A virus, parainfluenza virus, herpes virus 1 and 2, and hepatitis A and B. This activity appears to develop from crofelemer’s ability to bind to the viral envelope, preventing viral attachment and penetration of the host cell.Citation38
Pharmacokinetics
Oral crofelemer has little-to-no systemic absorption. Plasma concentrations are undetectable after oral administration of crofelemer.Citation39 Metabolites of crofelemer have not been identified. Food does not affect the efficacy or absorption of crofelemer, as coadministration with a fatty meal did not result in increased systemic exposure. Thus, crofelemer may be administered with or without food. Although in vitro studies show that crofelemer may inhibit cytochrome P450 isoenzyme 3A and the transporters multidrug resistance protein 2 and organic anion-transporting polypeptide 1A2, no clinically relevant drug–drug interactions exist with crofelemer. In particular, no drug–drug interactions were found between crofelemer and antiretrovirals such as nelfinavir, zidovudine, and lamivudine, although a 20% decrease in lamivudine exposure was observed in patients receiving crofelemer 500 mg four times daily. In addition, due to minimal absorption, crofelemer is unlikely to inhibit cytochrome P450 isoenzymes 1A2, 2A6, 2B6, 2C9, 2C19, 2D6, 2E1, and CYP3A4.Citation36 Pharmacokinetic dose-ranging studies have not been published for crofelemer, but clinical trials have used different dosing such as 125 mg to 500 mg every 6 to 12 hours. Recent FDA approval labeling for crofelemer recommends using the 125 mg delayed-release tablets twice daily for the treatment of symptomatic noninfectious diarrhea in adult HIV/AIDS patients on antiretroviral therapy.
Clinical efficacy
Crofelemer has been evaluated in clinical studies for the treatment of various types of secretory diarrhea, including cholera-related and acute infectious diarrhea as well as travelers’ diarrhea and IBS-D.Citation15–Citation17 In addition, the efficacy and safety of crofelemer has also been demonstrated in two pivotal studies for the treatment of HIV-associated diarrhea.Citation14,Citation35
A Phase II, randomized, double-blind, placebo-controlled study was designed to evaluate the safety and efficacy of crofelemer for the treatment of HIV-associated diarrhea.Citation14 HIV patients between 18 and 60 years of age and with chronic diarrhea were included in this study. Chronic diarrhea was defined as having at least three soft or watery stools with stool weight of more than 200 grams per day. Eligible patients were also required to have a diagnosis of AIDS as defined by the Centers for Disease Control and be on appropriate antiretroviral therapy for at least 2 weeks before screening and throughout the duration of the clinical trial. Study patients discontinued all antidiarrheal agents at least 24 hours prior to study initiation. Baseline stool weight and frequency were determined for each participant during a 24-hour observation period. Patients included in the study were randomized to one of two treatment arms: 500 mg (two 250 mg capsules) of crofelemer every 6 hours for 4 days, or two placebo capsules every 6 hours for 4 days. The primary outcome of this study was the efficacy of crofelemer, as assessed by parameters such as stool weight and frequency. Secondary outcomes included daily output of stool chloride and daily GI index score.Citation14
A total of 51 patients were enrolled in this Phase II trial, with 26 in the crofelemer arm and 25 in the placebo arm. However, six of the 51 patients did not meet at least one of the inclusion criteria. Baseline demographics were assessed and no significant differences between the two treatment arms were found. The mean baseline stool weight assessed during the 24-hour observation period was 914.8 g for the crofelemer arm and 813.9 g for the placebo arm. The mean baseline stool frequency assessed during the 24-hour observation period was 5.2 stools for both the crofelemer arm and placebo arm.Citation14
In comparisons of results at day 4 compared with baseline, patients treated with crofelemer had a greater average reduction in stool weight and frequency compared with the placebo group. The mean reduction in stool weight from baseline to day 4 was 451.3 g/day for the crofelemer arm and 150.7 g/day for the placebo arm (P = 0.14). The mean reduction in stool frequency from baseline to day 4 was 3.0 stools/day in the crofelemer arm and 2.0 stools/day in the placebo arm (P = 0.30). Regression models revealed that patients treated with crofelemer had a statistically significant decrease in stool weight (P = 0.008) and frequency (P = 0.04) at day 4. No significant differences in the daily GI index score were found between the two treatment arms. Patients in the crofelemer arm experienced a mean reduction in chloride concentration of 7.1 mEq/g (P = 0.037) after 4 days, whereas patients in the placebo arm experienced an increase of 3.4 mEq/g (P = 0.41).Citation14 The results of this Phase II trial illustrate that crofelemer may be useful in reducing stool weight and frequency in patients with HIV-associated diarrhea. Another important observation from this study is that 77% of patients were on PI-based antiretroviral regimens. This further supports the role of crofelemer in the treatment of diarrhea in HIV patients whose diarrhea has multifactorial causes, including HAART
The ADVENT study was a Phase III, randomized, double-blind placebo-controlled multicenter trial designed to evaluate the efficacy of crofelemer in the treatment of secretory diarrhea in HIV-infected patients.Citation35 For this study, diarrhea was defined as persistently loose stools even with regular use of antidiarrheal agents, or one or more watery stools per day without use of antidiarrheal agents. Eligible patients included in the study were those receiving stable antiretroviral therapy, had a history of diarrhea for at least 1 month, had CD4+ cell counts > 100 cells/μL, and had no evidence of infection with an intraluminal pathogen. Patients were excluded if they had a history of GI disease that caused diarrhea. The ADVENT study consisted of two stages: optimal dosing was established in stage 1 and safety and efficacy of crofelemer were assessed in stage 2. Each stage included two phases, a 4-week placebo-controlled phase and 5-month treatment-extension phase where all patients received crofelemer. A 10-day screening period, during which all patients received placebo, preceded the placebo-controlled phase. Randomization to the placebo-controlled phase occurred only if patients experienced one or more watery bowel movements per day on at least 5 of the last 7 days of the screening period. The primary efficacy endpoint of the study was the proportion of patients who demonstrated a response to crofelemer; clinical response was defined as no more than two watery bowel movements per week for at least 2 of the 4 weeks of the placebo-controlled phase.Citation35,Citation36
Three-hundred seventy-four patients were enrolled and randomized to the following treatment arms: 236 in the crofelemer arm and 138 in the placebo arm. Baseline demographics of these patients included the following characteristics: 85% male, 46% Caucasian, and 32% African-American, and a median age of 45 years (range, 21–68 years). Of the patients enrolled in the study, 39% had CD4+ cell counts <404, 81% had an undetectable HIV viral load, and 7%, 3%, and 9% had HIV viral loads of ≥1000, 400–999, and <400 HIV copies/mL, respectively. In addition, the median time since diagnosis of HIV was 12 years, the median time since the onset of diarrhea was 4 years, and the median number of daily watery bowel movements was 2.5 per day. PI based antiretroviral regimens were the most common of the treatment groups (64% in the crofelemer 125 mg twice daily group). Among these, 22% of patients in the crofelemer 125 mg twice daily group were receiving lopinavir/ritonavir, making it the most frequently used PI. Tenofovir/emtricitabine was the most commonly used NRTI antiretroviral backbone (33% in the crofelemer 125 mg twice daily group).Citation36
In stage 1, patients were randomized 1:1:1:1 to receive either crofelemer 125 mg, 250 mg, 500 mg, or placebo twice daily. Results from stage 1 of the ADVENT study revealed an optimal dosing regimen of crofelemer 125 mg twice daily, as patients in this arm experienced a better clinical response than those in the placebo group (20.5% versus 2%; P-value not reported). In stage 2, patients were randomized to receive either crofelemer 125 mg twice daily or placebo in the first phase. During this phase, 16.3% of patients in the crofelemer arm experienced a clinical response, compared with 11.4% in the placebo arm (P-value not reported). During the second phase, all patients received crofelemer (patients who were on placebo from the first phase in stage 2 crossed over to receive crofelemer). Combined data from both phases revealed that 17.6% of patients in the crofelemer arm demonstrated a significant clinical response versus only 8% in the placebo arm (one-sided P < 0.01). Patients who received the placebo during the 4-week phase and then crossed-over to crofelemer during the 5-month treatment extension phase showed considerable improvement after 1 month of use (36% versus 9%; odds ratio = 5.85, P < 0.0001). These patients were also found to have a greater chance of experiencing a clinical response in the remaining 4 months of the 5-month treatment extension phase. Treatment response was consistent among prespecified subgroups, including: duration of diarrhea, baseline number of daily watery bowel movements, use of PIs, CD4+ cell count, and age. However, crofelemer was found to be less effective in African-Americans than non-African-Americans when examining treatment-effect consistency across race subgroups.Citation35,Citation36 Results from the ADVENT study provide supportive evidence for the recent FDA approval of crofelemer 125 mg delayed-release tablets twice daily for the symptomatic relief of noninfectious diarrhea in adults with HIV/AIDS treated with antiretroviral therapy.
Safety and tolerability
Overall, oral crofelemer is a safe and tolerable drug as illustrated in clinical studies.Citation14–Citation17,Citation35 In the Phase II clinical trial evaluating the use of crofelemer in HIV-infected patients, no serious adverse effects were reported by patients and the treatment was well tolerated.Citation14 Similarly, preliminary data from the ADVENT study show that the safety profile of crofelemer is comparable to placebo in HIV-infected patients.Citation35 The number of patients experiencing adverse events was similar between the crofelemer group and placebo group (34.6% versus 32.8%, P-value not reported). No patients in the crofelemer group discontinued the study due to adverse events, whereas 3% discontinued in the placebo group. Serious adverse events were experienced by 1% of patients on crofelemer versus 3% of patients on placebo. Combined data from the ADVENT trial and an ongoing 48-week open-label safety study (N = 439) revealed that the most frequently experienced adverse effects in patients receiving crofelemer 125 mg twice daily include: upper respiratory tract infection (23.5%), urinary tract infection (7.8%), abdominal pain (6.2%), flatulence (5.6%), hemorrhoids (3.6%), and dyspepsia (2.5%).Citation35,Citation40 Combined data from three placebo-controlled trials studying the use of crofelemer 125 mg twice daily in a total of 229 HIV-infected patients revealed that the most common adverse reactions with incidence rates ≥3% included upper respiratory tract infections, bronchitis, cough, flatulence, and increased bilirubin.Citation36 In another study evaluating the efficacy and safety of crofelemer in patients with IBS-D, the most common adverse events included constipation (5%) in patients receiving crofelemer 125 mg, flatulence (7%) in patients receiving crofelemer 250 mg, and worsening IBS-D (5%) and abdominal pain (5%) in patients receiving crofelemer 500 mg.Citation16 Because systemic absorption of crofelemer is minimal, the occurrence of serious adverse events is low, indicating that crofelemer is a considerably safer option for HIV-infected patients on antiretroviral therapy who require treatment of secretory diarrhea.
Limitations
Few clinical studies have evaluated the efficacy and safety of crofelemer for the treatment of HIV-associated diarrhea.Citation14,Citation35 Some limitations from the Phase II clinical trial are the following: a small sample size, short treatment duration, predominantly male population, limited dietary options during admission to study unit, uncertainty about the use of prior antibiotics that could affect the course of diarrhea, suboptimal follow-up data, and insufficient safety data.Citation14 In particular, the safety data on the types of adverse events experienced and the percentages of patients experiencing those adverse effects were lacking in this study. On the contrary, the ADVENT study comprised a larger sample of patients, had patients receiving crofelemer for a much longer time, and recorded adequate safety data. As with the Phase II study, one limitation from the ADVENT study was the inclusion of a predominantly male population; thus, the generalizability to females is unknown.Citation35 Clinical trials evaluating the efficacy and safety of crofelemer in HIV-infected patients are needed to determine the long-term benefits for patients experiencing chronic secretory diarrhea caused by either the HIV disease state and/or HAART. In addition, head-to-head clinical trials comparing crofelemer with other antidiarrheal agents have not been evaluated in HIV-infected patients on antiretroviral therapy. These are warranted and may be useful to derive a better understanding of the role of crofelemer in clinical practice.
Clinical implications
Crofelemer is a novel agent used for the treatment of various types of secretory diarrhea, including HIV-associated diarrhea. Diarrhea is a common comorbidity in patients infected with HIV/AIDS for a variety of reasons, including opportunistic infections, malignancies, HIV enteropathy, and antiretroviral agents. Diarrheal illness is often intolerable, which creates a potential adherence barrier to antiretrovirals and follow-up medical care.Citation5 Because clinical studies have shown that crofelemer presents a viable option for the treatment of noninfectious diarrhea in adult HIV/AIDS patients on antiretroviral therapy, it is possible that adherence to HAART might improve with continued use of crofelemer.Citation14,Citation35 Adherence to antiretrovirals is critical because it is usually associated with better treatment outcomes, including higher CD4+ cell counts, lower viral load, preservation of current and future antiretroviral therapy, and reduced risk of transmission of HIV and resistant viral strains.
Crofelemer may offer a suitable treatment option for HIV-infected patients who use antiretroviral therapy consisting of a higher total daily dosing of ritonavir, such as lopinavir/ritonavir, and experience adverse effects, such as chronic diarrhea. The most common PI used by patients in the ADVENT study was lopinavir/ritonavir. In addition, the CASTLE study illustrates the difference in incidence rates of GI adverse events between two commonly used PI-based regimens: atazanavir/ritonavir and lopinavir/ritonavir.Citation20 Patients treated with lopinavir/ritonavir experienced intolerable GI adverse effects, such as diarrhea, more frequently than patients treated with atazanavir/ritonavir, resulting in more frequent antidiarrheal use (22% versus 9%). The IBS-Quality of Life tool was used to assess differences in quality of life between the two groups. Results show that the lopinavir/ritonavir group experienced a reduced mean IBS-Quality of Life.Citation20 Nonetheless, lopinavir/ritonavir is still a common PI-based antiretroviral regimen recommended for and used by HIV-infected patients worldwide. Because this regimen often requires concomitant treatment with antidiarrheals, crofelemer could have far-reaching clinical implications. Given that other antiretroviral classes such as NRTIs, non-NRTIs, and integrase strand transfer inhibitors are also associated with diarrhea (but usually less commonly than the PIs), crofelemer may also provide symptomatic relief of chronic diarrhea for HIV-infected patients on these antiretroviral-based regimens.
HIV-associated diarrhea is often secretory in nature, which supports the role of crofelemer due to its anti-secretory mechanism of action. Crofelemer may offer a viable therapeutic option for HIV-infected patients who experience diarrhea caused by HIV itself. In particular, patients may benefit even more so from using crofelemer during the acute phase of HIV infection, when HIV enteropathy occurs more commonly.Citation2,Citation14
Crofelemer may be more efficacious than presented in the ADVENT study, because even though the median number of daily watery bowel movements was 2.5 per day for patients, a strict criterion for the primary outcome requiring less than two watery stools per week for ≥2 of 4 weeks was enforced. The complete results from the ADVENT study have yet to be published and when they become available, they may offer a better understanding of the efficacy and safety of crofelemer in the HIV-infected patient population on antiretroviral therapy.
One of the strengths of the ADVENT study was the inclusion of a diverse ethnic population (46% Caucasians and 32% African Americans). It was noted in this study that crofelemer was less effective for African-Americans than non-African-Americans.Citation36 Reasons for this discrepancy in efficacy is unclear, but a possible underlying explanation may have a genetic basis, as the presence of CFTR and CaCC channels on the intestinal epithelium may vary by ethnicity, thus affecting the targeting effects of crofelemer.
Although no clinical trials have compared the efficacy of crofelemer with other commonly used antidiarrheal agents () for the symptomatic relief of diarrhea in HIV-infected patients, it is important to highlight the advantages of crofelemer.Citation2,Citation11–Citation13 These include minimal systemic absorption, low incidence of adverse events, and few drug–drug interactions. Furthermore, another significant advantage of crofelemer lies in its mechanism of action, as it is the only therapeutic agent that specifically inhibits both CFTR and CaCC, the activation of which is one of the primary causes of secretory diarrhea. Unlike other antidiarrheal agents, crofelemer does not interfere with peristalsis and does not cause the formation of a viscous liquid by holding water in stool, thereby limiting potential adverse effects such as bloating, constipation, and flatulence. In addition, several of the antidiarrheal agents available on the market, such as diphenoxylate/atropine, show inconsistent clinical efficacy results in this patient population. Although antidiarrheals such as polycarbophil, kaolin-pectin, attapulgite, and bismuth subsalicylate are commonly used to treat diarrhea, they have yet to be studied in patients with PI-associated diarrhea.Citation11
Conclusion
Crofelemer is a first-in-class oral natural agent that is useful for the treatment of secretory diarrhea because of its unique and targeted mechanism of action involving inhibition of both CFTR and CaCC. Clinical studies have shown it to be a very safe and well-tolerated agent. Crofelemer was recently approved by the FDA for the symptomatic relief of diarrhea in HIV/AIDS patients on antiretroviral therapy, because of its efficacy and safety observed in clinical studies. Crofelemer may provide a suitable treatment for noninfectious, chronic diarrhea associated with HIV and HAART. Furthermore, crofelemer could eliminate a potential barrier to antiretroviral treatment adherence, reducing the incidence of resistance to current antiretrovirals, preserving future antiretroviral options, and improving the overall quality of life for HIV-infected patients. Crofelemer may be especially useful for HIV-infected patients who experience chronic diarrhea from antiretroviral therapy, including PI-based regimens, reducing the need for modification of antiretroviral therapy and allowing for sustained treatment with potent HAART. Crofelemer may offer a more effective and safe alternative to other antidiarrheals. Head-to-head clinical trials comparing crofelemer to other antidiarrheals are needed. In addition, the long-term safety and efficacy of crofelemer should be explored in clinical studies that include larger, more-diverse HIV-infected populations, measurement of adherence to antiretroviral therapy, and analysis of subsequent treatment outcomes.
Disclosure
The authors report no conflicts of interest in this work.
References
- ZingmondDSKilbourneAMJusticeACDifferences in symptom expression in older HIV-positive patients: the Veterans Aging Cohort 3 Site Study and HIV Cost and Service Utilization Study experienceJ Acquir Immune Defic Syndr200333Suppl 2S84S9212853857
- MacArthurRDDuPontHLEtiology and pharmacologic management of noninfectious diarrhea in HIV-infected individuals in the highly active antiretroviral therapy eraClin Infect Dis201255686086722700829
- LorenzKAShapiroMFAschSMBozzetteSAHaysRDAssociations of symptoms and health-related quality of life: findings from a national study of persons with HIV infectionAnn Intern Med20011349 Pt 285486011346321
- SiddiquiUBiniEJChandaranaKPrevalence and impact of diarrhea on health-related quality of life in HIV-infected patients in the era of highly active antiretroviral therapyJ Clin Gastroenterol200741548449017450031
- HillABalkinARisk factors for gastrointestinal adverse events in HIV treated and untreated patientsAIDS Rev2009111303819290032
- SiegelKSchrimshawEWBrown-BradleyCJLekasHMSources of emotional distress associated with diarrhea among late middle-age and older HIV-infected adultsJ Pain Symptom Manage201040335336920579836
- TradtrantipLNamkungWVerkmanASCrofelemer, an antisecretory antidiarrheal proanthocyanidin oligomer extracted from Croton lechleri, targets two distinct intestinal chloride channelsMol Pharmacol2010771697819808995
- ZhangWFujiiNNarenAPRecent advances and new perspectives in targeting CFTR for therapy of cystic fibrosis and enterotoxin-induced secretory diarrheasFuture Med Chem20124332934522393940
- ThiagarajahJRVerkmanASCFTR inhibitors for treating diarrheal diseaseClin Pharmacol Ther201292328729022850599
- BarrettKEKeelySJChloride secretion by the intestinal epithelium: molecular basis and regulatory aspectsAnnu Rev Physiol20006253557210845102
- ShermanDSFishDNManagement of protease inhibitor-associated diarrheaClin Infect Dis200030690891410854364
- Berni CananiRSecondoAPassarielloAZinc inhibits calcium-mediated and nitric oxide-mediated ion secretion in human enterocytesEur J Pharmacol20106262–326627019819236
- PrimiMPBuenoLBaumerPBerardHLecomteJMRacecadotril demonstrates intestinal antisecretory activity in vivoAliment Pharmacol Ther199913Suppl 63710646045
- HolodniyMKochJMistalMA double blind, randomized, placebo-controlled phase II study to assess the safety and efficacy of orally administered SP-303 for the symptomatic treatment of diarrhea in patients with AIDSAm J Gastroenterol199994113267327310566728
- DiCesareDDuPontHLMathewsonJJA double blind, randomized, placebo-controlled study of SP-303 (Provir) in the symptomatic treatment of acute diarrhea among travelers to Jamaica and MexicoAm J Gastroenterol200297102585258812385443
- MangelAWChaturvediPEvaluation of crofelemer in the treatment of diarrhea-predominant irritable bowel syndrome patientsDigestion200878418018619092244
- BardhanPSharmaABolmallCSafety and efficacy of a novel anti-secretory anti-diarrheal agent crofelemer (NP-303), in the treatment of adult acute infectious diarrhea and cholera, with or without the use of antibioticsProceedings of the US–Japan CMSP: 13th International Conference on Emerging Infectious Diseases (EID) in the Pacific Rim – Focused on Enteric DiseasesApril 6–9, 2009Kolkata, India
- KartalijaMSandeMADiarrhea and AIDS in the era of highly active antiretroviral therapyClin Infect Dis1999284701705 quiz 706–70710825021
- BrenchleyJMDouekDCHIV infection and the gastrointestinal immune systemMucosal Immunol200811233019079157
- MalanNSuJManciniMCASTLE Study TeamGastrointestinal tolerability and quality of life in antiretroviral-naive HIV-1-infected patients: data from the CASTLE studyAIDS Care201022667768620467943
- JohnsonMGrinsztejnBRodriguezCAtazanavir plus ritonavir or saquinavir, and lopinavir/ritonavir in patients experiencing multiple virological failuresAIDS200519768569415821394
- JohnsonMGrinsztejnBRodriguezC96-week comparison of once-daily atazanavir/ritonavir and twice-daily lopinavir/ritonavir in patients with multiple virologic failuresAIDS200620571171816514301
- MolinaJMAndrade-VillanuevaJEchevarriaJCASTLE Study TeamOnce-daily atazanavir/ritonavir versus twice-daily lopinavir/ritonavir, each in combination with tenofovir and emtricitabine, for management of antiretroviral-naive HIV-1-infected patients: 48 week efficacy and safety results of the CASTLE studyLancet2008372963964665518722869
- MolinaJMAndrade-VillanuevaJEchevarriaJCASTLE Study TeamOnce-daily atazanavir/ritonavir compared with twice-daily lopinavir/ritonavir, each in combination with tenofovir and emtricitabine, for management of antiretroviral-naive HIV-1-infected patients: 96-week efficacy and safety results of the CASTLE studyJ Acquir Immune Defic Syndr201053332333220032785
- BánhegyiDKatlamaCda CunhaCAWeek 96 efficacy, virology and safety of darunavir/r versus lopinavir/r in treatment-experienced patients in TITANCurr HIV Res201210217118122339125
- MadrugaJVBergerDMcMurchieMTITAN Study GroupEfficacy and safety of darunavir-ritonavir compared with that of lopinavir-ritonavir at 48 weeks in treatment-experienced, HIV-infected patients in TITAN: a randomised controlled phase III trialLancet20073709581495817617272
- MillsAMNelsonMJayaweeraDOnce-daily darunavir/ritonavir vs lopinavir/ritonavir in treatment-naive, HIV-1-infected patients: 96-week analysisAIDS200923131679168819487905
- OrtizRDejesusEKhanlouHEfficacy and safety of once-daily darunavir/ritonavir versus lopinavir/ritonavir in treatment-naive HIV-1-infected patients at week 48AIDS200822121389139718614861
- WalmsleySAvihingsanonASlimJGemini: a noninferiority study of saquinavir/ritonavir versus lopinavir/ritonavir as initial HIV-1 therapy in adultsJ Acquir Immune Defic Syndr200950436737419214123
- EronJJrYeniPGatheJJrKLEAN Study TeamThe KLEAN study of fosamprenavir-ritonavir versus lopinavir-ritonavir, each in combination with abacavir-lamivudine, for initial treatment of HIV infection over 48 weeks: a randomised non-inferiority trialLancet2006368953447648216890834
- PulidoFEstradaVBarilJGLong-term efficacy and safety of fosamprenavir plus ritonavir versus lopinavir/ritonavir in combination with abacavir/lamivudine over 144 weeksHIV Clin Trials2009102768719487177
- HicksCBDeJesusESloanLMCOL100758 Study TeamComparison of once-daily fosamprenavir boosted with either 100 or 200 mg of ritonavir, in combination with abacavir/lamivudine: 96-week results from COL100758AIDS Res Hum Retroviruses200925439540319320570
- Braga NetoMBAguiarCVMacielJGEvaluation of HIV protease and nucleoside reverse transcriptase inhibitors on proliferation, necrosis, apoptosis in intestinal epithelial cells and electrolyte and water transport and epithelial barrier function in miceBMC Gastroenterol2010109020701796
- RufoPALinPWAndradeADiarrhea-associated HIV-1 APIs potentiate muscarinic activation of Cl-secretion by T84 cells via prolongation of cytosolic Ca2+ signalingAm J Physiol Cell Physiol20042865C998C100815075198
- MacArthurRHawkinsTBrownSLaMarcaAChaturvediPErnstJADVENT Trial: crofelemer for the treatment of secretory diarrhea in HIV+ individualsProceedings of the 19th Conference on Retroviruses and Opportunistic InfectionsMarch 5–8, 2012Seattle, WA
- Fulyzaq® (crofelemer) [prescribing Information]Raleigh, NCSalix Pharmaceuticals, Inc2013 Available from: http://www.fulyzaq.com/Accessed April 1, 2013
- FischerHMachenTEWiddicombeJHA novel extract SB-300 from the stem bark latex of Croton lechleri inhibits CFTR-mediated chloride secretion in human colonic epithelial cellsJ Ethnopharmacol2004932–335135715234776
- UbillasRJoladSBrueningRCSP-303, an antiviral oligomeric proanthocyanidin from the latex of Croton lechleri (Sangre de Drago)Phytomedicine1994127710623195881
- GabrielSEDavenportSESteagallRJVimalVCarlsonTRozhonEJA novel plant-derived inhibitor of cAMP-mediated fluid and chloride secretionAm J Physiol19992761 Pt 1G58G639886979
- MacArthurRHawkinsTBrownSSafety and tolerability of crofelemer 125 mg for treating non-infectious diarrhea in HIV+ individuals: results from double-blind and open-label studiesProceedings of the 20th Conference on Retroviruses and Opportunistic InfectionsMarch 3–6, 2013Atlanta, GA